Lentilactobacillus buchneri Preactivation Affects the Mitigation of Methane Emission in Corn Silage Treated with or without Urea
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Chemical and Microbiological Analyses
2.3. Determination of Aerobic Stability
2.4. Determination of in vitro Gas Production, Methane Content, Metabolizable Energy, Organic Matter Digestibility, and Protein Degradability
2.5. Statistical Analysis
3. Results and Discussion
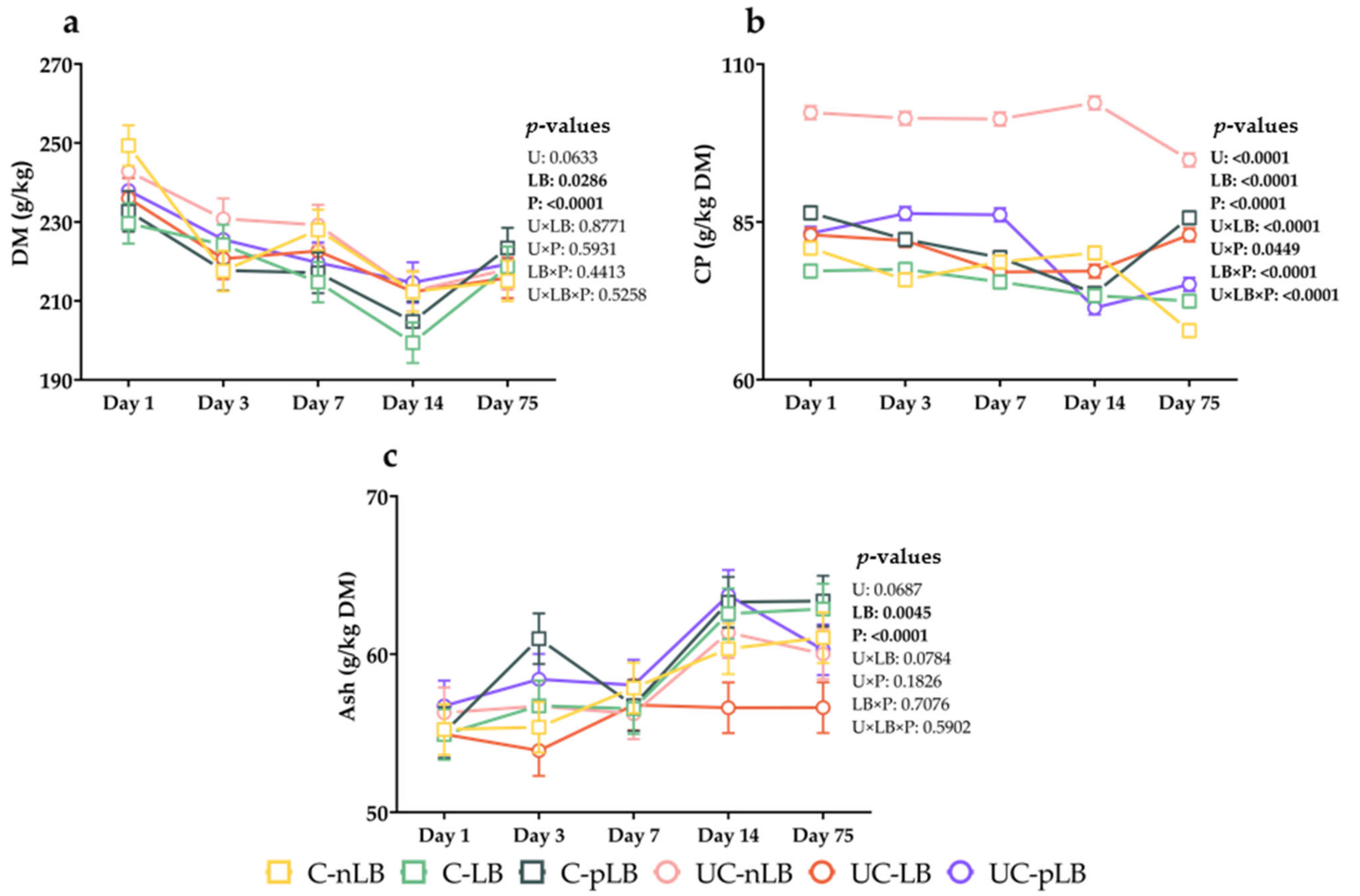
3.1. Effect of Urea-Treatment and Preactivation of L. buchneri on Chemical Compositions of Corn Silage
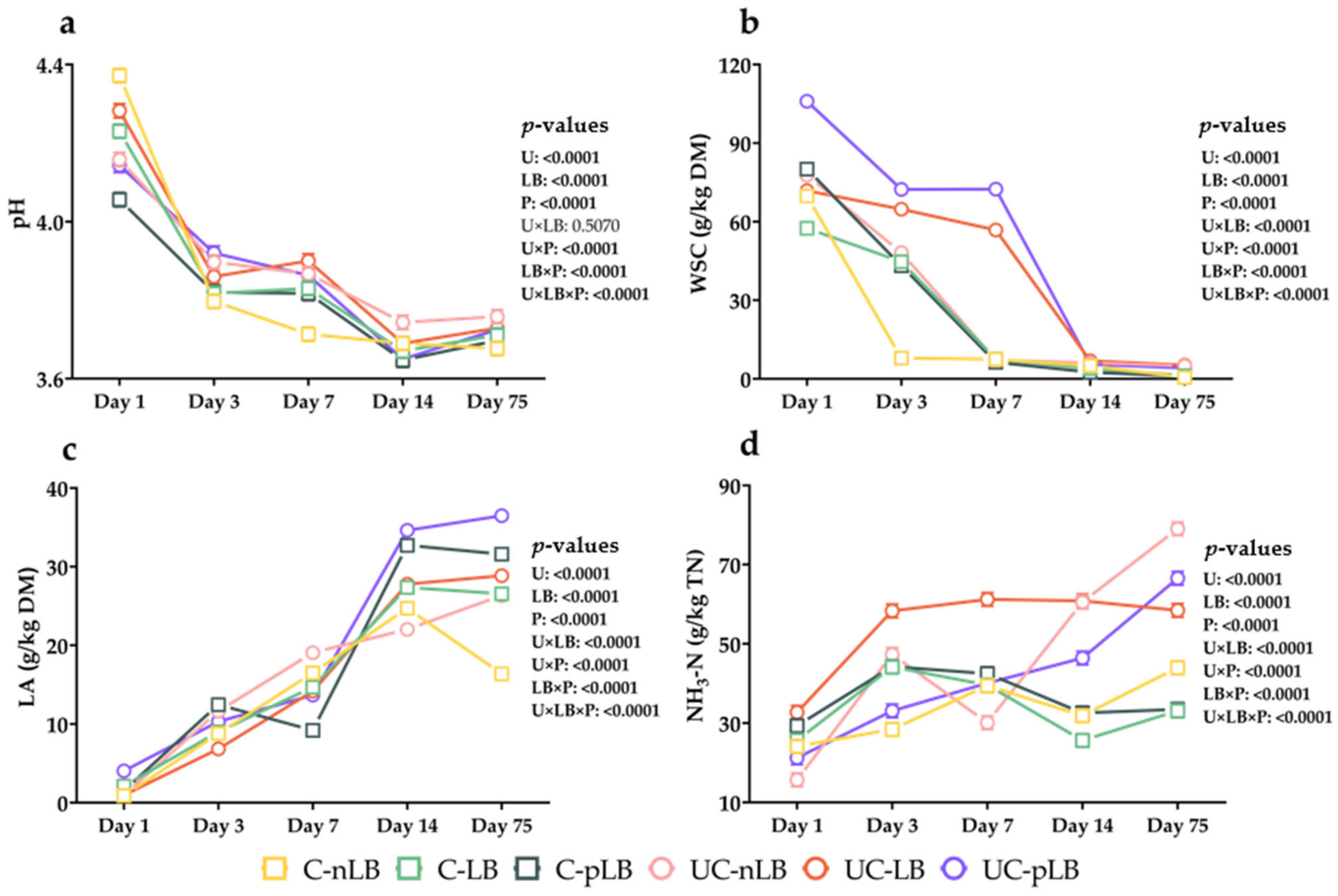
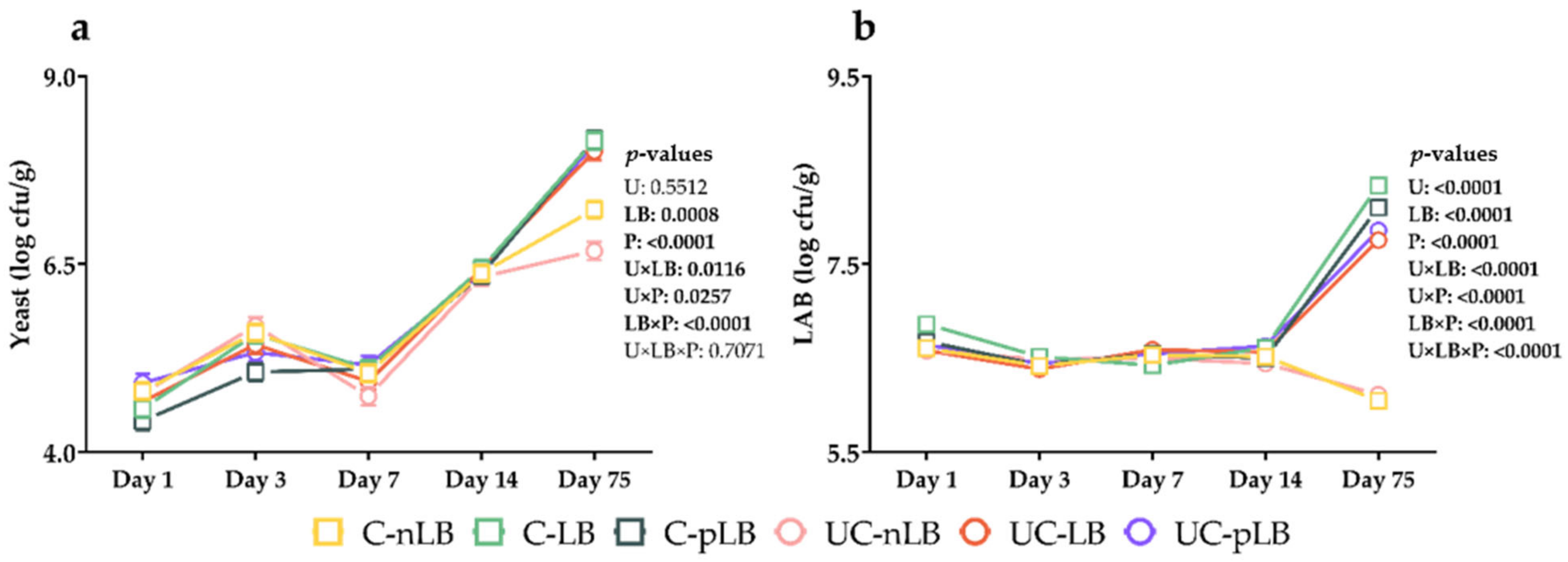
3.2. Effect of Urea-Treatment and Preactivation of L. buchneri on Fermentation Characteristics of Corn Silage
3.3. Effect of Urea Treatment and L. buchneri Forms on Aerobic Stability of Corn Silage
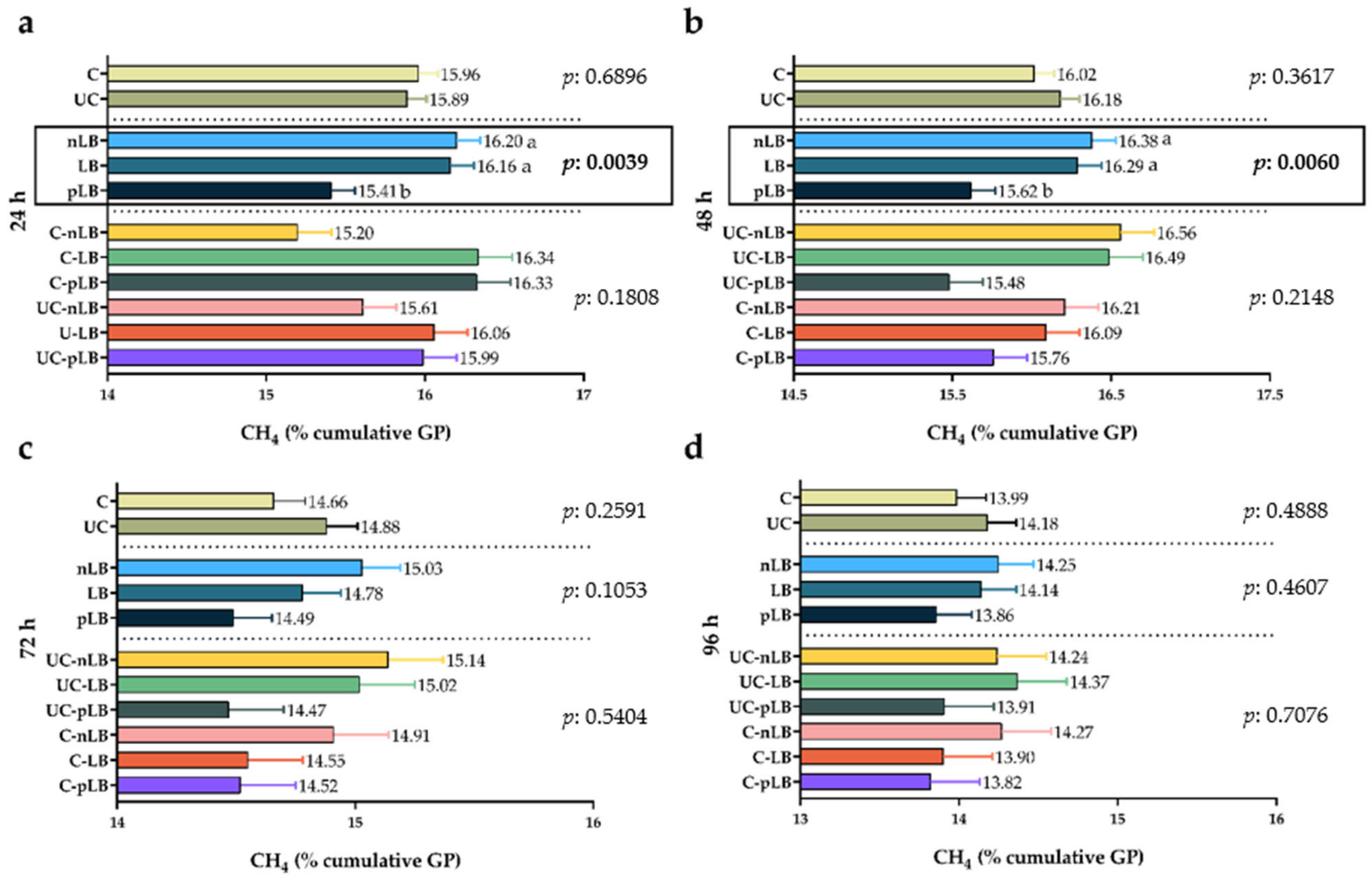
3.4. Effect of Urea-Treatment and Preactivation of L. buchneri on Total Production of Gas and Methane from Corn Silage
3.5. Factors Affecting In Vitro Gas Production and Methane Ratio in Corn Silages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samarappuli, D.; Berti, M.T. Intercropping Forage Sorghum with Maize Is a Promising Alternative to Maize Silage for Biogas Production. J. Clean. Prod. 2018, 194, 515–524. [Google Scholar] [CrossRef]
- Lan, W.; Yang, C. Science of the Total Environment Ruminal Methane Production: Associated Microorganisms and the Potential of Applying Hydrogen-Utilizing Bacteria for Mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Gislon, G.; Colombini, S.; Borreani, G.; Sandrucci, A.; Galassi, G. Milk Production, Methane Emissions, Nitrogen, and Energy Balance of Cows Fed Diets Based on Different Forage Systems. J. Dairy Sci. 2020, 103, 8048–8061. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane Mitigation in Ruminants: From Microbe to the Farm Scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef]
- Kader Esen, V.; Palangi, V. Genetic Improvement and Nutrigenomic Management of Ruminants to Achieve Enteric Methane Mitigation: A Review. Methane 2022, 1, 342–354. [Google Scholar] [CrossRef]
- Besharati, M.; Palangi, V.; Moaddab, M.; Nemati, Z.; Pliego, A.B.; Salem, A.Z.M. Influence of Cinnamon Essential Oil and Monensin on Ruminal Biogas Kinetics of Waste Pomegranate Seeds as a Biofriendly Agriculture Environment. Waste Biomass Valorization 2021, 12, 2333–2342. [Google Scholar] [CrossRef]
- Besharati, M.; Palangi, V.; Taghizadeh, A.; Kaya, A.; Abachi, S. Determining the Effect of Natural Inhibitors on Sesame Meal Degradability Using In Vitro Three Step Method. Vet. Arh. 2021, 91, 513–521. [Google Scholar] [CrossRef]
- Esen, S.; Okuyucu, B.; Koç, F.; Özdüven, M.L. Determination of Nutritional Quality and Aerobic Stability of Sorghum, Maize, and Sorghum-Maize Mixture Silages. J. Tekirdag Agric. Fac. 2022, 19, 61–69. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Muck, R.E. Ensiling in 2050: Some Challenges and Opportunities. Grass Forage Sci. 2019, 74, 178–187. [Google Scholar] [CrossRef]
- Boland, T.M.; Kiely, P.O. Grass and Forage Science In Vitro Rumen Methane Output of Grasses and Grass Silages Differing in Fermentation Characteristics Using the Gas-Production Technique (GPT). Grass Forage Sci. 2012, 68, 228–244. [Google Scholar] [CrossRef]
- Hassanat, F.; Gervais, R.; Julien, C.; Massé, D.I.; Lettat, A.; Chouinard, P.Y.; Petit, H.V.; Benchaar, C. Replacing Alfalfa Silage with Corn Silage in Dairy Cow Diets: Effects on Enteric Methane Production, Ruminal Fermentation, Digestion, N Balance, and Milk Production. J. Dairy Sci. 2013, 96, 4553–4567. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Hassanat, F.; Gervais, R.; Chouinard, P.Y.; Petit, H.V.; Massé, D.I. Methane Production, Digestion, Ruminal Fermentation, Nitrogen Balance, and Milk Production of Cows Fed Corn Silage- or Barley Silage-Based Diets. J. Dairy Sci. 2014, 97, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.C.; Adesogan, A.T.; Lara, E.C.; Rabelo, C.H.S.; Berchielli, T.T.; Teixeira, I.A.M.A.; Siqueira, G.R.; Reis, R.A. Effects of Feeding Corn Silage Inoculated with Microbial Additives on the Ruminal Fermentation, Microbial Protein Yield, and Growth Performance of Lambs. J. Anim. Sci. 2014, 92, 5640–5650. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, C.H.S.; Basso, F.C.; McAllister, T.A.; Lage, J.F.; Gonçalves, G.S.; Lara, E.C.; Oliveira, A.A.; Berchielli, T.T.; Reis, R.A. Influence of Lactobacillus buchneri as Silage Additive and Forage: Concentrate Ratio on the Growth Performance, Fatty Acid Profile in Longissimus Muscle, and Meat Quality of Beef Cattle. Can. J. Anim. Sci. 2016, 96, 550–562. [Google Scholar] [CrossRef]
- Rabelo, C.H.S.; Basso, F.C.; Lara, E.C.; Jorge, L.G.O.; Härter, C.J.; Mari, L.J.; Reis, R.A. Effects of Lactobacillus buchneri as a Silage Inoculant or Probiotic on In Vitro Organic Matter Digestibility, Gas Production and Volatile Fatty Acids of Low Dry-Matter Whole-Crop Maize Silage. Grass Forage Sci. 2017, 72, 534–544. [Google Scholar] [CrossRef]
- Aworh, O.C. African Traditional Foods and Sustainable Food Security. Food Control 2023, 145, 109393. [Google Scholar] [CrossRef]
- Ding, Z.T.; Xu, D.M.; Bai, J.; Li, F.H.; Adesogan, A.T.; Zhang, P.; Yuan, X.J. Characterization and Identification of Ferulic Acid Esterase-Producing Lactobacillus Species Isolated from Elymus nutans Silage and Their Application in Ensiled Alfalfa. J. Appl. Microbiol. 2019, 127, 985–995. [Google Scholar] [CrossRef]
- Dos Santos, A.P.M.; Santos, E.M.; de Araújo, G.G.L.; de Oliveira, J.S.; de Zanine, A.M.; Pinho, R.M.A.; Cruz, G.F.d.L.; Ferreira, D.d.J.; Perazzo, A.F.; Pereira, D.M.; et al. Effect of Inoculation with Preactivated Lactobacillus buchneri and Urea on Fermentative Profile, Aerobic Stability and Nutritive Value in Corn Silage. Agriculture 2020, 10, 335. [Google Scholar] [CrossRef]
- Fang, J.; Matsuzaki, M.; Suzuki, H.; Cai, Y.; Horiguchi, K.I.; Takahashi, T. Effects of Lactic Acid Bacteria and Urea Treatment on Fermentation Quality, Digestibility and Ruminal Fermentation of Roll Bale Rice Straw Silage in Wethers. Grassl. Sci. 2012, 58, 73–78. [Google Scholar] [CrossRef]
- Wanapat, M.; Kang, S. Improvement of Whole Crop Rice Silage Nutritive Value and Rumen Degradability by Molasses and Urea Supplementation. Trop. Anim. Health Prod. 2013, 45, 1777–1781. [Google Scholar] [CrossRef]
- Wahyono, T.; Sholikin, M.M.; Konca, Y.; Obitsu, T.; Sadarman, S.; Jayanegara, A. Effects of Urea Supplementation on Ruminal Fermentation Characteristics, Nutrient Intake, Digestibility, and Performance in Sheep: A Meta-Analysis. Vet. World 2022, 15, 331–340. [Google Scholar] [CrossRef]
- Kara, K. Estimated Ruminal Digestion Values and Digestion End-Products of Concentrated Mix Feed after In Vitro Treatment with Propionic Acid. Vet. Med. 2018, 63, 537–545. [Google Scholar] [CrossRef]
- Bharanidharan, R.; Arokiyaraj, S.; Kim, E.B.; Lee, C.H.; Woo, Y.W.; Na, Y.; Kim, D.; Kim, K.H. Ruminal Methane Emissions, Metabolic, and Microbial Profile of Holstein Steers Fed Forage and Concentrate, Separately or as a Total Mixed Ration. PLoS ONE 2018, 13, e0202446. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the Safety and Efficacy of Lactobacillus brevis (DSM 23231), Lactobacillus buchneri (DSM 22501), Lactobacillus buchneri (NCIMB 40788–CNCM I-4323), Lactobacillus buchneri (ATCC PTA-6138) and Lactobacillus buchneri (ATCC PTA-2494) as silage additives for all species. EFSA J. 2013, 11, 3168. [Google Scholar] [CrossRef]
- Esen, S.; Cabi, E.; Koç, F. Effect of Freeze-Dried Kefir Culture Inoculation on Nutritional Quality, In Vitro Digestibility, Mineral Concentrations, and Fatty Acid Composition of White Clover Silages. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Koç, F.; Özkan Ünal, E.; Okuyucu, B.; Esen, S.; Işık, R. Effect of Different Kefir Source on Fermentation, Aerobic Stability, and Microbial Community of Alfalfa Silage. Animals 2021, 11, 2096. [Google Scholar] [CrossRef]
- Ülger, I.; Beyzi, S.B.; Kaliber, M.; Konca, Y. Chemical, Nutritive, Fermentation Profile and Gas Production of Citrus Pulp Silages, Alone or Combined with Maize Silage. S. Afr. J. Anim. Sci. 2020, 50, 161–169. [Google Scholar] [CrossRef]
- Koç, F.; Coçkuntuna, L. Silo Yemlerinde Organik Asit Belirlemede İki Farklı Metodun Karşılaştırması. Hayvansal Üretim 2003, 44, 37–46. [Google Scholar]
- Koç, F.; Eser, S.; Okuyucu, B.; Esen, S. Effectiveness of Enzymes and Inoculants on Biological Pretreatment of Different High Dry Matter Lignocellulosic Materials. Bioresour. Technol. Rep. 2021, 16, 100836. [Google Scholar] [CrossRef]
- Ashbell, G.; Weinberg, Z.G.; Azrieli, A.; Hen, Y.; Horev, B. A Simple System to Study the Aerobic Determination of Silages. Can. Agric. Eng. 1991, 34, 171–175. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the Energetic Feed Value Obtained from Chemical Analysis and In Vitro Gas Production Using Rumen Fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Goel, G.; Makkar, H.P.S.; Becker, K. Effects of Sesbania sesban and Carduus pycnocephalus leaves and Fenugreek (Trigonella foenum-graecum L.) Seeds and Their Extracts on Partitioning of Nutrients from Roughage- and Concentrate-based Feeds to Methane. Anim. Feed Sci. Technol. 2008, 147, 72–89. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The Estimation of Protein Degradability in the Rumen from Incubation Measurements Weighted According to Rate of Passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor In Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Aufrere, J.; Cartailler, D. Mise au Point d’une Methode de Laboratoire de Prevision de la Degradabilite des Proteines Alimentaires des Aliments Concentres dans le Rumen. Ann. Zootect. 1988, 37, 255–270. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.84; CRAN. 2017. Available online: https://github.com/taiyun/corrplot (accessed on 4 January 2022).
- Da Silva, É.B.; Costa, D.M.; Santos, E.M.; Moyer, K.; Hellings, E.; Kung, L. The Effects of Lactobacillus hilgardii 4785 and Lactobacillus buchneri 40788 on the Microbiome, Fermentation, and Aerobic Stability of Corn Silage Ensiled for Various Times. J. Dairy Sci. 2021, 104, 10678–10698. [Google Scholar] [CrossRef]
- Sinclair, L.A.; Jackson, M.A.; Huntington, J.A.; Readman, R.J. The Effects of Processed, Urea-Treated Whole-Crop Wheat or Maize Silage and Supplementation of Whole-Crop Wheat on the Performance of Dairy Cows. Livest. Prod. Sci. 2005, 95, 1–10. [Google Scholar] [CrossRef]
- Gómez-vázquez, A.; Pinos-rodríguez, J.M.; García-lópez, J.C.; De Cruz-lázaro, E.; Luna-palomera, C.; Sánchez-hernández, R. Nutritional Value of Sugarcane Silage Enriched with Corn Grain, Urea, and Minerals as Feed Supplement on Growth Performance of Beef Steers Grazing Stargrass. Trop. Anim. Health Prod. 2011, 43, 215–220. [Google Scholar] [CrossRef]
- Canbolat, Ö. Effect of Supplementation of Urea on the Nutritive Value and Fermentation Characteristics of Apple Pulp Silages. J. Agric. Sci. 2022, 28, 430–437. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Arriola, K.G.; Vyas, D.; Kim, D.; Agarussi, M.C.N.; Silva, V.P.; Flores, M.; Jiang, Y.; Yanlin, X.; Pech-cervantes, A.A.; Ferraretto, L.F.; et al. Effect of Lactobacillus hilgardii, Lactobacillus buchneri, or Their Combination on the Fermentation and Nutritive Value of Sorghum Silage and Corn Silage. J. Dairy Sci. 2021, 104, 9664–9675. [Google Scholar] [CrossRef]
- Ren, H.; Sun, W.; Yan, Z.; Zhang, Y.; Wang, Z.; Song, B.; Zheng, Y.; Li, J. Bioaugmentation of Sweet Sorghum Ensiling with Rumen Fluid: Fermentation Characteristics, Chemical Composition, Microbial Community, and Enzymatic Digestibility of Silages. J. Clean. Prod. 2021, 294, 126308. [Google Scholar] [CrossRef]
- Buettner, M.R.; Lechtenberg, V.L.; Hendrix, K.S.; Hertel, J.M. Composition and digestion of ammoniated tall fescue (Festuca arundinacea Schreb.) hay. J. Anim. Sci. 1982, 54, 173–178. [Google Scholar] [CrossRef]
- Islam, M.; Enishi, O.; Purnomoadi, A.; Higuchi, K.; Takusari, N.; Terada, F. Energy and Protein Utilization by Goats Fed Italian Ryegrass Silage Treated with Molasses, Urea, Cellulase or Cellulase + Lactic Acid Bacteria. Small Rumin. Res. 2001, 42, 49–60. [Google Scholar] [CrossRef]
- Blajman, J.E.; Páez, R.B.; Vinderola, C.G.; Lingua, M.S.; Signorini, M.L. A Meta-Analysis on the Effectiveness of Homofermentative and Heterofermentative Lactic Acid Bacteria for Corn Silage. J. Appl. Microbiol. 2018, 125, 1655–1669. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Dong, Z.; Li, J.; Shao, T. Characteristics of Lactic Acid Bacteria Isolated from Different Sources and Their Effects on the Silage Quality of Oat (Avena sativa L.) Straw on the Tibetan Plateau. Grassl. Sci. 2018, 64, 128–136. [Google Scholar] [CrossRef]
- Rabelo, C.H.S.; Basso, F.C.; Lara, E.C.; Jorge, L.G.O.; Härter, C.J.; Mesquita, L.G.; Silva, L.F.P.; Reis, R.A. Effects of Lactobacillus Buchneri as a Silage Inoculant and as a Probiotic on Feed Intake, Apparent Digestibility and Ruminal Fermentation and Microbiology in Wethers Fed Low-Dry-Matter Whole-Crop Maize Silage. Grass Forage Sci. 2018, 73, 67–77. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; Mcallister, T.A.; Santos, M.C.; Kung, L., Jr. Silage Review: Recent Advances and Future Uses of Silage Additives 1. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Restelatto, R.; Novinski, C.O.; Pereira, L.M.; Silva, E.P.A.; Volpi, D.; Zopollatto, M.; Schmidt, P.; Faciola, A.P. Chemical Composition, Fermentative Losses, and Microbial Counts of Total Mixed Ration Silages Inoculated with Different Lactobacillus Species. J. Anim. Sci. 2019, 97, 1634–1644. [Google Scholar] [CrossRef]
- Wu, P.; Li, L.; Jiang, J.; Sun, Y.; Yuan, Z.; Feng, X.; Guo, Y. Effects of Fermentative and Non-Fermentative Additives on Silage Quality and Anaerobic Digestion Performance of Pennisetum purpureum. Bioresour. Technol. 2020, 297, 122425. [Google Scholar] [CrossRef] [PubMed]
- Witzig, M.; Lengowski, M.B.; Zuber, K.H.R.; Jens, M.; Rodehutscord, M. Anaerobe Effects of Supplementing Corn Silage with Different Nitrogen Sources on Ruminal Fermentation and Microbial Populations In Vitro. Anaerobe 2018, 51, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.L.S.; Carvalho, B.F. Silage Fermentation—Updates Focusing on the Performance of Micro-Organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of Metabolome and Bacterial Community in Whole Crop Corn Silage by Inoculating Homofermentative Lactobacillus plantarum and Heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 10, 3299. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Qi, M.; Smiley, B.; Rutherford, W.; Wang, Y.; McAllister, T.A. Impact of Saccharomyces cerevisiae and Lactobacillus buchneri on Microbial Communities during Ensiling and Aerobic Spoilage of Corn Silage. J. Anim. Sci. 2019, 97, 1273–1285. [Google Scholar] [CrossRef]
- Gallo, A.; Bernardes, T.F.; Copani, G.; Fortunati, P.; Giuberti, G.; Bruschi, S.; Bryan, K.A.; Nielsen, N.G.; Witt, K.L.; Masoero, F. E Ff Ect of Inoculation with Lactobacillus buchneri LB1819 and Lactococcus lactis O224 on Fermentation and Mycotoxin Production in Maize Silage Compacted at Different Densities. Anim. Feed Sci. Technol. 2018, 246, 36–45. [Google Scholar] [CrossRef]
- Bai, J.; Xu, D.; Xie, D.; Wang, M.; Li, Z.; Guo, X. Effects of Antibacterial Peptide-Producing Bacillus subtilis and Lactobacillus buchneri on Fermentation, Aerobic Stability, and Microbial Community of Alfalfa Silage. Bioresour. Technol. 2020, 315, 123881. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Lin, X.W.Y.; Zhang, Q.; Yang, X.C.F. Fermentation Quality and Aerobic Stability of Mulberry Silage Prepared with Lactic Acid Bacteria and Propionic Acid. Anim. Sci. J. 2019, 90, 513–522. [Google Scholar] [CrossRef]
- Guo, L.; Lu, Y.; Li, P.; Chen, L.; Gou, W.; Zhang, C. Effects of Delayed Harvest and Additives on Fermentation Quality and Bacterial Community of Corn Stalk Silage. Front. Microbiol. 2021, 12, 687481. [Google Scholar] [CrossRef]
- Negri, M.; Bacenetti, J.; Fiala, M.; Bocchi, S. Evaluation of Anaerobic Degradation, Biogas and Digestate Production of Cereal Silages Using Nylon-Bags. Bioresour. Technol. 2016, 209, 40–49. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological Strategies for Enhanced Hydrolysis of Lignocellulosic Biomass during Anaerobic Digestion: Current Status and Future Perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef]
- Ansah, T.; Sahoo, A.; Rahman, N.A.; Kumawat, P.K.; Bhatt, R.S. In Vitro Digestibility and Methane Gas Production of Fodder from Improved Cowpea (Vigna unguiculata L.) and Groundnut (Arachis hypogaea L.) Varieties. Sci. Afr. 2021, 13, e00897. [Google Scholar] [CrossRef]
- Singh, S.; Kushwaha, B.P.; Nag, S.K.; Mishra, A.K.; Singh, A.; Anele, U.Y. In Vitro Ruminal Fermentation, Protein and Carbohydrate Fractionation, Methane Production and Prediction of Twelve Commonly Used Indian Green Forages. Anim. Feed Sci. Technol. 2012, 178, 2–11. [Google Scholar] [CrossRef]



| Item | C | UC | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nLB | LB | pLB | nLB | LB | pLB | SEM | U | LB | U×LB | |
| AA (g/kg DM) | 7.69 d | 9.14 c | 6.13 e | 10.96 b | 13.12 a | 4.46 f | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| PA (g/kg DM) | 0.48 a | 0.41 bc | 0.34 d | 0.38 cd | 0.42 bc | 0.44 ab | 0.01 | 0.6903 | 0.0058 | <0.0001 |
| BA (g/kg DM) | ND | ND | ND | ND | ND | ND | - | - | - | - |
| LA/AA ratio | 2.13 d | 2.90 c | 5.15 b | 2.40 cd | 2.20 d | 8.18 a | 0.11 | <0.0001 | <0.0001 | <0.0001 |
| Item | C | UC | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nLB | LB | pLB | nLB | LB | pLB | SEM | U | LB | U×LB | |
| DM (g/kg) | 220.1 | 221.7 | 224.7 | 222.0 | 220.9 | 223.4 | 3.27 | 0.9748 | 0.6044 | 0.8682 |
| pH | 5.90 b | 5.77 bc | 5.03 d | 5.54 c | 6.31 a | 5.08 d | 0.07 | 0.2346 | <0.0001 | 0.0002 |
| Yeast (log cfu/g) | 9.81 a | 9.46 b | 9.13 d | 9.41 bc | 9.34 c | 9.07 d | 0.02 | <0.0001 | <0.0001 | <0.0001 |
| CO2 (mL) | 125.0 a | 117.2 b | 86.4 c | 120.1 ab | 51.8 d | 41.0 e | 1.38 | <0.0001 | <0.0001 | <0.0001 |
| Aerobic stability (h) | 132 b | >168 a | >168 a | >168 a | >168 a | >168 a | 0.47 | <0.0001 | <0.0001 | <0.0001 |
| Item | C | UC | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nLB | LB | pLB | nLB | LB | pLB | SEM | U | LB | U×LB | |
| 3 h, mL | 16.42 a | 13.79 b | 16.51 a | 15.14 ab | 17.15 a | 15.19 ab | 0.84 | 0.7145 | 0.8898 | 0.0237 |
| 6 h, mL | 24.97 | 23.20 | 25.10 | 23.90 | 27.58 | 24.64 | 1.19 | 0.3498 | 0.7293 | 0.0801 |
| 12 h, mL | 35.03 | 32.28 | 34.71 | 32.98 | 36.66 | 34.43 | 1.73 | 0.6377 | 0.9417 | 0.2007 |
| 24 h, mL | 48.44 | 44.72 | 48.19 | 44.76 | 47.77 | 45.91 | 2.31 | 0.6166 | 0.9414 | 0.3405 |
| 48 h, mL | 58.50 | 54.81 | 57.79 | 54.52 | 57.52 | 55.02 | 2.57 | 0.5325 | 0.9908 | 0.4086 |
| 72 h, mL | 64.03 | 60.19 | 61.84 | 59.23 | 63.57 | 59.75 | 2.85 | 0.6249 | 0.9239 | 0.3737 |
| 96 h, mL | 68.05 | 62.88 | 64.37 | 61.92 | 66.60 | 62.78 | 2.87 | 0.5811 | 0.8722 | 0.2682 |
| c | 0.045 | 0.047 | 0.051 | 0.048 | 0.045 | 0.052 | 0.0029 | 0.8815 | 0.1744 | 0.7584 |
| a, mL | 10.62 ab | 8.38 b | 9.78 b | 9.70 b | 12.78 a | 9.43 b | 0.93 | 0.1960 | 0.5941 | 0.0280 |
| b, mL | 56.36 | 53.84 | 53.84 | 51.55 | 53.31 | 52.04 | 2.64 | 0.2912 | 0.9274 | 0.7129 |
| a + b, mL | 66.98 | 62.22 | 63.62 | 61.25 | 66.09 | 61.47 | 2.86 | 0.5771 | 0.8164 | 0.2746 |
| ME, MJ/kg DM | 9.17 | 8.70 | 9.24 | 8.83 | 9.17 | 8.87 | 0.32 | 0.7580 | 0.9284 | 0.3459 |
| OMD, % DM | 61.40 | 58.31 | 61.99 | 59.33 | 61.44 | 59.46 | 2.05 | 0.7772 | 0.9180 | 0.3429 |
| IVCPD, % CP | 88.68 c | 91.16 bc | 93.89 ab | 93.84 ab | 92.39 ab | 94.67 a | 0.66 | 0.0008 | 0.0013 | 0.0110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bağcık, C.; Koç, F.; Erten, K.; Esen, S.; Palangi, V.; Lackner, M. Lentilactobacillus buchneri Preactivation Affects the Mitigation of Methane Emission in Corn Silage Treated with or without Urea. Fermentation 2022, 8, 747. https://doi.org/10.3390/fermentation8120747
Bağcık C, Koç F, Erten K, Esen S, Palangi V, Lackner M. Lentilactobacillus buchneri Preactivation Affects the Mitigation of Methane Emission in Corn Silage Treated with or without Urea. Fermentation. 2022; 8(12):747. https://doi.org/10.3390/fermentation8120747
Chicago/Turabian StyleBağcık, Caner, Fisun Koç, Kadir Erten, Selim Esen, Valiollah Palangi, and Maximilian Lackner. 2022. "Lentilactobacillus buchneri Preactivation Affects the Mitigation of Methane Emission in Corn Silage Treated with or without Urea" Fermentation 8, no. 12: 747. https://doi.org/10.3390/fermentation8120747
APA StyleBağcık, C., Koç, F., Erten, K., Esen, S., Palangi, V., & Lackner, M. (2022). Lentilactobacillus buchneri Preactivation Affects the Mitigation of Methane Emission in Corn Silage Treated with or without Urea. Fermentation, 8(12), 747. https://doi.org/10.3390/fermentation8120747








