Abstract
Recycling bioresources is the only way to sustainably meet a growing world population’s food and energy needs. One of the ways to do so is by using agro-industry wastewater to cultivate microalgae. While the industrial production of microalgae requires large volumes of water, existing agro-industry processes generate large volumes of wastewater with eutrophicating nutrients and organic carbon that must be removed before recycling the water back into the environment. Coupling these two processes can benefit the flourishing microalgal industry, which requires water, and the agro-industry, which could gain extra revenue by converting a waste stream into a bioproduct. Microalgal biomass can be used to produce energy, nutritional biomass, and specialty products. However, there are challenges to establishing stable and circular processes, from microalgae selection and adaptation to pretreating and reclaiming energy from residues. This review discusses the potential of agro-industry residues for microalgal production, with a particular interest in the composition and the use of important primary (raw) and secondary (digestate) effluents generated in large volumes: sugarcane vinasse, palm oil mill effluent, cassava processing waster, abattoir wastewater, dairy processing wastewater, and aquaculture wastewater. It also overviews recent examples of microalgae production in residues and aspects of process integration and possible products, avoiding xenobiotics and heavy metal recycling. As virtually all agro-industries have boilers emitting CO2 that microalgae can use, and many industries could benefit from anaerobic digestion to reclaim energy from the effluents before microalgal cultivation, the use of gaseous effluents is also discussed in the text.
1. Introduction
The beginnings of modern microalgae biotechnology were surrounded by significant excitement because of the enormous potential extrapolated from the high productivity achieved in laboratories. However, while the search for algal biofuels since the 1980s has evolved a lot, it has not been able to consistently provide products to the market. Conversely, nutritional and nutraceutical biomass products have flourished, as shown by the FDA’s GRAS certifications (generally recognized as safe certifications by the U.S. Food and Drug Administration) and the growing offer of consumer products. The market for microalgae products today has wildly diverse estimates, but on the same order of magnitude of USD 1 to 5 billion by 2027 with a CAGR (compound annual growth rate) of 4–7% [1,2]. Even if the market remains in the lower figures, the projected growth is enormous and is driving research and development in the area.
The unsuccess of biofuels relative to specialty products does not mean that fuels have not evolved. However, their sales value is not yet capable of covering production costs. The use of agro-industrial residues, produced in huge volumes in agro-industrial and agro-energy processing, could be a way of enabling biofuel production. The idea is to couple a need with an opportunity: the need to carry out biological treatments of agro-industrial liquid effluents and returning clean water to the environment and the opportunity of reclaiming nutrients and water by cultivating microalgae culture in these residues, in raw or minimally treated form [3,4,5].
Liquid agro-industrial effluents are processing and washing waters produced during the industrialization of energy or food crops or livestock. Today, most waste undergoes simple biological treatment with the eventual production of biogas and the use of the final liquid effluent for fertigation [6,7,8]. However, this process does not entirely take advantage of the large amount of nutrients available in the waste. The final steps of biological treatment, namely stabilization ponds, typically already have native microalgae growing [9,10]. Agro-industry residues are produced in large amounts and have low toxicity compared to other industrial effluents but also have a high eutrophication potential because of the availability of nutrients, especially nitrogen and phosphorus.
Effluents produced in small amounts can also be used for microalgal cultivation and the logic is the same—taking advantage of the leftover, diluted nutrients. However, small processing plants have a proportionally higher CAPEX (capital expenditures) than large-scale plants. That is why most interest goes to a few effluents produced in staggering amounts, such as vinasses. The large volume of residues can be inferred from agro-industrial production [7]: in 2020, crop production worldwide was estimated at 9.3 billion tons, including food, energy, and animal feed crops [11] (Figure 1). Some of these crops, such as potatoes and tomatoes, are only minimally or partially processed and others do not generate large volumes of liquid waste, such as cereals. However, much of cassava, palm, and sugarcane are extensively processed into starch, palm oil, and ethanol, generating large volumes of waste.
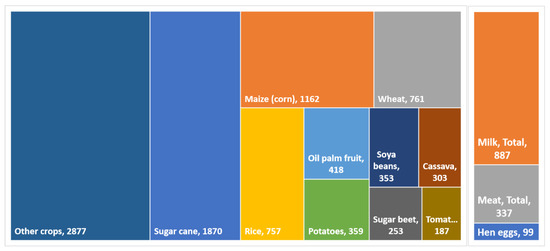
Figure 1.
Food and energy crops and animal products in the world in 2020, in million tons. Data compiled from the FAO database [11]. Egg production was converted assuming 60 g/egg.
Liquid agro-industry wastes are usually too diluted for burning or composting but are also too concentrated to be directly discarded. Microalgae can be cultivated in such residues [12,13,14] after residue pretreatment, microalgae adaptation, or both. Three main effluent streams could be available from industries: two liquid and one gaseous fraction. The two liquid fractions are (1) the primary effluents, derived directly from the process (wash waters and residue streams, such as vinasses from ethanol production), with high COD (chemical oxygen demand), and (2) the secondary effluents, the product of the previous treatment of the primary effluents. This treatment can be aerobic, but because of the high CODs, it is more common to use anaerobic digestion. Therefore, secondary effluents are also termed digestates. Secondary effluents usually have a lower COD since part of it was removed during the primary treatment. Both residues have variable concentrations of several macro- and micronutrients. The gaseous effluent fraction—commonly called flue gases—is the product of the combustion of fuels that heat boilers and generate steam. Flue gases are composed of air, CO2, and minor amounts of NOx, SOx, and CO. These gases cannot sustain microalgal growth alone but can be a source of CO2 [15]. A final source of CO2 for microalgae cultivation in circular processes could be the biogas produced in anaerobic digestion, which is not a residue but can be upgraded to biomethane by removing the CO2.
Microalgae can produce a multitude of products of industrial interest, from biomass and nutraceutical fractions to biofuels; selecting an appropriate product can add value to waste and increase the industry income and the circularity of the process. By providing nutrients at a meager cost, using residues can reduce expenses in algal production. This is advantageous for already established products, such as PC (phycocyanin, a blue-colored protein from cyanobacteria) and AX (astaxanthin, a carotenoid), and enables the production of new products, such as biofuels and PHAs (polyhydroxyalkanoates, biopolymers produced by some cyanobacteria) [16,17,18].
Some bottlenecks to the massive production of microalgal biomass, such as the low cell concentration in cultures and the consequent need for large cultivation areas and large volumes of culture media and nutrients, can be solved using agro-industry wastewaters. However, these residues bring other challenges, such as the high COD and its sometimes-intense color and turbidity, which can reduce light availability. As a result, microalgal cultures—rarely axenic—can be dominated by bacteria and other microorganisms that compete for nutrients or even predate microalgae [19]. This means that pre-treatments may be necessary to ensure high productivity. Microalgae selection and adaptation are also required.
Nevertheless, the advantages of including a microalgal production step in biorefineries are many and can overcome various difficulties, as can be perceived from the intensive recent research in the area. Recycling bioresources, reducing COD through microalgal cultivation, reusing water, producing new products, and creating value through a circular approach are tendencies in bioprocesses. The following sections discuss the most relevant aspects of the use of agro-industrial residues in the production of microalgae products, starting with an overview of larger-volume residues—those that are produced in a more concentrated and favorable way for the development of large-scale processes. The text proceeds to describe successful cases and approaches in producing microalgae in these residues. Then, it discusses the important issue of using gaseous effluents as a carbon source and as an opportunity to mitigate emissions from the agro-industry. Finally, it presents possible algal products that can be targeted for integrated processes and discusses new process development and future perspectives.
2. Agro-Industrial Wastewaters Overview
Liquid effluents can be efficiently used as culture media for microalgae because of their nutrient availability, comparable to that of classical media, such as Zarrouk, BBM (Bold’s basal medium), or BG-11 (blue-green medium). One of the main difficulties in the cultivation of microalgae on a large scale is the high consumption of water and nutrients for medium, which leads to high costs [20], so the use of effluent could be a strategy for the dissemination of microalgae cultivation and sustainability in production processes [21,22].
Agro-industrial effluents are wastewaters generated by processing raw materials from agriculture, forestry, or fishing. They can be generated as a by-product of the process or derived from the cleaning procedures of lines and equipment. Among the effluents that are generated as by-products are cassava processing wastewater (CPW), palm oil mill effluent (POME), sugarcane vinasse (SV), abattoir wastewater (AW), aquaculture wastewater (AqW), and dairy wastewater (DW).
Sugarcane vinasse (SV) is wastewater generated through the separation of alcohol from the fermentation broth [7]. According to [23], sugarcane vinasse is generated in a proportion of 12–15 m3/m3 ethanol. Considering that, in 2021, 124.15 million m3 of bioethanol were produced worldwide [24], it can be inferred that around 1.86 billion m3 of vinasse were generated. In the case of POME and CPW, 3.45 tons POME/ton of oil and 0.6 m3 CPW/ton of cassava were generated, respectively; thus, it can be inferred that 285.45 million tons of POME and 75.6 million m3 CPW were generated in the year 2021 [25,26].
Global meat production in 2020 reached almost 337.18 million tonnes for cattle, poultry, pigs, and sheep, according to the meat statistics from the United Nations Food and Agriculture Organization, 2021. About 27% of the freshwater used by humans is consumed by animal production. Meat production requires between 6 and 20 times more water than cereals and vegetables or fruits and generates from 1.5 to 18 m3 of effluents/ton of meat produced [27]. Meat processing plants produce large quantities of abattoir wastewater (AW) from slaughtering and processing animals and cleaning the facilities. The effluent composition varies by type of animal and process specificities [28]. Organic matter, suspended particles, oils and greases, blood, manure, urine, and meat tissues make up the effluent from slaughterhouses. Blood is one of the main dissolved contaminants, and its separation efficiency in processes is essential for ensuring lower effluent loads [29]. This effluent is characterized by high BOD, COD, high oil concentrations, total solids, total suspended solids, total nitrogen, and chlorides [30]. Nitrogen compounds are one of the main nutrients in wastewater; nitrates accelerate plant growth in water bodies and can be easily reduced to nitrites, threatening human health and the environment when treated incorrectly [31].
The growing demand for dairy products has resulted in the development of many dairy industries, generating wastewater with high polluting potential [32]. It is estimated that world milk production will grow by 1.6% per year over the next decade (reaching about 997 Mt by 2029), and the world per capita consumption of fresh dairy products is projected to increase by 1.0% per year by 2030. Wastewater in the dairy industry (DW) is composed of carbohydrates, proteins, grease or oil, and high phosphate and nitrate concentrations [33]—so much so that in a Tetradesmus obliquus culture, the substitution of 20% (v/v) of Bold’s basal medium (BBM) by acid whey permeate was considered the best compensation for biomass growth, enzyme production, and nutrient utilization [34].
Aquaculture will account for 52% of all fish production by 2030, reaching 105 Mt, according to a report by the Organization for Economic Co-operation and Development (OECD)/Food and Agriculture Organization (FAO) [35]. Aquaculture wastewater (AqW) comprises dissolved and particulate organic matter and dissolved solids, such as phosphorus and nitrogen compounds. Food and feces are present in aquaculture effluents and cause environmental deterioration of both receiving water bodies and sediments. They cause the increased metabolic activity of aerobic bacteria, and the scarcity of oxygen by bacterial action leads to the destruction of the most vulnerable aerobic life forms in rivers and lakes [36].
Wastewater is a major concern in the world due to the negative aspects and impacts it can generate on the environment. The effects of agro-industrial effluents on the environment depend mainly on their physical-chemical composition. In general, agro-industrial effluents have high organic loads (measured as COD), reducing the amount of dissolved oxygen in water bodies if incorrectly disposed of, thus affecting aquatic ecosystems. The high organic loads of agro-industrial effluents are explained by high concentrations of carbohydrates, proteins, and even fats [7]. These effluents are also a significant source of macronutrients, such as nitrogen, phosphorus, and potassium (Table 1).
According to [37,38], excess nitrogen and phosphorus in water bodies induce eutrophication processes, that is, the accelerated growth of plants or microalgae that affect the balance of the aquatic ecosystem. Agro-industrial effluents are also a significant source of micronutrients, such as chromium, cobalt, copper, iron, zinc, manganese, molybdenum, nickel, and selenium. These compounds are present in small amounts in wastewater. However, they are essential in the catabolic reactions developed by microorganisms. SV, for example, is a source of different micronutrients, including Co, Fe, Mo, Se, and Zn. POME and CPW are also sources of micronutrients, such as Fe, Zn, and Mn.
These effluents are also a source of amino acids, such as proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, histidine, lysine, and arginine. [39,40,41]. The presence of amino acids in effluents can improve microbial growth or the production efficiency of some byproducts, as they can alter metabolic pathways [42].
Agro-industrial wastewaters also contain significant amounts of toxic compounds, such as heavy metals, which can bioaccumulate in the food chain. For example, SV contains heavy metals in significant concentrations, such as Cd, Cu, Cr, Ni, and Pb, in concentrations of 200 ppb, 200 ppb, 100 ppb, 2000 ppb, and 5000 ppb, respectively. [43]. Other metals, such as As, Hg, and Zn, have also been found in this effluent [44]. Metals, such as Cd, Cr, Hg, and Cu, have also been found in POME and CPW effluents. [45,46]. In the case of CPW, it also contains significant amounts of cyanide, which is highly toxic [18]. While microalgae can transform low-cost raw nutrients from wastewater into valuable biomass components [47], the excess of certain metals can be harmful or lead to bioaccumulation, as discussed in Section 7 of this text.

Table 1.
Physical-chemical composition of agro-industrial effluents that are produced in large volumes.
Table 1.
Physical-chemical composition of agro-industrial effluents that are produced in large volumes.
| Parameter | SV, Sugarcane Vinasse | POME, Palm Oil Mixed Effluent | CPW, Cassava Processing Wastewater | AW, Abattoir Wastewater | DW, Dairy Wastewater | AqW, Aquaculture Wastewater | |
|---|---|---|---|---|---|---|---|
| Main effluent characteristics | COD (gO2 L−1) | 27.7–299.5 | 22.65–85.71 | 1.4–141.3 | 190–12800 | 91.6–4000 | 6.1–165 |
| BOD5 (gO2 L−1) | 5.04–47.63 | 34.71–52.67 | 1.96–44.6 | 53–2250 | 90–1814 | 8.5 | |
| pH | 4.04–6.5 | 4.3–5.2 | 3.9–7.2 | 6.4–7.7 | 4.33–7.35 | 7–8.2 | |
| Total solids (g L−1) | 7.5–36.6 | 47–64.6 | 5.4–92.9 | - | 10.8–9290 | - | |
| Fixed solids (g L−1) | 0.27–14.16 | - | - | 2594 | - | ||
| Volatile solids (g L−1) | 4.7–26.3 | 24.84–30.92 | 3.5 | - | - | - | |
| Nutrients | Ammonia (mg L−1) | 18–118.1 | 77–101 | 61.53 | 6.5–532.3 | 53–115 | ≤0.1–6.25 |
| TKN (mg L−1) | 122–540 | 180–1400 | 180 | 22–2209 | |||
| Nitrate (mg L−1) | 0,1–45.3 | 109–136 | 4 | 11.97–140 | 18.05 | 0.35–152.8 | |
| Nitrite (mg L−1) | 0.1–0.4 | - | - | 13.7–54 | 0.398 | 0.3–24.7 | |
| Sulfate(mg L−1) | 669–3070 | - | 63.77 | 12.63–500 | 17 | 420.6 | |
| Phosphorus (mg L−1) | 44–232 | 109–136 | 0.4 -3000 | 7–108 | 11.6–3055 | 16.1 | |
| Magnesium (mg L−1) | 343–616 | 279–296 | 17.68–43.08 | 18.64 | 18 | 39.6–49.75 | |
| Calcium (mg L−1) | 344–609 | 282–290 | 18.41–86.49 | 4.9–67 | 129 | 168.9–118 | |
| Potassium (mg L−1) | 1542–3652 | 1696–2043 | 15.92–435.75 | 8.1–90 | - | 195.1 | |
| Sodium (mg L−1) | 27–57 | 94–113 | 16.42–17.68 | 621 | - | 246.4 | |
| Fluoride(mg L−1) | 0.14–0.44 | - | - | - | |||
| Chloride (mg L−1) | 209–3548 | 94–113 | 52 | 352 | 1204 | 147.3 | |
| Metals | Aluminum (mg L−1) | 1.13–11.9 | - | 1.12 | - | - | - |
| Barium (mg L−1) | 0.23–0.56 | - | - | - | - | - | |
| Cadmium (mg L−1) | 0.08–0.027 | 0.01–0.02 | - | 0.009 | - | - | |
| Arsenic (mg L−1) | 0.098–0,14 | - | - | - | - | - | |
| Chrome (mg L−1) | 0.028–0.084 | 0.05–0.43 | - | - | - | - | |
| Cobalt (mg L−1) | 0.011–0.035 | 0.04–0.06 | - | - | - | - | |
| Copper (mg L−1) | 0.19–1.16 | 0.8–1.6 | 3.26 | - | - | - | |
| Iron (mg L−1) | 5.8–18.6 | 65–164 | 72,65 | 0.9–21 | - | - | |
| Lead (mg L−1) | 0.01–0.59 | 0.72 | 0.5–4 | - | - | ||
| Manganese (mg L−1) | 1.04–4.62 | 2.1–4.4 | 3.3 | - | - | - | |
| Mercury (mg L−1) | 0.01 | - | - | - | - | - | |
| Molybdenum (mg L−1) | 0.016–0.066 | - | - | - | - | - | |
| Nickel (mg L−1) | 0.038–0.12 | 0.1–3.6 | - | - | - | - | |
| Selenium (mg L−1) | 0.02–0.076 | - | - | - | - | ||
| Zinc (mg L−1) | 0.2–1.19 | 1.2–2.72 | 25,11 | 0.178 | - | - | |
| Compounds | Lipids (g L−1) | - | 8.81–37.88 | - | - | 34.217 | - |
| Glycerol (mg L−1) | 3333 | - | - | - | - | - | |
| Reducing sugar (mg L−1) | - | 228–236 | 1300 | - | - | - | |
| Cyanide (mg L−1) | - | - | 46,75 | - | - | - | |
| References | [40,44] | [48,49,50] | [51,52,53] | [28,29,30,31,54,55,56,57] | [58,59,60,61,62] | [63,64,65,66,67] | |
BOD5, biochemical oxygen demand in 5 days of incubation; TKN, total nitrogen measured via the Kjeldahl method.
3. Direct Microalgae Cultivation in Residues—Description and Examples
3.1. Vinasse
The utilization of vinasse for growing microalgae is limited due to the high color and turbidity of this agro-industrial effluent, which can reduce light penetration in the algal culture, reducing photosynthesis [68]. A common practice consists of diluting the vinasse to enable microalgae cultivation [69,70,71], although the use of diluted vinasse as a culture medium is not a desirable feature at the industrial scale [72]. These issues can also be solved by clarifying the vinasse using chemical clarifying agents, such as Al2(SO4), FeCl3, and Ca(OH)2 [73], or natural alternatives, such as Moringa oleifera seeds with activated charcoal [68]. Other alternatives to clarify vinasse are the mechanical filtration, centrifugation, decantation, and decomposition of the organic matter using electrochemical, oxidative, and biological means [74]. These methods, however, are rarely applied on a large scale due to the related costs [68]. Another well-established treatment for vinasse before microalgae cultivation is anaerobic digestion (AD). It is usually carried out in up-flow anaerobic sludge blanket (UASB) reactors since they can be cost-effective due to simple operation and high efficiency in removing the organic load. Moreover, AD allows biogas collection, which can be used both for its energetic and CO2 content [75]. Usually, vinasse goes through a pretreatment before entering the UASB, when the acidic pH is adjusted to ensure the stability of the anaerobic digestion process [76]. Additionally, the removal of suspended solids from the vinasse before AD may be necessary to avoid system acidification due to the excess of volatile fatty acids (VFAs) [75]. In the study by [77], this strategy was used to cultivate Chlorella vulgaris, reaching a productivity of 70 mg L−1 day −1. In this study, the combination of anaerobic digestion and C. vulgaris cultivation showed a significant reduction of vinasse organics, N, and P contents with a COD and turbidity removal greater than 80%.
Table 2 presents other microalgae cultivated in raw or highly concentrated vinasse media. Some studies demonstrate higher productions of chlorophyll a and b as well as proteins and carbohydrates associated with the presence of determined percentages of vinasse in the media [78,79]. Inoculating a microalgae culture typically cultivated in synthetic media into the agro-industrial residue can lead to a culture crash [3]. This is due to the high concentrations of certain compounds in effluents, which can damage the microalgae osmoregulation and metabolic processes [80]. It is necessary, thus, to slowly adapt the cultures through sequential inoculations into media with increasing effluent concentrations, as was the case, for example, of the study of Sydney et al. [81], in which microalgae of the genus Botryococcus, Synechococcus, Chlorella, Neochloris, Scenedesmus, and Arthrospira were inoculated initially in 10% vinasse media and afterward in 20% and 30% concentrations. High inoculum concentrations can also help with culture competition, and previous adaptation to external conditions can be made before inoculation into large volumes of effluent [82].
3.2. POME, Palm Oil Mixed Effluent
POME usually contains high amounts of suspended solids that can adversely affect microalgae cultivation because of low light penetration. Particulate matter can also reduce nutrient availability to the cells due to the adsorption or absorption of nutrients [83,84]. Similarly to other wastewaters, POME may contain inhibitors, such as excessive amounts of residual oil, which can lead to microalgae sedimentation and, consequently, a reduction in treatment efficiency due to biomass losses [85]. For those reasons, pretreatments such as anaerobic digestion of POME may be critical before microalgae cultivation [83], with AD eventually followed by centrifugation for solids [86]. To improve light access during cultivation, processes such as coagulation or absorption may be considered. Adsorption can use activated carbon extracted produced in situ from palm kernel shells [87,88]. Acid–heat treatment is another option, being especially useful for reducing the dark color observed in the effluent due to lignin removal and also increasing the concentration of sugars that can contribute to mixotrophic microalgae growth [87]. Overall, filtration, autoclaving, or exposure to UV light [89] can be performed to treat the wastewater regarding microbiological levels before microalgae cultivation.
Another option to treat wastewater is to utilize immobilized microalgae. It is the case of Immobilized Nannochloropsis oculata in down-flow hanging sponge (DHS) reactors for treating POME [90]. The efficiencies of removal of COD, ammoniacal nitrogen (NH3-N), and phosphate were of 73%, 80%, and 83%, respectively, with high color removal as well. Only minerals (CaCl2.2HO, MgSO4.7H2O, Fe2SO4.5H2O), trace elements (CuSO4, NiCl2.6H2O, CoCl2.6H2O), and phosphate buffer (KH2PO4, K2HPO4) were supplemented to the microalgae to guarantee the pollutants removal [90]. In another study, immobilized Chlorococcum oleofaciens in alginate beads (as in the previously mentioned case) were able to remove total phosphorus, nitrogen, ammonia nitrogen, and soluble chemical oxygen by 90.43%, 93.51%, 91.26%, and 50.72%. However, during the process, the beads suffered degradation; thus, COD values increased, showing that bead stability is an important factor in the treatment [91]. Immobilized microalgae can also be used to treat other types of agro-industrial effluents. It is the case of Synechococcus pevalekii, which was used to treat rice vinasse, a decrease in nitrate content and total solid removal of 91 and 29% was observed after 10 days, with significant Mg2+, K+, Cl−, and PO43− removals. Subsequently, the treated vinasse was used as culture media (69% v/v) for cultivating Dunaliella salina in mixotrophic cultivation, with a 175% increase in cell number when compared to 100% Conway medium [92].
3.3. CPW, Cassava Processing Wastewater
The use of cassava processing waste (CPW) as a culture medium for microalgae production has increased due to the volume generated and its nutritional composition, allowing the production of various microalgae and metabolites [93]. It presents high levels of carbohydrates, proteins, and fats, with high organic loads—the COD is, on average, 55 g O2 L−1 and the BOD (biochemical oxygen demand) is 10 g O2 L−1 [7].
Due to the high concentration of some components, such as phosphate and cyanide (Table 1), it is not always possible to CPW directly. Typical CPW treatment consists of sieving, primary sedimentation (separation of sand and other particles), and an anaerobic lagoon and facultative lagoon [18]. Currently, processes such as filtration, flocculation, and sterilization are also being evaluated as pretreatment for this effluent. However, the most promising current research is using the anaerobic digestion of CPW [17,94]. After pretreatment, the effluent CPW (or its digestate) still contains high amounts of nitrogen, phosphate, and soluble sugars, thus allowing the mixotrophic or heterotrophic microalgae production for bioproducts, such as biomass, biodiesel, or pigments [95]. Table 2 describes several products produced using CPW as a substrate.
Similarly to CPW, the cultivation of microalgae in CPW requires an adaptation stage of the culture. The most common conditions are (I) the dilution of the effluent (challenging the culture with growing residue concentrations) and (II) the isolation and selection of resistant strains. For instance, four possible strains of Chlorella sp., Scenedesmus sp., Monoraphidium sp., and Golenkinia sp. were isolated from a secondary effluent of a cassava processing industry and evaluated using non-sterile CPW, sterile CPW, and pre-treated CPW by anaerobic digestion at different concentrations. The crude CPW is not viable; diluted CPW could be favorable. In the case of sterilized CPW, the appropriate dilution was 30%, while in the case of pure digested CPW, the growth of Monoraphidium sp., Golenkinia sp., and Scenedesmus sp. was identified. For Chlorella sp., the adequate dilution was 40%. The cell values obtained were 2 ×, 4 ×, and 7 × 105 cells mL−1 for Monoraphidium sp., Golenkinia sp., and Scenedesmus sp., respectively, and 1 × 105 for Chlorella sp., [18].
The dilution adaptation process can be carried out using synthetic culture media or water. In a process development for Haematococcus production, BBM was used until the culture reached the exponential phase. Then, for mixotrophic and heterotrophic growth, CPW was added to the BBM medium [96].
3.4. Abattoir Wastewaters
Most studies of microalgae cultivation in slaughterhouse effluents were performed after anaerobic digestion. Acid treatment for precipitation is another pretreatment option: by reducing the pH value of the effluent from 6–7 to 4, about 80% of the total COD was removed as sludge [97]. Afterward, Chlorella vulgaris was cultivated in the effluent and 83% of the remaining COD was removed, reaching a biomass concentration of 1.2 g L−1. Microalgae can be especially useful for removing the high ammonium and phosphate contents in slaughterhouse effluents [98], and parameters such as the pH of the wastewater can be modified to suit the microalgae of interest, as was the case in the study by Katırcıoğlu et al. [98], in which effluent pH values from 9 to 11 were tested. The pH of 10.5 was considered the best for optimizing nutrient removal and obtaining total lipid and fatty acid methyl esters in Chlorella vulgaris comparable to cultivation in BG-11. Moreover, this alkaline pH inhibited the presence of rotifers. At the end of the twelfth day, ammonium and phosphate removals were 99% and 96.43%, respectively [98]. In the study by [99], the filamentous microalgae Stigeoclonium FAUBA-10 achieved biomass productivity of 0.45 g L−1 d−1 in semicontinuous cultivation in slaughterhouse effluent, with a nutrient removal above 92% in 5 days. This microalga also has the operational advantage of easy harvesting by filtration through mesh screens (<341 μm). Aside from pH, CO2 injection may play an important role in nutrient removal from ADAE (anaerobically digested abattoir effluent), since it participates in the pH equilibrium in the medium and avoids ammonia stripping caused by the deionization of ammonium (NH4+) to ammonia (NH3), which, at high concentrations, is toxic to microalgae cells [100,101].
Microalgae typically coexist with certain bacteria species through symbiotic interactions, and this relationship can be significant in wastewaters [102]. Together, both groups can interact and perform better towards environmental stresses. Microbial diversity depends on the wastewater source and environmental conditions [80]. When treating dairy farm and poultry slaughterhouse wastewaters, the microalgae Tetradesmus obliquus and the bacterium Variovorax paradoxus were co-cultivated. They attained higher microalgal growth with efficient nutrient removal and the highest reduction in COD (83–87%) compared to mono or sterile cultivations. It was evident that not only this microalgal–bacterial interaction happened but further interactions with other native bacterial communities in the tested wastewaters also increased bioremediation [80]. Additionally, the microalgae Coelastrella sp., Chlamydomonas sp., and Scenedesmus sp. were cultivated in POME and showed better phycoremediation in the unsterilized residue due to microalgae–bacteria consortia interaction, with the highest values of COD and NH4+ removal when compared to sterilized POME. Evidence of shifting in the bacteria community was seen, with Actinobacteria, Bacteroidetes, Planctomycetes, and Proteobacteria being the major phyla found [86].
3.5. Dairy Processing Wastewater
The dairy industry is one of the most polluting due to the variety of products generated, i.e., milk, yogurt, cheese, cream, butter, ice cream, and others. For this reason, the composition of the wastewater presents high concentrations of nutrients, fats, chlorides, sulfates, and lactose [103]. For instance, the milk effluent analysis showed COD values of 85–95,000 mg L−1, BOD of 40–48,000 mg L−1, total nitrogen of 14–830 mg L−1, and a total phosphorus of 9–280 mg L−1 [104].
Nevertheless, these nutrients are not readily fermentable, so they need processes that facilitate the presence of simple sugars. Some pretreatments described are anaerobic fermentation, filtration, sedimentation, sterilization, ultrasound, and pH variations [105,106]. The effluent from a dairy industry used for Chlorella production was pretreated by the addition of NaOH (5M) up to pH 7, heating (≈80 °C), and centrifugation [107]. Other pretreatments include the use of homogenization tanks, which act as grease traps in addition to allowing solids to settle [108].
In addition to pretreatment, inoculum adaptation is necessary before starting microalgae production using effluents. One technique currently used is the isolation of extremophilic microalgae. For instance, potential microalgae were isolated from dairy effluents. During this stage, effluent samples were cultivated in artificial media such as BG-11 or BBM [109,110]. Because many of the processes are performed with commercial strains, an adaptation step is necessary before cultivation. In experiments conducted with Chlorella sorokiniana UTEX 1230 (CCAP, Oban, UK), the microalgae were gradually adapted to medium-strength DW digestate for eight weeks. For another six weeks, the proportion of wastewater was increased from 10% to 90% in order to finally use the undiluted effluent [111].
3.6. Aquaculture Wastewater
Effluents generated from the aquaculture industry are of great interest due to the high degree of contamination from the excessive use of artificial nutrients, chemicals, and antibiotics. The use of microalgae in this effluent has several benefits (I) treatment of the effluent from the massive production of algae and (II) production of biomass or bioproducts [112] that are of particular interest in the aquaculture industry.
The quantity and quality of this effluent vary according to the species cultivated, the scale of production, the type and location of the system, and the technology used for treatment. In general terms, AW is composed of suspended solids (i.e., fish excreta and feed waste) and inorganic nutrients, such as ammonia (NH3), nitrate (NO3−), nitrite (NO2−), and phosphorus (as PO43−), whose concentrations depend on the type and cycles of production [21]. For example, the average chemical oxygen demand (COD) is approximately 300 mg L−1.
The systems most used as pretreatments for this effluent are sedimentation, precipitation to remove phosphorus, and biological denitrification [113]. Due to their characteristics and natural composition, diatoms are used to cultivate fish farm effluents, efficiently eliminating excess nutrients in 3–5 days (open pond cultures) [112].

Table 2.
Microalgae growth in agro-industrial wastewater.
Table 2.
Microalgae growth in agro-industrial wastewater.
| Agro-Industrial Effluent | Microalgae | Medium Additives | Productivity (g L−1 day−1) | Biomass (g L−1) | COD Reduction (%) | BOD Reduction (%) | Bio-Products | Reference |
|---|---|---|---|---|---|---|---|---|
| Vinasse | Chlorella vulgaris | 10% saline medium is composed by (g L−1):(0.25) Mg (NO3)2·6H2O; (0.025) CaCl2·2H2O; (0.0075) MgSO4·7H2O; (0.015) KH2PO4 (0.0025) NaCl and 1 mL L−1 of trace elements. 540 mg L−1 oxytetracycline 450 mg L−1 of ampicillin | 2.1 | 10.5 | 49.1 | 70.0 | Biomass | [114] |
| Vinasse | Scenedesmus bajacalifornicus | - | 0.325 | 3.9 | 51.9 | 60.3 | Biomass | [115] |
| Vinasse | Desmodesmus subspicatus | - | 1.450 | 2.9 | 66 (TOC*) | - | Biomass | [116] |
| Vinasse | Arthrospira maxima | 70% water | 0.150 | 2.25 | 81 | 89.2 | Peptide fractions (57% of proteins in biomass) | [117] |
| Vinasse | Desmodesmus sp. | - | 2.426 | 4.0 | 36.2 | - | Biomass | [118] |
| Vinasse | Chlorella vulgaris | - | 0.073 | 2.7 | 15.6 | - | Biomass(10% of lipids in biomass) | [119] |
| Vinasse | Scenedesmus sp. | - | 0.055 | 1.06 | 41.5 | - | Biomass | [82] |
| Slaughterhouse | Chlorella vulgaris | - | 0.575 | 1.15 | 85 | - | Biomass | [120] |
| Slaughterhouse | Chlorella vulgaris | - | 0.433 | 1.3 | 19.4 | - | Chlorophyll-a (6.8 mg L−1) | [121] |
| Slaughterhouse | Chlorella vulgaris | - | 0.194 | 5.4 | 94 | 99 | Biomass | [99] |
| Slaughterhouse | Tetradesmus obliquus | - | 0.234 | 6.6 | 96 | 99 | Biomass | [99] |
| Slaughterhouse | Chlorella vulgaris | - | 0.424 | 1.2 | 81 | - | Biomass | [97] |
| Slaughterhouse | Chlorella sp. and Scenedesmus sp. (1:1) | - | 0.033 | 0.231 | 13.4 | - | Biomass | [57] |
| Slaughterhouse | Chlorella sp. (Trebouxiophyceae) | 1% CO2 at a flow rate of 0.5 L min−1 | 0.030 | 0.18 | 49.4 | - | Biomass | [57] |
| POME | Chlorella sorokiniana and Pseudomonas sp. (1:1) | 70% (v/v) water; 200 mg L−1 of glucose; 2.5% urea and glycerol 0.1 vvm CO2 aeration. | 0.409 | 5.74 | 93.7 | - | Lipids(14.43% of the biomass) | [122] |
| POME | Scenedesmus sp. | - | 0.018 | 0.55 | 48 | - | Biomass | [86] |
| POME | Chlorella pyrenoidosa | 75% (v/v) waterurea and TSP (2:1) | - | - | 90.42 | - | Lipids(36% of the biomass) | [123] |
| POME | Arthrospira platensis | - | 0.032 | 0.19 | 15 | - | Biomass | [124] |
| POME | Nannochloropsis sp. | 20% Walne’s medium | 0.05 | 0.7 | 47.5 | 47.2 | Lipids(45% of the biomass) | [125] |
| POME | Chlorella sp. | 50% BBM | - | - | 57.6 | - | Biomass | [126] |
| POME | Chlorophyceae (not identified) | 25% water | 0.047 | 0.7 | 89.6 | 99.4 | Biomass | [127] |
| POME | Scenedesmus sp. | - | 0.027 | 0.8 | 60 | - | Biomass | [86] |
| Cassava | Desmodesmus subspicatus LC172266 | 10% BBM 10% Trace elements solution | 0.041 | 0.63 | 89.04 | 85.85 | Lipids (21.40%) | [128] |
| Cassava | Desmodesmus subspicatus LC172266 | 10% BBM 10% Trace elements solution | 0.07 | 1.04 | 51.39 | 62.22 | Lipids (24.70%) | [128] |
| Cassava | Desmodesmus armatus | 10% BBM 10% Trace elements solution | 0.07 | 0.73 | 92 | 87 | Lipids | [94] |
| Cassava | Chlorella sorokiniana | - | 0.021 | - | - | 90 | Biomass | [95] |
| Cassava | Scenedesmus sp. | - | - | - | 72 | 74 | Lipids | [129] |
| Cassava | Chlorella sorokiniana P21 | - | 0.48 | 2.11 | 88 | - | Biomass (58% lipid) | [130] |
| Cassava | Chlorella sorokiniana P21 | - | 0.18 | 2.56 | 73.78 | - | Biomass | [130] |
| Cassava | Chlorella sorokiniana WB1DG | - | 0.049 | 1.3 | 63.42 | - | Biomass | [130] |
| Aquaculture | Tetraselmis suecica | 0.02g L−1 N 0.01g L−1 P | 0.068 | 0.9 | - | - | Biomass, carbohydrate (10.62%), lipids (25.06%) proteins (50.20%) | [63] |
| Aquaculture | Chlorella vulgaris | - | - | - | 71 | 55.72 | - | [113] |
| Aquaculture | Chlorella vulgaris | - | 0.465 | - | 98.10 | - | Biomass | Gao,2021 |
| Aquaculture | Spirulina sp. | 25% Zarrouk medium | 0.2 | 1.1 | 90 | - | Biomass, protein (63.73%), phycocyanin (16.60 mg ml−1), polyunsaturated fatty acids (38.20%) and C18:3n6 (38.20%) | [131] |
| Aquaculture | Chlorella sorokiniana | - | 0.353 | 4.02 | 71.88 | - | Biomass, Lipids, Protein, Carbohydrate | [132] |
| Aquaculture | Ankistrodesmus falcatus | - | 0.16 | 2.25 | 61 | - | Biomass, Lipids, Protein, | [133] |
| Aquaculture | Chlorella sorokiniana | - | 0.107 | 1.51 | 69 | - | Biomass, Lipids, Proteins | [133] |
| Dairy | Chlorella sorokiniana | - | not show | 17 | 93 | - | Protein, lipids, biomass | [111] |
| Dairy | Chlamydomonas polypyrenoideum | 25% water | not show | 7.7 | 55.7 | - | Lipids (42%) | [134] |
| Dairy | Chlorella sp. | - | 0.000175 | 0.26 | 80.63 | - | Lipids | [109] |
| Dairy | Chlorella sorokiniana SU-1 | - | 1.96 | 0.153 | 67.6 | - | Lipids | [20] |
| Dairy | Chlorella sorokiniana | - | 1.667 | 0.108 | 57.17 | 56.95 | Biomass | [135] |
| Dairy | Chlorella vulgaris | - | 0.42 | 0.02 | - | - | Lipids | [136] |
| Dairy | Chlorella sp. T4 | 40% water | 0.0085 | 0.85 | 59.7 | - | Lipids | [137] |
| Dairy | Chlorella vulgaris | 25% water | 0.225 | 2.43 | 81.48 | - | Lipids | [138] |
4. Anaerobic Digestion as Pretreatment and Energy Recovery Strategy
4.1. Anaerobic Digestion as a Pretreatment Step
Anaerobic digestion (AD) is an effective waste treatment technology, especially for the wastewater or solid waste of high organic content. It can be used to recover organic compounds and produce biogas or other high-added-value products. Anaerobic systems are cheaper and relatively simple to operate but less efficient at removing organic matter than aerobic systems [54]. In contrast to conventional methods of landfilling or incineration, which are more polluting and do not allow the recovering of any material, anaerobic digestion has been used with the possibility of producing bioenergy and nutrient recycling [139]. The anaerobic digestion process decreases the organic waste load (both COD and BOD) [140]; organic matter is mainly converted into methane, while ammonia and phosphate are not consumed during the process [139]. The residual liquid effluent, called digestate, is the co-product generated in the AD process. It has a high content of nutrients (N and P) and cannot be released directly into nature [20]. Disposing and treating large amounts of digestate is an important problem to solve, as improper disposal can cause adverse environmental impacts. Due to the requirements of equipment and high energy consumption to reduce COD and other components of this digestate, nutrient recovery becomes attractive and has the potential for various applications [141,142]. Alternatively, some microalgae species can tolerate high nutrient content and other toxic pollutants from the digestate, and this can be used as a low-cost substrate for microalgae cultivation. Anaerobic digestion plays a vital role in the sustainable development of microalgae-based integrated biorefinery [143].
Wastewater systems significantly impact global water, energy, climate, and sustainability. Due to the high organic loads reported in effluents, such as POME, vinasse, cassava wastewater, dairy wastewater, slaughterhouses, and aquaculture, anaerobic digestion efficiently reduces this organic load. Still, it has the limitation of not removing phosphorus and nitrogen. Crude wastewater contains a very high concentration of organic compounds. It is characterized by high turbidity and low transparency, nutrients in the form of complex mixtures and is challenging regarding direct use by microalgae. The anaerobic digestion of wastewaters as a pre-treatment for the cultivation of microalgae has the advantage of the partial removal of organic compounds, besides the transformation of compounds into easily absorbed mineral forms and the reduction of turbidity, to improve the availability of light that enables photosynthesis [144].
The anaerobic digestion process mineralizes phosphorus and nitrogen into phosphate and ammonium, which are suitable forms for the assimilation by microalgae. The tertiary effluent generated after the cultivation of microalgae can be disposed of in an environmentally safe manner [145]. The integration of wastewater treatment plants and microalgae cultivation can be a resource for producing algae biomass, value-added compounds, and nutrient recycling [47]. This integration is also beneficial for the ability of microalgae to capture carbon dioxide and, consequently, reduce carbon dioxide emissions [146]. The CO2 content present in biogas generally ranges from 20 to 60%, and microalgae tolerant to high CO2 concentrations are preferred. Some microalgae may be more or less tolerant to the presence of CH4, for example, a mutant Chlorella sp. MM2 tolerates up to 80% of CH4, while a native strain of Nannochloropsis gaditana can tolerate 100% CH4 without significant changes in biomass production and growth [147], meaning that biogas can be bubbled through culture media for CO2 absorption (biogas upgrading is discussed in Section 5). The capacity of nutrient assimilation of these photosynthetic microorganisms reduces the risk of contamination of water bodies by effluent releases [148].
4.2. Secondary Effluents Composition
Raw effluents generally have physicochemical characteristics that limit their use directly in the production of microalgae, which is why pre-treatment steps of the effluents are necessary [72,149]. In the anaerobic digestion process, some compounds or nutrients are removed, mainly carbon sources, such as carbohydrates, and some lipids are practically depleted. As a result, up to 90% of the DOC (dissolved organic carbon) is removed. [40,150]. The secondary effluent or digestate from the AD process is left with a residual COD and important nutrients, such as ammonia and phosphate. Volatile fatty acids, such as acetic, butyric, propionic, and lactic acids [151,152], are coproducts of AD and are also responsible for the residual COD of the effluent and can be used as alternative sources of carbon. In addition, complex polymers produced in AD can be bioavailable to be used or harnessed in other processes.
Dairy effluent presents high-polluting potential due to the high concentrations of organic matter and nutrients, such as potassium, nitrogen, and phosphorus, low pH, and high corrosiveness [153]. Dairy wastewater can be treated by integrating anaerobic digestion and microalgal remediation. Reports mention the COD removal of up to 80% in dairy effluent after anaerobic digestion [154]. Kusmayadi et al. [20] tested Chlorella sorokiniana SU-1 and the effect of inoculum sizes using dairy wastewaters. They found that AD effectively reduced the concentration of COD from 4000 to 978 mg L−1 and total phosphorus content from 11.6 to 8.6 mg L−1. Total nitrogen content was increased by anaerobic bacteria due to the transformation of organic nitrogen to NH4. As microalgae primarily use inorganic nitrogen sources, this is particularly advantageous for subsequent microalgae cultivation. For vinasse and cheese whey, 89% COD removal efficiency can be obtained via the AD process [155]. The presence of nutrients in cassava wastewater, nitrogen and phosphorus, and carbohydrates characterize their potential as substrates in fermentative processes. They have a high COD of about 25,000 to 50,000 mg L−1, and anaerobic digestion is commonly applied to decrease this load [156]. The effluent from slaughterhouses presents high organic loads (500–15,900 mg L−1 COD) and a significant amount of nutrients, which are attractive to the treatment by anaerobic digestion. In studies with anaerobic digestion of slaughterhouse residues, removal percentages were obtained from between 16 and 22% [157] to 80% of COD [158].
Vadivelo et al. [57] reported microalgae cultivation in two raw, undiluted anaerobic digestates. A monoculture of Chlorella sp. and a mixed culture of Chlorella sp. and Scenedesmus sp. were found to be effective in treating these secondary effluents. The microalgae were cultivated in anaerobically digested municipal centrate and anaerobically digested abattoir effluent. Chlorella sp. Removed around 95% of NH3-N and 58% of PO43−, successfully demonstrating the use of the anaerobic digestates as culture media for microalgae.
The anaerobic digestion of aquaculture wastewater still concerns few studies, but its application has been considered an advantageous treatment alternative [159,160]. In cultivation systems that use recirculating aquaculture systems (RAS), the solid waste collected, mainly containing fecal matter and uneaten feed, can be anaerobically treated. Besides the reduction of solids and nutrients, there is a potential for methane production compared to other manure sources. Factors, such as the type of feed and species cultivated, impact the characteristics of the waste and its anaerobic biodegradability [161]. Diets rich in lipids can positively impact methane production, while lignocellulosic materials can limit the hydrolysis step of the anaerobic digestion process [161]. Table 3 presents certain secondary effluents commonly used for microalgae cultivation after AD.

Table 3.
Physicochemical characteristics of secondary effluents from the anaerobic digestion of wastewaters that can be considered for microalgae cultivation.
5. CO2 Fixation and Biogas Upgrading
Beyond wastewater and solid residues in the agro-industry, discussions can expand to the issue of gaseous effluents and the development of bioprocesses that play an important role in the treatment, since they are considered one of the primary sources of emissions and contamination in the industrial process [81,165]. One of the emission sources originates from burning biomass or fossil fuels, where coal is widely used as an energy source [166,167,168,169,170]. Another source of emissions of gaseous effluents is biodigesters, which can be used to produce methane to generate electricity and heat and can emit high concentrations of CO2 and H2S [171]. In this scenario of the biological treatment of gaseous effluent, microalgae represent a promising strategy for the photosynthetic fixation of CO2 and other compounds (e.g., H2S, sulfur oxides—SOx, and nitrogen oxides—NOx) present in the gas streams and offer the possibility of producing microalgae-based bioproducts and biofuels [165].
5.1. Microalgae Potential for Flue Gases Fixation
The photosynthetic apparatus of algae has been discussed as a potential strategy for GHG (greenhouse gas) mitigation and as a natural alternative for gaseous effluent treatment, since it simultaneously generates biomass that can be used in the recovery of high-value-added products. In this scenario, different microalgae species have been explored for industrial gas fixation to achieve greener processes and economic gain, strengthening the circular economy concept.
Most large industries rely heavily on fossil fuels, mainly coal and diesel, that emit a vast amount of flue gases, whose CO2 concentration is typically above 10% [172,173,174]. Aside from CO2 emissions, industrial flue gases are also often accompanied by other pollutants that aggravate the polluting potential of emissions, such as NOx, SOx, hydrocarbons, particulate matter, and trace pollutants. For instance, coal-burning generates flue gases with approximately 8 to 15% CO2 content and varying concentrations of SOx and NOx, with reports of 30 to 200 ppm and 60 to 210 ppm, respectively [169,175,176,177].
The presence of these compounds in flue gas can be a challenge for microalgae-mediated gaseous effluent treatment because photosynthetic mechanisms (passive diffusion or enzymatic pathway [178,179]) responsible for CO2 transport and assimilation in the cells are sensitive to changes in environmental conditions. Therefore, different microalgae species will have different biofixation potentials since they differ in their ability to tolerate the CO2 concentrations and other compounds in the flue gas [180,181]. From another perspective, some genera can assimilate sulfur and nitrogen compounds in the flue gases as nutrient sources. There are reports of tolerance to SOx concentrations above 90 ppm by microalgae of the genus Chlorella that have been extensively studied for flue gas fixation [182]. In continuous mixotrophic cultivation conducted with municipal wastewater and coal-fired flue gas, Chlorella vulgaris showed a tolerance capacity of 180 ppm SOx and 250 ppm NOx, removing 59% and 55% of the compounds, respectively, and exhibited effluent treatment efficiency by reducing the organic matter concentration and CO2 content (72%) [176].
Another aspect to be considered during flue gas biofixation is that the increase of CO2 mole fractions in the inlet stream of the cultures leads to an intense reduction in pH, resulting from the dissolution of CO2, NOx, and SOx in the aqueous medium, which induces the formation of acidic species [179,183]. This process causes metabolic inhibition, which can cause the cell death of the microalgae or can extend the lag phase [183]. To improve the practical application of gaseous effluent treatment methods, several species of microalgae with tolerance to high CO2 concentrations have been investigated, in addition to efforts to elucidate the pathways for inhibition of the compounds present in the gases and strategies to minimize these problems [184,185,186].
It should be noted that tolerance and biofixation capacity are two different bioprocesses. Tolerating high flue gas concentrations will not necessarily result in efficient biofixation processes. The tolerance to different compounds and concentrations present in the flue gas was evaluated for microalgae species, with concentrations above 15% CO2 [182,187,188]. However, higher biofixation capacities are reported at concentrations of approximately 10% CO2 and have encouraged the development of strategies such as the intermittent injection of gas into the cultures or dilution of the gases, as reported by Yadav et al. [183], Moheimani et al. [169], and Matito-Martos et al. [189]. Another strategy used to minimize the toxic effect of high concentrations of CO2 (15.6%), and in this specific case, the presence of hydrogen sulfide gas (H2S) (120 ppm), is the use of scrubbers [187] and the serial connection of photobioreactors [187,190]. Recently, Chauhan et al. [180] demonstrated that the pre-acclimatization of microalgae could improve biofixation performance in the presence of SOx and NOx.
Developing mixotrophic cultivation strategies by integrating processes that use waste resources for microalgae growth and bioproduct recovery can reduce the operating costs of biological systems and improve process performance by increasing biomass productivity [191,192,193,194]. In addition, integrating CO2 biofixation processes combined with wastewater treatment can provide more efficient treatment than individual operations [184,194]. This was observed in treating POME associated with CO2 fixation using the microalgae Chlorella sp. and Scenedesmus sp. Furthermore, the authors suggested that the organic load present in the wastewater improved the stability of the pH even with CO2 injection [184]. Table 4 presents recent studies that have conducted evaluations in microalgal cultures with wastewater and gas injection.
When a stable and well-established system is extant, it can be affirmed that mixotrophic cultures with wastewater and CO2 injection favor the increase of biomass productivity due to the improvement of the photosynthetic process and the activation of metabolisms associated with the assimilation of organic carbon present in the wastewater [183]. The presence of other compounds or the restriction of nutrient sources can stress the cells and enhance the production of biomolecules such as carotenoids [180,188,192]. Song et al. [188] have shown that in the presence of 15% CO2 and 80 ppm SO2, the microalgae Arthrospira sp. increased pigment production compared to experiments without SO2, demonstrating that the sulfur present in the flue gas acted as a nutrient source for the microalgae.
Developing flue gas biofixation integrated with wastewater treatment to obtain microalgal biomass is a complex process. Although the experimental analysis is especially relevant, technical and economic feasibility and environmental and social aspects are fundamental to evaluating the interaction of the different fields that comprise the process [193,195]. The recovery of bioproducts from microalgal biomass after wastewater treatment is an interesting strategy for developing an economically feasible approach that addresses the concept of a circular economy. There is a rapidly expanding market for products derived from microalgal biomass. However, when using wastewater and flue gas in cultivation, the produced biomass may not be adequate for producing food, feed, or nutraceuticals. Still, it can be used for chemicals, biofuel, and biofertilizer production [193].

Table 4.
Overview of reports that have evaluated integrated mixotrophic culture with wastewater treatment and CO2 injection.
Table 4.
Overview of reports that have evaluated integrated mixotrophic culture with wastewater treatment and CO2 injection.
| Microalgae | Wastewater | Flue Gases | CO2 Biofixation | Considerations | Reference | ||
|---|---|---|---|---|---|---|---|
| Origin | Composition | CO2 Content Injected | |||||
| Chlorella sp. GD | Aquaculture | Natural gas | 8% CO2 57% NO | 8% CO2 | 2333 mg L−1 d−1 | Growth is higher when aerated with flue gas compared to equivalent pure CO2 | [179] |
| Chlorella sp. L166 | Soybean processing | Simulated | n.a. | 5% CO2 | 25% | Diluted effluent (5x) and pure CO2 injection | [196] |
| Chlorella vulgaris | Industrial wastewater (textile and food) | Coal-fired | 10% CO2 0.554% CO 61 ppm NO2 30 ppm SOx 9 ppm HC | 5% CO2 | 187.65 mg L−1 d−1 | Increased lipid and carbohydrate accumulation | [183] |
| Phormidium valderianum BDU 20041 | Ossein effluent (Gelatin industry) | Coal-fired | 15% CO2 | 15% CO2 | 56.4 mg L−1 d−1 | High cell density to overcome metabolic stress | [197] |
| Scenedesmus sp. UKM9 Chlorella sp. UKM2 | Palm oil mill effluent (POME) | Simulated | n.a. | 10% CO2 | 829 mg L−1 d−1 | Process conducted in two steps: effluent treatment and CO2 fixation | [184] |
5.2. Biogas Upgrading
Biogas is a biofuel derived from the anaerobic digestion of biomass that has attracted attention in industries because it is a clean and economically low-cost energy generation process. The gas generated is composed chiefly of methane (40–70%) and CO2 (20–60%), and other gases, such as nitrogen (0–3%), oxygen (0–1%), H2S (0–10,000 ppm), ammonia, and trace compounds [198,199,200,201,202]. The calorific value of biogas is limited to methane, which reduces the calorific value in the presence of other gases and leads to undesired emissions during combustion. Therefore, biogas upgrading is conducted to increase the calorific value of the gas by primary cleaning to remove CO2 and H2S content, concentrating methane at the process output [203]. Any reduction in non-methane gases increases the calorific value of the biogas. However, biogas upgrading usually aims for a methane content higher than 85–90%.
Over the last decade, much has been explored concerning the microalgae-based upgrading process that could benefit biogas with high CO2 content on a microalgae production and nutrient removal platform. Besides the CO2 removal, other undesired compounds in the biogas can be removed—especially H2S, which generates acid oxides during burning and leads to equipment corrosion [201,204].
Generally, biogas upgrading is conducted in absorption column reactors connected to photobioreactors for microalgal cultivation. Biogas is injected into the column, which contains a highly alkaline solution (pH > 9.0) and microalgae cells, where CO2 and H2S are efficiently dissociated in the alkaline liquid phase of the column and a strongly pH-dependent reaction forms predominantly bicarbonate and hydrosulfides, respectively. [201,203]. The outflow of purified methane gas occurs from the top of the column, and the liquid is recirculated from the photobioreactor [205,206,207,208,209,210]. The oxygen presence can facilitate the oxidation of H2S to SO42−, and these compounds can be a source of sulfur for the cells but can also inhibit growth when in concentrations above 200 ppm [201,211]. Frequently, removals higher than 95% of H2S are reported, which occur mainly via oxidation, resulting in systems with high absorption in the liquid solution (SO42−) and secondarily through fixation by the microalgae cells, which play a crucial role in CO2 fixation [200,201,207,212]. Table 5 presents H2S and CO2 removal results in microalgae-based biogas upgrading processes.

Table 5.
Biogas upgrading using microalgae-based biological systems.
In microalgae cultivation, raceway photobioreactors are often used, which makes axenic cultivation difficult, and this encourages the use of microorganism consortia. Many reports evaluate the effect of symbiosis between bacteria and microalgae [202,208,213,214]. This technique has shown promising results at the laboratory scale and recently was assessed at a pilot scale with a consortium of microalgae and bacteria and showed efficiency higher than 90% for CO2 and H2S removal and methane content higher than 85%. [202,209]. Tubular reactors can be a strategy to improve biomass productivity in upgrading processes [210]. However, they are more expensive than open reactors, requiring greater control of parameters and engineering design complexity. Although axenic cultures are not frequently used, the microalgae of the genera Scenedesmus and Chlorella are often highlighted because of their versatility, tolerance to high CO2 content (20–60%), and potential for capture. They have been reported to predominate even in non-axenic cultures. [207,208].
In addition to the above configuration, there is the possibility of using photobioreactors with biogas injection for CO2 removal, where the culture is intermittently fed with biogas during the active photosynthesis phase [215,216,217]. To optimize biogas upgrading with direct injection into the photobioreactor, it is necessary to have sufficient residence time for CO2 dissolution, uptake by the microalgae cells, and nutrient removal [203]. The optimization of multiple parameters can be fundamental to the performance of the reactor, such as luminosity that directly influences the growth rate of the microalgae and demonstrates impacts on nutrient removal when integrated with wastewater treatment [217].
Photosynthetic biogas upgrading in a commercial system must operate continuously with the control of CO2 content, which ideally should be kept in a range of up to 6% and with low O2 content to avoid a potentially explosive environment. There is a challenge in microalgae-mediated biogas upgrading, since higher CO2 contents are present in biogas, which may require larger project areas [218].
Beyond that, oxygen is generated during photosynthesis, which can increase the content of biomethane [203]. One strategy applied to reduce the oxygen content of the biogas is recirculation during the dark phase of cultivation, where the photosynthetic step is interrupted and the increased respiration of the microalgae occurs [219]. In addition to environmental parameters, reducing the hydraulic retention time of biogas in reactors can provide engineering benefits and operational strategies with airlift configuration absorption unit with an evaluation of bubble dispersion and system behavior enabled short hydraulic retention time (10 min) that resulted in approximately 14% improvement in biogas calorific value with complete H2S removal [206].
In the context of effluent treatment and biogas upgrading, the integration of biological processes has resulted in the co-culture of microalgae and fungi with emphasis on the fungus Ganoderma lucidum, which presents a high capacity for nutrient removal from effluents and friendly coexists in the environment with microalgae, having been recently evaluated for Scenedesmus obliquus and some species of Chlorella [220,221,222]. The technology is still being assessed on a small scale and may result in higher energy expenditure and more unit operations. Even so, it shows promising results for nutrient (phosphorus, COD, nitrogen) and CO2 removal at approximately 89% and 75%, respectively [222].
Recent reports have emerged aiming to improve the efficiencies of biogas upgrading processes for stable performance on a large scale. One of the strategies applied was to use oxidizing bacteria to act on the oxidation of H2S concomitant with CO2 fixation by microalgae. This technique has shown promising results on a small scale and recently was evaluated at a pilot scale with a consortium of microalgae and bacteria and showed efficiency above 99% for removing CO2 and H2S [202].
The impact of biogas upgrading integrated with wastewater treatment was evaluated by Rajendran et al. [218], who compared nine scenarios with different biogas upgrading technologies, which showed that microalgae-based upgrading technology would cost between 0.24 and 0.37 EUR/m3. One challenge to the process scale-up is the requirement for reduced gas flow, compared to physical and chemical methods, in addition to the variation in yield and gas flow rate. This directly affects reactor design, area footprint, process control, and production cost [203,218]. It is worth emphasizing that the scale of the unit directly impacts the costs [223]. However, there is the possibility of cost reduction through the commercialization of bioproducts recovered from algal biomass in a circular economy approach that develops a process based on the management of waste with high environmental impact while expanding the commercial portfolio of industries for bioproducts from microalgae [224]. There are still gaps to be filled in terms of system optimization and scale-up that could reduce the costs of operation and production.
6. Tertiary Liquid Residues Destination and Water Reuse
Freshwater is a limiting resource in microalgae cultivation, and it is undeniable that the use of industrial wastewater is an interesting strategy to reduce the water footprint of these systems. It has already been demonstrated in the previous topics that besides improving the microalgae productivity and growth rate, there are concurrent processes of organic matter reduction present in the wastewater that can lead to serious environmental problems if disposed of inappropriately. Therefore, it is essential to consider that after cultivation, it is necessary to dispose of this wastewater safely, and environmental parameters must be considered.
However, there is a lack of research evaluating wastewater composition after microalgae cultivation. Most approaches are limited to assessing parameters required to comply with legislation for disposal into water bodies for the fertigation, or even recirculation, of the culture medium in algae cultivation, which will eventually also lead to disposal. The use of industrial wastewater as a culture medium for microalgae contributes to improving water quality for application in agricultural irrigation, often in compliance with environmental legislation in force and minimizing environmental impacts, or depending on fewer unit operations for downstream treatment, usually involving effluent polishing processes before discharge (e.g., pH adjustment; ultraviolet light; etc.) [225,226].
In a recent evaluation of wastewater composition after microalgae cultivation, Morais et al. [225] performed a comparative analysis of physicochemical parameters regarding current European laws, finding that the most significant impact of water reuse is inferred to pathogens, such as Escherichia coli. The limitations of reuse in urban areas were discussed, such as the necessary reduction of suspended solids and turbidity, as well as pH corrections, since the culture is conducted in an alkaline medium. In a non-conservative scenario, the water could be used for seed production (fertigation) [225]. The use for washing floors or animal stalls after the decantation and disinfection processes can also be a possible destination [216].
The recirculation of the medium in the algal culture represents a strategy for reducing the water input to the system and could be an insight into implementing “zero waste” processes [227,228]. Wastewater from aquaculture or shrimp farms can be strategically used in the microalgae growth to reduce the nutrient load and can subsequently be treated by filtration and disinfection to return to the animal cultivation process, reducing the water footprint of the system, and acting in closed production cycles [228]. Industrial wastewater used in cultivation can also be used in recycling systems, as in a study by Daneshvar [227] with dairy effluent and the microalgae Scenedesmus quadriculata and Tetraselmis suecica. When recycling tertiary wastewater from cultivation, some losses in the quality of the microalgal biomass can occur, which affects the growth rate or changes in the biochemical compositions of the algae [227]. Despite being a potential strategy, when the primary wastewater is generated constantly and in large volumes by the industry, there may be a high treatment demand, making recycling unnecessary or even problematic.
Insights into value-added resource recovery are lacking in the field, and this step could play an important role in increasing microalgae-based processes’ economic and environmental viability. One possible insight is the recovery of exopolysaccharides excreted by microalgae. These compounds possess bioactivity and can be applied in structuring an integrated system as building blocks for various commercial applications [229]. Exopolysaccharide recovery systems have already been evaluated in effluents such as POME and the microalgae Phaeodactylum tricornutum [230] and in systems with the microalgae Arthrospira platensis and Chlamydomonas asymmetrica with CO2 injection in the culture [231]. The integration of processes with liquid effluents and gas injections to produce algal biomass and the recovery of exopolysaccharides can be targeted. The main challenge of these systems is the specificity of production from microalgae, which may limit the use of some species in integrated processes.
In an integrated project, industrial wastewater can be a source of carbon (combined with gas injection) in the mixotrophic cultivation of microalgae, which results in the recovery of bioproducts from biomass. In a zero-waste concept, the integration of a process to recover exopolysaccharides from the culture water could be proposed, provided that the engineering design is conditioned from the beginning to parameter adjustments for satisfactory yields. In addition, when all the possibilities are exhausted, water can be applied in washing systems, in fertigation, or destined for water bodies if following the adequate emission limits.
SDG 6 from the list of Sustainable Development Goals (SDGs) of the United Nations deals with increasing water use efficiency in all sectors by 2030 [232]. It emphasizes the relevance of studies that are concerned with evaluating the feasibility of reducing the use of freshwater in bioprocesses and with attention to the parameters of the wastewater generated from cultivation and, if possible, a feasible approach beyond microalgae cultivation and the recovery of algae-based bioproducts.
7. Fate of Xenobiotics and Heavy Metals
The use of microalgae in the bioremediation of xenobiotic compounds is a promising technology due to the microalgae’s capacity to eliminate different types of compounds, including emerging ones [233]. Besides nutrients, such as carbon, nitrogen, phosphorus, and potassium, which are considered contaminants due to their potential impact on water bodies, other pollutants have been removed using microalgae: heavy metals, pharmaceutical compounds, and radioactive compounds, among others [234].
Microalgae cultures remove xenobiotic compounds using four main mechanisms: (i) biodegradation (Figure 2A), in which decomposition reactions reduce the toxicity of the pollutant; hydrolysis and oxidation reactions generally predominate, and the process can be intra- or extracellular [235]. This mechanism was observed in the microalgae Scenedesmus obliquus and Chlorella pyrenoidosa in the biodegradation of pharmaceutical compounds [236]. A second mechanism is (ii) bioaccumulation, when the pollutant accumulates inside the cells, which can change their structure (Figure 2B). The microalgae Desmodesmus subspicatus employs this mechanism in the bioremediation of radiolabeled 17a-ethinylestradiol. Researchers [237] also observed the bioaccumulation mechanisms in the carbamazepine bioremediation process using the microalgae Chlamydomonas mexicana and Scenedesmus obliquus. Additionally, some metals, such as Cu, Pb, Cd, and Co, can be eliminated by Cladophora glomerata and Oedogonium rivulare by short-term uptake and others, such as Ni, Cr, Fe, and Mn, in continuous uptake. The third mechanism is (iii) bioadsorption, where the pollutant attaches to the cell surface through electrostatic forces (Figure 2C). According to [235], microalgae cell walls are negatively charged due to the predominance of functional groups, such as carboxyl and phosphoryl, compared to amines. They also contain different polymers, which also facilitate the adsorption process. This mechanism was observed in dead Scenedesmus obliquus and Chlorella pyrenoidosa biomasses in removing progesterone and norgestrel [236]. Because bioadsorption is extracellular, the process is affected by the system’s hydrophobicity and the pollutants’ functional groups. Thus, xenobiotic compounds with cationic groups are highly attracted to the microalgae surface through electrostatic interactions [235]. The fourth mechanism for bioremediation is iv) bioaugmentation, based on the use of two or more species of microalgae or other types of microorganisms (Figure 2D). The main advantage of this bioremediation mechanism is that it generates synergy between the different microorganisms in the pollutant degradation process, in addition to expanding the spectrum of pollutants to be bioremediated. For instance, the symbiosis between Chlorella protothecoides and Brevundimonas diminuta promotes nutrient removal from wastewater. One of the approaches used for the bioaugmentation process is based on CO2 and O2 gas exchange in the effluent. However, the population interactions are not fully understood [238].
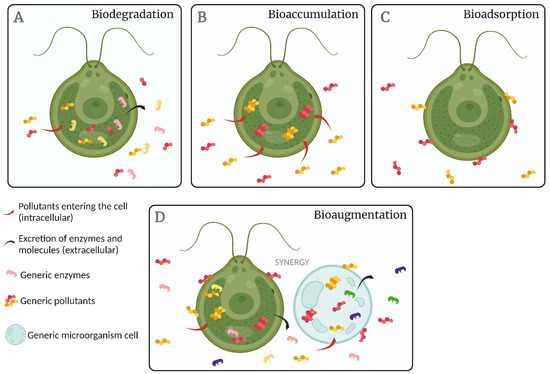
Figure 2.
Bioremediation mechanisms at play in microalgae cultures. (A) Biodegradation, where the cells directly degrade xenobiotics into innocuous molecules; (B) bioaccumulation, where the xenobiotics are not degraded but accumulate in living cells and can be removed with them; (C) bioadsorption, where molecules cling to the surface of living or dead cells and can be removed with the biomass; and (D) bioaugmentation, where pollutant molecules are degraded in an exchange of metabolites between microalgae and one or more symbionts.
8. Microalgae Products
Microalgae can be employed in the production of different commercially important biomolecules. Their potential contribution to the global bioeconomy includes a wide range of bioproducts, such as biofuels (bioethanol, biohydrogen, and biodiesel), bioplastics, biofertilizers, lipids, carotenoids, nutraceutical biomass, animal and human nutrition products, and others [239]. Bioremediation, nutrient recycling through wastewater treatment, and carbon fixation are generally carried out during the production process, which is an excellent advantage of these technologies [240]. Other benefits are the high growth speed, low production cost, high adaptability to a wide range of growth conditions, pH, and climate, a faster cycle of cultivation, processing, and harvesting, and the ability to grow in wastewater [241]. Another advantage is that microalgae can be produced using effluents and wastewater in high volumes and areas unsuitable for agriculture. A high diversity of photobioreactors models has been developed to produce different bioproducts, which creates new environmentally friendly solutions for the industry. However, some challenges include low biomass production, acclimatization, contamination (depending on the bioreactor model), harvesting, and downstream processes’ costs. Some recent works applied the biorefinery concept to convert the different fractions of algal biomass into high-value-added products [242]. In this context, microalgae biomass is integrally exploited as a substrate in the so-called zero waste-technology approaches where pretreatment and microbial fermentation techniques can be employed.
Bioplastics are alternative plastics obtained from renewable sources [243]. They can be produced from microalgae through different methods: (i) the blending of synthetic polymers and bioplastics with microalgae, such as proteins, starch, cellulose, and PHA [244,245]; (ii) biopolymers produced from microalgae cells [246] involving the intracellular accumulation of biomolecules, such as PHA, PHB, and starch, under certain conditions of nutrient deficiency, and its subsequent extraction [242]; and (iii) the conversion of microalgae into construction blocks suitable for polymerization [247]. Chlorella sp. and Spirulina sp. are the main microalgae species employed for bioplastic production. Genetic modifications in microalgae species have also been studied to improve the production of PHAs [248].
Microalgal lipids and fatty acids are important bioproducts that have attracted interest due to their high content of unsaturated lipid molecules. They can be used as feedstocks for biofuels and biomedical applications [249]. The content of lipids in oleogenic microalgae can vary from 30–70% of the weight of dry cell biomass [250]. Omega fatty acids (ω−3 and ω−6) are accumulated within microalgae cells through different environmental and/or nutritional stress [239]. However, only a small percentage of microalgae species can accumulate omega fatty acids. Milano et al. (2016) investigated biodiesel production from microalgae strains with high concentrations of oleic acid in their fatty acids. The lipid content of algal biomass affects the quality and presentation of biodiesel and its suitability as an alternative fuel to petroleum-based diesel [251]. Genetic engineering techniques and molecular biotechnology are applied to increase lipid productivity in microalgae for oil conversion and use for biofuel production.
Microalgae are a natural source of carotenoids, with β-carotene (provitamin A), astaxanthin, and lutein being the main commercially valuable carotenoids found in microalgae [252]. Chlorella vulgaris, Chlorella protothecoides, Scenedesmus almeriensis, Dunaliella salina, Haematococcus pluvialis, Porphyridium cruentum, and Haslea ostrearia have been reported for carotenoid production. Because it can be produced throughout the year, carotenoid degradation by long-term storage is eliminated [253].
The application of microalgae in biofuel production occurs through the photosynthesis process performed by most microalgae, which allows CO2 and solar energy to be converted into sugar and biomass [241]. Microalgae are the most potent species in biofuel generation and wastewater treatment compared to plant-based biofuel crops because they use them as sources of nitrogen, phosphorus, and micronutrients for growth [254,255]. Microalgae have high levels of different carbohydrates, such as glycogen, starch, agar, and cellulose, which can be converted into fermentable sugars for bioethanol production. Some carbohydrate-rich microalgae, such as Chlamydomonas reinhardtii and Chlorella vulgaris, have the potential to produce carbohydrates for bioethanol production [256]. In biohydrogen production, microalgae have been considered an inexpensive potential source. It was reported that the hydrogenase enzyme is responsible for biohydrogen production, in sulfur-deprived, selected microalgae species. In the generation of biogas from microalgae, the challenge is related to the raw material, seasonal variation, and the pretreatment of algal biomass [257]. The production of biogas from microalgae through anaerobic digestion has the advantages of using wastewater, the feasibility of nutrient recycling, maximum biomass utilization, lower operating costs, and lower energy consumption [258].
Wastewaters from various processing streams can produce algal biomass for different applications and bioproducts (Table 6).

Table 6.
Microalgal products from different residues.
9. Circular Bioeconomy in Microalgal Production from Agro-Industrial Wastes
Microalgae represent a promising strategy in the circular bioeconomy because of their ability to fix CO2 and to be cultivated in non-arable lands using aqueous residues, reducing their polluting potential, and recovering nutrients in their biomass. Besides promoting significant biochemical transformations that result in environmental benefits, microalgae are an important source of various bioproducts, including edible oils, biofuels, biopigments, nutritional protein, peptides, and biofertilizer, which in turn can enhance the economic performance of microalgal biorefineries.
The market value of some of the most important autotrophic microalgal species—Spirulina, Chlorella, Dunaliella, and Haematococcus—is between EUR 370 and 500 million, corresponding to an annual production of 11.180 tons/year to 15.950 tons/year and an average price of 227–530 EUR/kg on dry basis [267]
The diffusion of microalgal technologies in circular processes can promote significant advances in establishing a bio-based economy. Important indicators to be considered in this context include the life-cycle assessment (LCA) evaluating environmental impacts, such as global warming potential (GWP), fossil energy requirement (FER), greenhouse gas (GHG) emissions, water footprint, land transformation and use, abiotic depletion (ADP), eutrophication potential (EP), acidification potential (ACP), ozone depletion (OD), photochemical oxidation potential (POP), and toxicity parameters. Banu et al. [268] summarized the life-cycle analysis and environmental impacts of different microalgal biorefineries configurations. The most frequently analyzed environmental impacts were GHG (mainly CO2) emissions and GWP associated with microalgal biomass and biodiesel production. For example, the “well to tank” LCA reported by Monari et al. [269] to produce 1 MJ of biodiesel using Nannochloropsis sp. cultivated in photobioreactors showed that the use of wastewater is determinant to avoiding GHG emissions and energy demand. Otherwise, the microalgal biodiesel produced by conventional technology (i.e., using freshwater) had higher energy demand and GHG emissions than fossil diesel.
The evaluation of microalgal biorefineries from the economic point of view can be carried out through the analysis of life-cycle costing (LCC). It represents the aggregation of energy, installation, downstream, operating, maintenance, environmental, and decommissioning costs over the complete lifetime of the product and considers the operating cost (OC), the salvage value (SV), the maintenance cost (MC), and the capital cost (CC) [268]. Using agro-industrial wastewater in microalgal cultures reduces the operating costs associated with raw materials (water and nutrients). However, the presence of insoluble solids and undesired substances may require additional processing steps that can impact the economic performance of the process. It is challenging to elucidate the economic impact of agro-industrial wastewater in microalgal biorefineries since most studies focus on major process steps and equipment, such as downstream unit operations, cultivation modes, and bioreactor types. For example, the economic performance of microalgal biodiesel production (9.3 million gallons/year) using autotrophic mixed cultures was highly dependent on the reactor type. When comparing open ponds with closed tubular photobioreactors, the cost of lipid production to achieve a 10% return was USD 8.52/gal versus USD 18.10/gal respectively; when considering a hydrotreatment for fuel upgrade, the values rose to USD 9.84/gal and USD 20.53/gal, respectively [270]. Similarly, the operational costs of ꞵ-carotene and fertilizer production using Dunaliella salina increased from ca. 7.9 million EUR/year in open paddlewheel ponds to 13.2 million EUR/year in closed photobioreactors [271]. Economic studies focusing on agro-industrial wastewaters versus defined media should be developed, at least at a pilot scale, to demonstrate the viability and scalability of such processes.
The production of microalgal biomass and bioproducts in agro-industrial wastewaters still needs further development to reduce production costs so that these bioproducts can become economically competitive. The long cultivation time and the low yields are among the most cited obstacles. In a review of the potential of using swine, cattle, and poultry wastewater in microalgal cultures, Lopez-Sánchez et al. [272] pointed out that, although promising, the production of biomass using wastewater in a circular economy perspective is still limited to low yields because it depends, among other things, on the adaptability of the strain to the physicochemical conditions of the medium, which often needs to be sterilized.
Although there are challenges concerning process economics, the technical viability of microalgae cultivation using circular bioeconomy approaches has been demonstrated at laboratory-, pilot-, and large-scale. Lopez-Pacheco et al. [273] presented studies on the use of wastewater from swine farms in the cultivation of different species of microalgae both as wastewater treatment and to produce byproducts of commercial interest. Ciardi et al. [274] cultivated Scenedesmus almeriensis using pig slurry at 5%, reaching a biomass productivity of 0.68 g L−1 d−1. García-Galan et al. [275] studied the treatment of agricultural runoff from irrigation channels in rural areas in a full-scale horizontal tubular system with Pediastrum sp., Chlorella sp., Scenedesmus sp., and Gloeothece sp. The authors observed a 22.8% increase in biomass production in 4 days, with up to 72% removal of various organic pollutants.
Examples of pilot- to large-scale facilities where microalgae are cultivated in wastewaters applying circular economy approaches were summarized by Goswami et al. [276]. MaB-floc SBRs (microalgae-bacterial flocs in sequencing batch reactors) in Belgium used aquaculture wastewater (ca. 12 m3, 40 mg L−1 N, 2 mg L−1 P) to cultivate microalgae and bacteria in outdoor raceway reactors, with a biomass yield of 25 g m−2 day−1 and a nutrient removal efficiency above 50% for nitrogen and phosphorus. Researchers at Chilgokgun Agricultural Technology Center in South Korea cultivated Chlorella sp., Coelastrella sp., Acutodesmus sp., and Pseudopediastrum sp. in outdoor raceway ponds with different wastewaters (ca. 236,000 m3, 15–30 mg L−1 N, 3–6 mg L−1 P), producing 2612.7 kg biomass with nutrient removal efficiencies of 13.1 mg/kg biomass for nitrogen and 2.5 mg/kg biomass for phosphorus. NPDEAS, at the Federal University of Paraná in Curitiba, Brazil, treated 9.38 m3 of swine slaughterhouse wastewater in a closed photobioreactor producing 1.5 kg m−3 day−1 of microalgal biomass. The development of a microalgal biorefinery to be integrated into an ethanol industry was reported by Sydney et al. [81]. Sugarcane vinasse was used as a medium for chemical and biological CO2 fixation, the first achieved by the reaction of the gas with alkalinized vinasse to form carbonate and bicarbonate salts and the second by the cultivation of different autotrophic microalgal and cyanobacterial species, namely Botryococcus braunii, Synechococcus nidulans, Chlorella kessleri, Chlorella vulgaris, Neochloris oleoabundans, Scenedesmus obliquus, Arthrospira platensis, Arthrospira laxissima, and Arthrospira maxima. The previous chemical treatment was important to reduce turbidity and organic load, allowing the cultivation of the microorganisms at vinasse concentrations between 70 and 100%, with a biomass production of 2.25 g L−1 of B. braunii reached after 15 days.
10. Process Development and Perspectives
Coupling microalgae production with residue treatment is conceptually simple but operationally complex, because of the variable composition of wastewaters and the process parameters involved in massive microalgae cultures. However, there are vital aspects that, if carefully considered, can aid in process development. The key elements to be considered are effluent composition, availability, and the microalgae products of interest. From these initial constraints, the options can be narrowed down to a few process designs that can be compared on a techno-economical basis. Such considerations are complex and case-specific, but a few initial concerns are:
Production system: Residue volume and biomass use dictate the type of bioreactor to be used. Relatively small volumes can be processed in closed photobioreactors in a more controlled process that can lead to specialty algal products, since the risk of contamination is low and the quality of the product can be guaranteed. Large volumes, however, require large processing areas. In this case, open bioreactors are more cost-effective; however, the culture can have contaminants. This reduces the possible applications, unless downstream and fractionation guarantees the removal or inactivation of biological contaminants.
Residue COD and composition: Recovering energy as biogas can be profitable for the industry and will also lead to a digestate more adequate for autotrophic microalgal growth. However, dilute residues can be directly transferred to microalgal production. When a high amount of suspended or macromolecular solids is extant, as with CPW, minimal treatment (flocculation, removal of suspended solids) can recover the organic load for biodigestion while giving a clarified, less turbid supernatant effluent. The BMP, biomethane potential of residues, can be estimated at around 350 NLCH4. kg COD−1, assuming a substrate such as a carbohydrate or lactic or acetic acid with minimal formula CH2O is present; this initial estimation can be used to define whether it is worth including an AD step in the process.
Most secondary and tertiary residues also have large amounts of N- and P, but in imbalanced quantities, leaving unconverted nutrients. If the objective is simply to take advantage of excess nutrients, then the medium can be left unchanged. However, to maximize biomass production, a first improvement is adding a nitrogen or phosphate source to approach the Redfield ratio of 16:1 N to P (molar basis). The medium composition can be further adjusted for specific microalgae.
Residue toxicity and pathogenicity is a final concern that must be evaluated. The toxicity here refers to microalgae and cyanobacteria, which can be affected by cyanide from CPW, high salinity, concentrated transition metals such as Cu2+, and antibiotics or pesticides. Partial pretreatment and microalgae selection can tackle these problems. Still, these also affect the final biomass use—it is recommended that an orthogonal approach is used, i.e., the product from one agro-industry chain (e.g., vinasse) is applied in another chain (e.g., animal rearing) or is not cycled back to the field (e.g., microalgal biomass for energy can be simply co-fired). Fractions such as biodiesel, carotenoids, or bioactive extracts can guarantee the removal of residual xenobiotics and pathogens through downstream processing steps.
Microalgae selection: This critical step affects both productivity and process resilience. The options for specialty products, such as nutritional biomass, are few and depend on the envisaged market. Still, among a specific genus (e.g., Arthrospira), a few species and many strains can be tested to find a robust culture to grow in the residue. Adapting the culture is essential: inoculating a reactor cannot be simply carried out with a pure culture, which would lead to a shock. Slowly rising the effluent concentrations allow the culture to adapt, but even so, there may be a limit, thus requiring residue dilution.
When there is no product constraint, and the objective is to recover nutrients or reduce the effluent COD and eutrophication potential, a culture can be selected for its suitability to grow in the residue with high productivity. Such a selection is based on sampling microalgae from a similar environment and using classical microbiological techniques, careful work that pays by taking advantage of the enormous natural biodiversity.
Microalgae must be selected for their rapid growth and resistance to contamination, especially in open photobioreactors. An approach that is becoming more popular is using polycultures and microalgal-bacterial consortia, at least for biomass, that will be recycled for bioenergy production. This takes advantage of the adaptability of a mixed culture throughout the year and is especially suitable for simple open systems.
Stepwise development: Coupling microalgae production with agro-industry waste recycling is not complicated, but it is complex—in the sense that many factors are interlinked in the process, from residue and weather variability to the management and downstream processing of massive microalgal cultures. Therefore, a sensible approach is stepwise process development, starting with the laboratory evaluation of the suitability of residues to AD and algae growth, using microalgae from culture collections and even isolates or mixed cultures. The process can be conceptually evaluated from laboratory data through classical techno-economic analysis and, if seemingly promising, it can be developed as a pilot scale. From this proof of concept, it can be safer to develop an industrial process. Microalgae production can also be developed stepwise: even if the final aim is to produce a specialty microalgal fraction, starting with a robust or fast-growing culture such as Arthrospira or Chlorella, simply separating and drying biomass or recycling it to AD can allow the workforce to focus on production issues, and in time, other microalgae and more sophisticated fractionation processes can be tested.
Author Contributions
Conceptualization, J.C.C. and C.R.S.; writing—original draft preparation, J.C.C., D.T.M.-A., W.J.M.-B., S.G.K., M.C.M., A.B.P.M., C.R., T.S., L.P.S.V., S.V., A.L.W. and V.T.S.; writing—review and editing, J.C.C., S.G.K., A.B.P.M., C.R., L.P.S.V., A.L.W., V.T.S. and C.R.S.; visualization, T.S. and W.J.M.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The researchers were funded by the Coordination for the Improvement of Higher Education Personnel, CAPES foundation—PROEX Program, and the National Council for Scientific and Technological Development, CNPq, grant no.313276/2021-8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Show, P.L. Global Market and Economic Analysis of Microalgae Technology: Status and Perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef] [PubMed]
- Yellapu, S.K.; Klai, N.; Kaur, R.; Tyagi, R.D.; Surampalli, R.Y. Oleaginous Yeast Biomass Flocculation Using Bioflocculant Produced in Wastewater Sludge and Transesterification Using Petroleum Diesel as a Co-Solvent. Renew Energy 2019, 131, 217–228. [Google Scholar] [CrossRef]
- Carvalho, J.C.D.; Goyzueta-Mamani, L.D.; Molina-Aulestia, D.T.; Júnior, A.I.M.; Iwamoto, H.; Ambati, R.; Ravishankar, G.A.; Soccol, C.R. Microbial Astaxanthin Production from Agro-Industrial Wastes—Raw Materials, Processes, and Quality. Fermentation 2022, 8, 484. [Google Scholar] [CrossRef]
- Melo, J.M.; Ribeiro, M.R.; Telles, T.S.; Amaral, H.F.; Andrade, D.S. Microalgae Cultivation in Wastewater from Agricultural Industries to Benefit next Generation of Bioremediation: A Bibliometric Analysis. Environ. Sci. Pollut. Res. 2021, 29, 22708–22720. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Sydney, E.B.; Assú Tessari, L.F.; Soccol, C.R. Culture Media for Mass Production of Microalgae. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-Industrial Wastewater Reuse for Irrigation of a Vegetable Crop Succession under Mediterranean Conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Bittencourt Sydney, E.; Bianchi Pedroni Medeiros, A.; Magalhães, A.I.; de Carvalho, J.C.; Karp, S.G.; Porto de Souza Vandenberghe, L.; Junior Letti, L.A.; Thomaz Soccol, V.; de Melo Pereira, G.V.; et al. Agro-Industrial Wastewater in a Circular Economy: Characteristics, Impacts and Applications for Bioenergy and Biochemicals. Bioresour. Technol. 2021, 341, 125795. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A Review on Agro-Industrial Waste (AIW) Derived Adsorbents for Water and Wastewater Treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Vieira, G.E.G.; Cardoso, A.D.S.; Marques, A.K.; Pickler, A. Assessment of the Potential of Residuary Microalgae from Stabilization Ponds for the Production of Biofuel. WIT Trans. State Art Sci. Eng. 2014, 83, 25–35. [Google Scholar] [CrossRef]
- Florentino, A.P.; Costa, M.C.; Nascimento, J.G.S.; Abdala-Neto, E.F.; Mota, C.R.; dos Santos, A.B. Identification of Microalgae from Waste Stabilization Ponds and Evaluation of Electroflotation by Alternate Current for Simultaneous Biomass Separation and Cell Disruption. Eng. Sanit. E Ambient. 2019, 24, 177–186. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT-Database; FAO: Rome, Italy. Available online: https://www.fao.org/faostat/ (accessed on 28 October 2022).
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The Potential of Sustainable Algal Biofuel Production Using Wastewater Resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient Recovery from Wastewater Streams by Microalgae: Status and Prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic Cultures of Microalgae: Metabolism and Potential Products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, F.; Kuang, Y.; He, H.; Qin, A. Current Techniques of Growing Algae Using Flue Gas from Exhaust Gas Industry: A Review. Appl. Biochem. Biotechnol. 2016, 178, 1220–1238. [Google Scholar] [CrossRef]
- de Faria Ferreira Carraro, C.; Loures, C.C.A.; de Castro, J.A. Microalgae Technique for Bioremediation Treatment of Cassava Wastewater. Water Air Soil Pollut. 2021, 232, 281. [Google Scholar] [CrossRef]
- Sorgatto, V.G.; Soccol, C.R.; Molina-Aulestia, D.T.; de Carvalho, M.A.; de Melo Pereira, G.V.; de Carvalho, J.C. Mixotrophic Cultivation of Microalgae in Cassava Processing Wastewater for Simultaneous Treatment and Production of Lipid-Rich Biomass. Fuels 2021, 2, 521–532. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Borghetti, I.A.; Cartas, L.C.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. Biorefinery Integration of Microalgae Production into Cassava Processing Industry: Potential and Perspectives. Bioresour. Technol. 2018, 247, 1165–1172. [Google Scholar] [CrossRef]
- Molina, D.; de Carvalho, J.C.; Júnior, A.I.M.; Faulds, C.; Bertrand, E.; Soccol, C.R. Biological Contamination and Its Chemical Control in Microalgal Mass Cultures. Appl. Microbiol. Biotechnol. 2019, 103, 9345–9358. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Lu, P.H.; Huang, C.Y.; Leong, Y.K.; Yen, H.W.; Chang, J.S. Integrating Anaerobic Digestion and Microalgae Cultivation for Dairy Wastewater Treatment and Potential Biochemicals Production from the Harvested Microalgal Biomass. Chemosphere 2022, 291, 133057. [Google Scholar] [CrossRef]
- Dourou, M.; Dritsas, P.; Baeshen, M.N.; Elazzazy, A.; Al-Farga, A.; Aggelis, G. High-Added Value Products from Microalgae and Prospects of Aquaculture Wastewaters as Microalgae Growth Media. FEMS Microbiol. Lett. 2021, 367, fnaa081. [Google Scholar] [CrossRef]
- Dixit, R.; Singh, S.; Enamala, M.K.; Patel, A. Effect of Various Growth Medium on the Physiology and De Novo Lipogenesis of a Freshwater Microalga Scenedesmus Rotundus-MG910488 under Autotrophic Condition. Clean Technol. 2022, 4, 733–751. [Google Scholar] [CrossRef]
- Sydney, E.B.; Carvalho, J.C.D.; Letti, L.A.J.; Magalhães, A.I.; Karp, S.G.; Martinez-Burgos, W.J.; Candeo, E. de S.; Rodrigues, C.; Vandenberghe, L.P.D.S.; Neto, C.J.D.; et al. Current Developments and Challenges of Green Technologies for the Valorization of Liquid, Solid, and Gaseous Wastes from Sugarcane Ethanol Production. J. Hazard. Mater. 2021, 404, 124059. [Google Scholar] [CrossRef] [PubMed]
- Statista Fuel Ethanol Production Worldwide in 2021, by Country. 2022. Volume 2022. Available online: https://www.statista.com/statistics/281606/ethanol-production-in-selected-countries/ (accessed on 15 October 2022).
- World-In-Data Cassava Production. 2022. pp. 2003–2005. Available online: Https://Ourworldindata.Org/Grapher/Cassava-Production?Tab=table&time=1961..2018 (accessed on 15 October 2022).
- Statista Palm Oil Industry Worldwide-Statistics & Facts. 2022. Available online: Https://Www.Statista.Com/Topics/6079/Global-Palm-Oil-Industry/#dossierKeyfigures (accessed on 15 October 2022).
- FAO. Meat Market Review: Emerging Trends and Outlook. 2021. Available online: https://www.fao.org/3/cb7886en/cb7886en.pdf (accessed on 15 October 2022).
- Bustillo-Lecompte, C.; Mehrvar, M.; Quiñones-Bolaños, E. Slaughterhouse Wastewater Characterization and Treatment: An Economic and Public Health Necessity of the Meat Processing Industry in Ontario, Canada. Int. Conf. Environ. Pollut. Public Health EPPH 2016, 4, 175–186. [Google Scholar] [CrossRef]
- Ansari, A. Assessment of Laboratory Scale Cylindrical Sequencing Batch Reactor for the Treatment of Abattoir Effluent. Innov. Infrastruct. Solut. 2022, 7, 100. [Google Scholar] [CrossRef]
- Ohale, P.E.; Onu, C.E.; Nwabanne, J.T.; Aniagor, C.O.; Okey-Onyesolu, C.F.; Ohale, N.J. A Comparative Optimization and Modeling of Ammonia–Nitrogen Adsorption from Abattoir Wastewater Using a Novel Iron-Functionalized Crab Shell. Appl. Water Sci. 2022, 12, 193. [Google Scholar] [CrossRef]
- Konneh, M.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Adsorption and Desorption of Nutrients from Abattoir Wastewater: Modelling and Comparison of Rice, Coconut and Coffee Husk Biochar. Heliyon 2021, 7, e08458. [Google Scholar] [CrossRef]
- Bella, K.; Rao, P.V. Anaerobic Digestion of Dairy Wastewater: Effect of Different Parameters and Co-Digestion Options—A Review. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Kaur, N. Different Treatment Techniques of Dairy Wastewater. Groundw. Sustain. Dev. 2021, 14, 100640. [Google Scholar] [CrossRef]
- Bentahar, J.; Doyen, A.; Beaulieu, L.; Deschênes, J.S. Acid Whey Permeate: An Alternative Growth Medium for Microalgae Tetradesmus Obliquus and Production of β-Galactosidase. Algal Res. 2019, 41, 101559. [Google Scholar] [CrossRef]
- OECD. Agricultural Outlook 2021–2030. In OECD-FAO Agricultural Outlook 2021–2030; OECD: Paris, France, 2021; ISBN 978-92-5-134608-2. [Google Scholar]
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture Wastewater Treatment Technologies and Their Sustainability: A Review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Sun, C.; Wang, S.; Wang, H.; Hu, X.; Yang, F.; Tang, M.; Zhang, M.; Zhong, J. Internal Nitrogen and Phosphorus Loading in a Seasonally Stratified Reservoir: Implications for Eutrophication Management of Deep-Water Ecosystems. J. Environ. Manag. 2022, 319, 115681. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.C.; Ridoutt, B.G.; Wang, X.C.; Ren, P.A. Nitrogen and Phosphorus Losses and Eutrophication Potential Associated with Fertilizer Application to Cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Junios, J.R.; Medeiros, A.B.P.; Herrmann, L.W.; Sydney, E.B.; Soccol, C.R. Biohydrogen Production from Agro-Industrial Wastes Using Clostridium Beijerinckii and Isolated Bacteria as Inoculum. Bioenergy Res. 2021, 15, 987–997. [Google Scholar] [CrossRef]
- Parsaee, M.; Kiani Deh Kiani, M.; Karimi, K. A Review of Biogas Production from Sugarcane Vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Gozan, M.; Aulawy, N.; Rahman, S.F.; Budiarto, R. Techno-Economic Analysis of Biogas Power Plant from POME (Palm Oil Mill Effluent). Int. J. Appl. Eng. Res. 2018, 13, 6151–6157. [Google Scholar]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; de Carvalho, J.C.; Molina, D.; Soccol, C.R. Hydrogen Production by Dark Fermentation Using a New Low-Cost Culture Medium Composed of Corn Steep Liquor and Cassava Processing Water: Process Optimization and Scale-Up. Bioresour. Technol. 2021, 320, 124370. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Rodrigues, I.J.; Garcia, M.L.; Fuess, L.T.; Rodrigues, I.J.; Garcia, M.L. Fertirrigation with Sugarcane Vinasse: Foreseeing Potential Impacts on Soil and Water Resources through Vinasse Characterization. J. Environ. Sci. Health 2017, 52, 1063–1072. [Google Scholar] [CrossRef]
- de Godoi, L.A.G.; Camiloti, P.R.; Bernardes, A.N.; Sanchez, B.L.S.; Torres, A.P.R.; da Conceição Gomes, A.; Botta, L.S. Seasonal Variation of the Organic and Inorganic Composition of Sugarcane Vinasse: Main Implications for Its Environmental Uses. Environ. Sci. Pollut. Res. 2019, 26, 29267–29282. [Google Scholar] [CrossRef]
- Olaoye, R.A.; Afolayan, O.D.; Adeyemi, K.A.; Ajisope, L.O.; Adekunle, O.S. Adsorption of Selected Metals from Cassava Processing Wastewater Using Cow-Bone Ash. Sci. Afr. 2020, 10, e00653. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chong, C.C.; Lam, M.K.; Leong, W.H.; Chuah, L.F.; Yusup, S.; Setiabudi, H.D.; Tang, Y.; Lim, J.W. Identification of Microbial Inhibitions and Mitigation Strategies towards Cleaner Bioconversions of Palm Oil Mill Effluent (POME): A Review. J. Clean. Prod. 2021, 280, 124346. [Google Scholar] [CrossRef]
- Malik, S.; Shahid, A.; Betenbaugh, M.J.; Liu, C.; Mehmood, A. A Novel Wastewater-Derived Cascading Algal Biorefinery Route for Complete Valorization of the Biomass to Biodiesel and Value-Added Bioproducts. Energy Convers. Manag. 2022, 256, 115360. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Arisht, S.N.; Jahim, J.M.; Takriff, M.S.; Tan, J.P.; Luthfi, A.A.I.; Abdul, P.M. Enhancement of Biohydrogen Production from Palm Oil Mill Effluent (POME): A Review. Int. J. Hydrog. Energy 2021, 47, 40637–40655. [Google Scholar] [CrossRef]
- Loh, S.K.; Nasrin, A.B.; Mohamad Azri, S.; Nurul Adela, B.; Muzzammil, N.; Daryl Jay, T.; Stasha Eleanor, R.A.; Lim, W.S.; Choo, Y.M.; Kaltschmitt, M. First Report on Malaysia’s Experiences and Development in Biogas Capture and Utilization from Palm Oil Mill Effluent under the Economic Transformation Programme: Current and Future Perspectives. Renew. Sustain. Energy Rev. 2017, 74, 1257–1274. [Google Scholar] [CrossRef]
- Rosa, D.; Medeiros, A.B.P.; Martinez-Burgos, W.J.; Do Nascimento, J.R.; De Carvalho, J.C.; Sydney, E.B.; Soccol, C.R. Biological Hydrogen Production from Palm Oil Mill Effluent (POME) by Anaerobic Consortia and Clostridium beijerinckii. J. Biotechnol. 2020, 323, 17–23. [Google Scholar] [CrossRef] [PubMed]
- HO, O.; Eruteyan, O. A Study on the Effects of Cassava Processing Wastes on the Soil Environment of a Local Cassava Mill. J. Pollut. Eff. Control 2016, 4, 4–7. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; de Paula, D.R.; Medeiros, A.B.P.; de Carvalho, J.C.; Soccol, V.T.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Biohydrogen Production in Cassava Processing Wastewater Using Microbial Consortia: Process Optimization and Kinetic Analysis of the Microbial Community. Bioresour. Technol. 2020, 309, 123331. [Google Scholar] [CrossRef]
- Costa, R.C.; Ramos, M.D.N.; Fleck, L.; Gomes, S.D.; Aguiar, A. Critical Analysis and Predictive Models Using the Physicochemical Characteristics of Cassava Processing Wastewater Generated in Brazil. J. Water Process Eng. 2022, 47, 102629. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Hazzwan Bohari, M.; Sicilia, A.; Avignone-Rossa, C.; Bussemaker, M.; Saroj, D.; Lee, J. Tertiary Treatment of Real Abattoir Wastewater Using Combined Acoustic Cavitation and Ozonation. Ultrason. Sonochem. 2020, 64, 104986. [Google Scholar] [CrossRef]
- Oyeniran, D.O.; Sogbanmu, T.O.; Adesalu, T.A. Antibiotics, Algal Evaluations and Subacute Effects of Abattoir Wastewater on Liver Function Enzymes, Genetic and Haematologic Biomarkers in the Freshwater Fish, Clarias gariepinus. Ecotoxicol. Environ. Saf. 2021, 212, 111982. [Google Scholar] [CrossRef]
- Okey-Onyesolu, C.F.; Onukwuli, O.D.; Ejimofor, M.I.; Okoye, C.C. Kinetics and Mechanistic Analysis of Particles Decontamination from Abattoir Wastewater (ABW) Using Novel Fish Bone Chito-Protein (FBC). Heliyon 2020, 6, e04468. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Foster, L.; Kwambai, C.; Bahri, P.A.; Moheimani, N.R. Microalgae Cultivation for the Treatment of Anaerobically Digested Municipal Centrate (ADMC) and Anaerobically Digested Abattoir Effluent (ADAE). Sci. Total Environ. 2021, 775, 145853. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Guerreiro, R.C.S.; Jerónimo, E.; Luz, S.; Pinheiro, H.M.; Prazeres, A.R. Cheese Manufacturing Wastewater Treatment by Combined Physicochemical Processes for Reuse and Fertilizer Production. J. Environ. Manag. 2020, 264, 110470. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.D.C.S.D.; Maranduba, H.L.; Hafner, M.B.; Rodrigues, L.B.; de Almeida Neto, J.A. Life Cycle Thinking Applied to Phytoremediation of Dairy Wastewater Using Aquatic Macrophytes for Treatment and Biomass Production. J. Clean. Prod. 2020, 267, 122006. [Google Scholar] [CrossRef]
- Ekka, B.; Mieriņa, I.; Juhna, T.; Turks, M.; Kokina, K. Quantification of Different Fatty Acids in Raw Dairy Wastewater. Clean. Eng. Technol. 2022, 7, 100430. [Google Scholar] [CrossRef]
- Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola-Albores, F.; Lagunas-Rivera, S.; Cruz-Rodríguez, R.I.; Cruz-Salomón, K.D.C.; Hernández-Méndez, J.M.E.; Domínguez-Espinosa, M.E. Treatment of Cheese Whey Wastewater Using an Expanded Granular Sludge Bed (EGSB) Bioreactor with Biomethane Production. Processes 2020, 8, 931. [Google Scholar] [CrossRef]
- Andreotti, V.; Solimeno, A.; Rossi, S.; Ficara, E.; Marazzi, F.; Mezzanotte, V.; García, J. Bioremediation of Aquaculture Wastewater with the Microalgae Tetraselmis suecica: Semi-Continuous Experiments, Simulation and Photo-Respirometric Tests. Sci. Total Environ. 2020, 738, 139859. [Google Scholar] [CrossRef]
- Tejido-Nuñez, Y.; Aymerich, E.; Sancho, L.; Refardt, D. Treatment of Aquaculture Effluent with Chlorella vulgaris and Tetradesmus Obliquus: The Effect of Pretreatment on Microalgae Growth and Nutrient Removal Efficiency. Ecol. Eng. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Meril, D.; Piliyan, R.; Perumal, S.; Sundarraj, D.K.; Binesh, A. Efficacy of Alginate Immobilized Microalgae in the Bioremediation of Shrimp Aquaculture Wastewater. Process Biochem. 2022, 122, 196–202. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Treatment of Real Aquaculture Wastewater from a Fishery Utilizing Phytoremediation with Microalgae. J. Chem. Technol. Biotechnol. 2019, 94, 900–910. [Google Scholar] [CrossRef]
- Akmukhanova, N.R.; Sadvakasova, A.K.; Torekhanova, M.M.; Bauenova, M.O. Feasibility of Waste—Free Use of Microalgae in Aquaculture. J. Appl. Phycol. 2022, 34, 2297–2313. [Google Scholar] [CrossRef]
- Nunes, N.S.P.; de Almeida, J.M.O.; Fonseca, G.G.; de Carvalho, E.M. Clarification of Sugarcane (Saccharum Officinarum) Vinasse for Microalgae Cultivation. Bioresour. Technol. Rep. 2022, 19, 101125. [Google Scholar] [CrossRef]
- Cea Barcia, G.E.; Imperial Cervantes, R.A.; Torres Zuniga, I.; van den Hende, S. Converting Tequila Vinasse Diluted with Tequila Process Water into Microalgae-Yeast Flocs and Dischargeable Effluent. Bioresour. Technol. 2020, 300, 122644. [Google Scholar] [CrossRef]
- Megawati, M.; Damayanti, A.; Bahlawan, Z.A.S.; Nurunia, E.; Agustina, F.J.E.; Fidyawati, F.; Hanifah, H. Growth rate and biochemical characterization of Chlorella pyrenoidosa cultivated in sugarcane vinasse medium. ASEAN Eng. J. 2022, 12, 127–134. [Google Scholar] [CrossRef]
- Candido, C.; Lombardi, A.T. Mixotrophy in Green Microalgae Grown on an Organic and Nutrient Rich Waste. World J. Microbiol. Biotechnol. 2020, 36, 20. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.C.; Bonomi, A.; Filho, R.M. Integration of Microalgae Production with Industrial Biofuel Facilities: A Critical Review. Renew. Sustain. Energy Rev. 2018, 82, 1376–1392. [Google Scholar] [CrossRef]
- Santana, H.; Cereijo, C.R.; Teles, V.C.; Nascimento, R.C.; Fernandes, M.S.; Brunale, P.; Campanha, R.C.; Soares, I.P.; Silva, F.C.P.; Sabaini, P.S.; et al. Microalgae Cultivation in Sugarcane Vinasse: Selection, Growth and Biochemical Characterization. Bioresour. Technol. 2017, 228, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Candido, C.; Bernardo, A.; Lombardi, A.T. Optimization and Qualitative Comparison of Two Vinasse Pre-Treatments Aiming at Microalgae Cultivation. Eng. Sanit. Ambient. 2021, 26, 359–367. [Google Scholar] [CrossRef]
- Siqueira, J.C.; Braga, M.Q.; Ázara, M.S.; Garcia, K.J.; Alencar, S.N.M.; Ramos, T.S.; Siniscalchi, L.A.B.; Assemany, P.P.; Ensinas, A.V. Recovery of Vinasse with Combined Microalgae Cultivation in a Conceptual Energy-Efficient Industrial Plant: Analysis of Related Process Considerations. Renew. Sustain. Energy Rev. 2022, 155, 111904. [Google Scholar] [CrossRef]
- Fu, S.F.; Xu, X.H.; Dai, M.; Yuan, X.Z.; Guo, R.B. Hydrogen and Methane Production from Vinasse Using Two-Stage Anaerobic Digestion. Process Saf. Environ. Prot. 2017, 107, 81–86. [Google Scholar] [CrossRef]
- Marques, S.S.I.; Nascimento, I.A.; de Almeida, P.F.; Chinalia, F.A. Growth of Chlorella vulgaris on Sugarcane Vinasse: The Effect of Anaerobic Digestion Pretreatment. Appl. Biochem. Biotechnol. 2013, 171, 1933–1943. [Google Scholar] [CrossRef]
- Trevisan, E.; Godoy, R.F.B.; Radomski, F.A.D.; Crisigiovanni, E.L.; Branco, K.B.Z.F.; Arroyo, P.A. Chlorella vulgaris Growth in Different Biodigested Vinasse Concentrations: Biomass, Pigments and Final Composition. Water Sci. Technol. 2020, 82, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef]
- Perera, I.A.; Abinandan, S.; Panneerselvan, L.; Subashchandrabose, S.R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Co-Culturing of Microalgae and Bacteria in Real Wastewaters Alters Indigenous Bacterial Communities Enhancing Effluent Bioremediation. Algal Res. 2022, 64, 102705. [Google Scholar] [CrossRef]
- Sydney, E.B.; Neto, C.J.D.; de Carvalho, J.C.; Vandenberghe, L.P.D.S.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Soccol, V.T.; Woiciechowski, A.L.; Medeiros, A.B.P.; et al. Microalgal Biorefineries: Integrated Use of Liquid and Gaseous Effluents from Bioethanol Industry for Efficient Biomass Production. Bioresour. Technol. 2019, 292, 121955. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Long Term Outdoor Microalgal Phycoremediation of Anaerobically Digested Abattoir Effluent. J. Environ. Manag. 2022, 323, 116322. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A.; Wan Maznah, W.O.; Morad, N.; Lalung, J.; Ismail, N.; Talebi, A.; Oyekanmi, A.A. Advances in POME Treatment Methods: Potentials of Phycoremediation, with a Focus on South East Asia. Int. J. Environ. Sci. Technol. 2022, 19, 8113–8130. [Google Scholar] [CrossRef]
- Khalid, A.A.H.; Yaakob, Z.; Abdullah, S.R.S.; Takriff, M.S. Assessing the Feasibility of Microalgae Cultivation in Agricultural Wastewater: The Nutrient Characteristics. Environ. Technol. Innov. 2019, 15, 100402. [Google Scholar] [CrossRef]
- Jasni, J.; Arisht, S.N.; Mohd Yasin, N.H.; Abdul, P.M.; Lin, S.K.; Liu, C.M.; Wu, S.Y.; Jahim, J.M.; Takriff, M.S. Comparative Toxicity Effect of Organic and Inorganic Substances in Palm Oil Mill Effluent (POME) Using Native Microalgae Species. J. Water Process Eng. 2020, 34, 101165. [Google Scholar] [CrossRef]
- Mohd Udaiyappan, A.F.; Hasan, H.A.; Takriff, M.S.; Abdullah, S.R.S.; Maeda, T.; Mustapha, N.A.; Mohd Yasin, N.H.; Nazashida Mohd Hakimi, N.I. Microalgae-Bacteria Interaction in Palm Oil Mill Effluent Treatment. J. Water Process Eng. 2020, 35, 101203. [Google Scholar] [CrossRef]
- Takriff, M.S.; Zakaria, M.Z.; Sajab, M.S.; Teow, Y.H. Pre-Treatments Anaerobic Palm Oil Mill Effluent (POME) for Microalgae Treatment. Indian J. Sci. Technol. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Low, S.S.; Bong, K.X.; Mubashir, M.; Cheng, C.K.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Munawaroh, H.S.H.; Show, P.L. Microalgae Cultivation in Palm Oil Mill Effluent (Pome) Treatment and Biofuel Production. Sustainability 2021, 13, 3247. [Google Scholar] [CrossRef]
- Phang, Y.K.; Tey, L.H.; Aminuzzaman, M.; Akhtaruzzaman, M.; Watanabe, A. Investigation of the Growth of Chlorella vulgaris and Chlamydomonas Reinhardtii Cultivated in Pre-Treated Palm Oil Mill Effluent (POME) as the Culture Medium. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 945. [Google Scholar]
- Minturo, G.J.; Noorain, R.; Hitam, S.M.S.; Shoiful, A.; Azni, M.E.; Ernawati, L.; Abdullah, R.; Mohamad, R. Immobilized Nannochloropsis Oculata in a Down-Flow Hanging Sponge (DHS) Reactor for the Treatment of Palm Oil Mill Effluent (POME). In IOP Conference Series: Earth and Environmental Science; Institute of Physics: London, UK, 2022; Volume 1017. [Google Scholar]
- Tan, K.A.; Lalung, J.; Morad, N.; Ismail, N.; Omar, W.M.W.; Khan, M.A.; Sillanpää, M.; Rafatullah, M. Post-Treatment of Palm Oil Mill Effluent Using Immobilised Green Microalgae Chlorococcum Oleofaciens. Sustainability 2021, 13, 1562. [Google Scholar] [CrossRef]
- Colusse, G.A.; Santos, A.O.; Rodrigues, J.M.; Barga, M.C.; Duarte, M.E.R.; de Carvalho, J.C.; Noseda, M.D. Rice Vinasse Treatment by Immobilized Synechococcus Pevalekii and Its Effect on Dunaliella Salina Cultivation. Bioprocess Biosyst. Eng. 2021, 44, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti Pessôa, L.; Pinheiro Cruz, E.; Mosquera Deamici, K.; Bomfim Andrade, B.; Santana Carvalho, N.; Rocha Vieira, S.; Alves Da Silva, J.B.; Magalhães Pontes, L.A.; Oliveira De Souza, C.; Druzian, J.I.; et al. A Review of Microalgae-Based Biorefineries Approach for Produced Water Treatment: Barriers, Pretreatments, Supplementation, and Perspectives. J. Environ. Chem. Eng. 2022, 10, 108096. [Google Scholar] [CrossRef]
- Okpozu, O.O.; Ogbonna, I.O.; Ikwebe, J.; Ogbonna, J.C. Phycoremediation of Cassava Wastewater by Desmodesmus Armatus and the Concomitant Accumulation of Lipids for Biodiesel Production. Bioresour. Technol. Rep. 2019, 7, 100255. [Google Scholar] [CrossRef]
- Melo, J.M.; Telles, T.S.; Ribeiro, M.R.; de Carvalho Junior, O.; Andrade, D.S. Chlorella sorokiniana as Bioremediator of Wastewater: Nutrient Removal, Biomass Production, and Potential Profit. Bioresour. Technol. Rep. 2022, 17, 100933. [Google Scholar] [CrossRef]
- Coutinho Rodrigues, O.H.; Itokazu, A.G.; Rörig, L.; Maraschin, M.; Corrêa, R.G.; Pimentel-Almeida, W.; Moresco, R. Evaluation of Astaxanthin Biosynthesis by Haematococcus Pluvialis Grown in Culture Medium Added of Cassava Wastewater. Int. Biodeterior. Biodegrad. 2021, 163, 105269. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Garcia Bustos, K.A.; Sanchez Vera, F.P.; Colina Andrade, G.J.; Pacheco Tanaka, D.A. Acid Precipitation Followed by Microalgae (Chlorella vulgaris) Cultivation as a New Approach for Poultry Slaughterhouse Wastewater Treatment. Bioresour. Technol. 2021, 335, 125284. [Google Scholar] [CrossRef]
- Katırcıoğlu Sınmaz, G.; Erden, B.; Şengil, I.A. Cultivation of Chlorella vulgaris in Alkaline Condition for Biodiesel Feedstock after Biological Treatment of Poultry Slaughterhouse Wastewater. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Evaluation of Microalgae as Bioremediation Agent for Poultry Effluent and Biostimulant for Germination. Environ. Technol. Innov. 2021, 24, 102048. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Matos, A.P.; Chaudry, S.; Bahri, P.A.; Moheimani, N.R. Effect of CO2 addition on Treating Anaerobically Digested Abattoir Effluent (ADAE) Using Chlorella sp. (Trebouxiophyceae). J. CO2 Util. 2020, 38, 273–281. [Google Scholar] [CrossRef]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Can CO2 Addition Improve the Tertiary Treatment of Anaerobically Digested Abattoir Effluent (ADAE) by Scenedesmus sp. (Chlorophyta)? Algal Res. 2021, 58, 102379. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; González-Martínez, A.; Rodelas, B.; González-López, J. Full-Scale Photobioreactor for Biotreatment of Olive Washing Water: Structure and Diversity of the Microalgae-Bacteria Consortium. Bioresour. Technol. 2017, 238, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater Based Microalgal Biorefinery for Bioenergy Production: Progress and Challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, N.; Zamani, H. Potential of Chlorella sorokiniana Cultivated in Dairy Wastewater for Bioenergy and Biodiesel Production. Bioenergy Res. 2022, 15, 334–345. [Google Scholar] [CrossRef]
- Rahman, D.Y.; Hidhayati, N.; Apriastini, M. Taufikurahman Utilization of Anaerobically Digested Dairy Manure Wastewater for Spirulina maxima Cultivation. IOP Conf. Ser. Earth Environ. Sci. 2022, 1038, 012022. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Isolation, Screening and Comprehensive Characterization of Candidate Microalgae for Biofuel Feedstock Production and Dairy Effluent Treatment: A Sustainable Approach. Bioresour. Technol. 2019, 293, 121998. [Google Scholar] [CrossRef]
- Ghobrini, D.; Brányik, T.; Yakoub-Bougdal, S.; Aïboud, K.; Kebbab, L.; Daoud, D.; Lahouel, N.; Bouarab, R.; Oumsalem, M.; Zanoun, L. Production of Biodiesel from the Locally Isolated Yellow Strain of Chlorella sp. Using Dairy Wastewater as a Growth Medium. AIP Conf. Proc. 2019, 2190, 020097. [Google Scholar] [CrossRef]
- Zapata, D.; Arroyave, C.; Cardona, L.; Aristizábal, A.; Poschenrieder, C.; Llugany, M. Phytohormone Production and Morphology of Spirulina platensis Grown in Dairy Wastewaters. Algal Res. 2021, 59, 102469. [Google Scholar] [CrossRef]
- Choi, Y.K.; Jang, H.M.; Kan, E. Microalgal Biomass and Lipid Production on Dairy Effluent Using a Novel Microalga, Chlorella sp. Isolated from Dairy Wastewater. Biotechnol. Bioprocess Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Pang, N.; Bergeron, A.D.; Gu, X.; Fu, X.; Dong, T.; Yao, Y.; Chen, S. Recycling of Nutrients from Dairy Wastewater by Extremophilic Microalgae with High Ammonia Tolerance. Environ. Sci. Technol. 2020, 54, 15366–15375. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, A.; Zkeri, E.; Panara, A.; Dasenaki, M.; Fountoulakis, M.S.; Thomaidis, N.S.; Stasinakis, A.S. Treatment of Different Dairy Wastewater with Chlorella sorokiniana: Removal of Pollutants and Biomass Characterization. J. Chem. Technol. Biotechnol. 2022, 97, 3193–3201. [Google Scholar] [CrossRef]
- Marella, T.K.; López-Pacheco, I.Y.; Parra-Saldívar, R.; Dixit, S.; Tiwari, A. Wealth from Waste: Diatoms as Tools for Phycoremediation of Wastewater and for Obtaining Value from the Biomass. Sci. Total Environ. 2020, 724, 137960. [Google Scholar] [CrossRef] [PubMed]
- Hesni, M.A.; Hedayati, A.; Qadermarzi, A.; Pouladi, M.; Zangiabadi, S.; Naqshbandi, N. Using Chlorella vulgaris and Iron Oxide Nanoparticles in a Designed Bioreactor for Aquaculture Effluents Purification. Aquac. Eng. 2020, 90, 102069. [Google Scholar] [CrossRef]
- Soto, M.F.; Diaz, C.A.; Zapata, A.M.; Higuita, J.C. BOD and COD Removal in Vinasses from Sugarcane Alcoholic Distillation by Chlorella vulgaris: Environmental Evaluation. Biochem. Eng. J. 2021, 176, 108191. [Google Scholar] [CrossRef]
- Heredia Falconí, J.H.; Soares, J.; Rocha, D.N.; Gomes Marçal Vieira Vaz, M.; Martins, M.A. Strain Screening and Ozone Pretreatment for Algae Farming in Wastewaters from Sugarcane Ethanol Biorefinery. J. Clean. Prod. 2021, 282, 124522. [Google Scholar] [CrossRef]
- Montaño Saavedra, M.D.; Paschino Bissoto, F.; de Souza, R.A.; Cárdenas Concha, V.O.; Gaspar Bastos, R. Growth of Desmodesmus subspicatus Green Microalgae and Nutrient Removal from Sugarcane Vinasse Clarified by Electrocoagulation Using Aluminum or Iron Electrodes. Dyna 2019, 86, 225–232. [Google Scholar] [CrossRef]
- Montalvo, G.E.B.; Thomaz-Soccol, V.; Vandenberghe, L.P.S.; Carvalho, J.C.; Faulds, C.B.; Bertrand, E.; Prado, M.R.M.; Bonatto, S.J.R.; Soccol, C.R. Arthrospira Maxima OF15 Biomass Cultivation at Laboratory and Pilot Scale from Sugarcane Vinasse for Potential Biological New Peptides Production. Bioresour. Technol. 2019, 273, 103–113. [Google Scholar] [CrossRef]
- de Mattos, L.F.A.; Bastos, R.G. COD and Nitrogen Removal from Sugarcane Vinasse by Heterotrophic Green Algae Desmodesmus sp. Desalination Water Treat. 2016, 57, 9465–9473. [Google Scholar] [CrossRef]
- Serejo, M.L.; Ruas, G.; Braga, G.B.; Paulo, P.L.; Boncz, M. Chlorella vulgaris Growth on Anaerobically Digested Sugarcane Vinasse: Influenceofturbidity. An. Acad. Bras. Cienc. 2021, 93, 1–10. [Google Scholar] [CrossRef]
- Hilares, R.T.; Vera, F.P.S.; Andrade, G.J.C.; Meza, K.T.; García, J.C.; Tanaka, D.A.P. Continuous Cultivation of Microalgae in Cattle Slaughterhouse Wastewater Treated with Hydrodynamic Cavitation. Water 2022, 14, 1288. [Google Scholar] [CrossRef]
- Chawla, P.; Gola, D.; Dalvi, V.; Sreekrishnan, T.R.; Ariyadasa, T.U.; Malik, A. Design and Development of Mini-Photobioreactor System for Strategic High Throughput Selection of Optimum Microalgae-Wastewater Combination. Bioresour. Technol. Rep. 2022, 17, 100967. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Yap, Y.J.; Mohd Zaid, H.F.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Tao, Y. Enhancing Microalga Chlorella sorokiniana CY-1 Biomass and Lipid Production in Palm Oil Mill Effluent (POME) Using Novel-Designed Photobioreactor. Bioengineered 2020, 11, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Elystia, S.; Muria, S.R.; Erlangga, H.F. Cultivation of Chlorella pyrenoidosa as a Raw Material for the Production of Biofuels in Palm Oil Mill Effluent Medium with the Addition of Urea and Triple Super Phosphate. Environ. Health Eng. Manag. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Garcia, G.M.; Boelen, P.; Buma, A.G.J. Influence of Photodegradation on the Removal of Color and Phenolic Compounds from Palm Oil Mill Effluent by Arthrospira platensis. J. Appl. Phycol. 2021, 33, 901–915. [Google Scholar] [CrossRef]
- Resdi, R.; Lim, J.S.; Idris, A. Batch Kinetics of Nutrients Removal for Palm Oil Mill Effluent and Recovery of Lipid by Nannochloropsis sp. J. Water Process Eng. 2021, 40, 101767. [Google Scholar] [CrossRef]
- Ng, F.L.; Phang, S.M.; Thong, C.H.; Periasamy, V.; Pindah, J.; Yunus, K.; Fisher, A.C. Integration of Bioelectricity Generation from Algal Biophotovoltaic (BPV) Devices with Remediation of Palm Oil Mill Effluent (POME) as Substrate for Algal Growth. Environ. Technol. Innov. 2021, 21, 101280. [Google Scholar] [CrossRef]
- Elvitriana, E.; Munir, E.; Delvian, D.; Wahyuningsih, H. Organic Substances Reduction in Palm Oil Mill Effluent (POME) After Cultivation with Locally Isolated Microalgae. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 80, 98–105. [Google Scholar] [CrossRef]
- Ogbonna, I.O.; Okpozu, O.O.; Ikwebe, J.; Ogbonna, J.C. Utilisation of Desmodesmus subspicatus LC172266 for Simultaneous Remediation of Cassava Wastewater and Accumulation of Lipids for Biodiesel Production. Biofuels 2019, 10, 657–664. [Google Scholar] [CrossRef]
- Romaidi; Hasanudin, M.; Kholifah, K.; Maulidiyah, A.; Putro, S.P.; Kikuchi, A.; Sakaguchi, T. Lipid Production from Tapioca Wastewater by Culture of Scenedesmus sp. with Simultaneous BOD, COD and Nitrogen Removal. J. Phys. Conf. Ser. 2018, 1025, 012075. [Google Scholar] [CrossRef]
- Padri, M.; Boontian, N.; Teaumroong, N.; Piromyou, P.; Piasai, C. Co-Culture of Microalga Chlorella sorokiniana with Syntrophic Streptomyces Thermocarboxydus in Cassava Wastewater for Wastewater Treatment and Biodiesel Production. Bioresour. Technol. 2022, 347, 126732. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.G.; Duarte, J.H.; Costa, J.A.V.; de Jesus Assis, D.; Lemos, P.V.F.; Druzian, J.I.; de Souza, C.O.; Nunes, I.L.; Chinalia, F.A. Spirulina sp. as a Bioremediation Agent for Aquaculture Wastewater: Production of High Added Value Compounds and Estimation of Theoretical Biodiesel. Bioenergy Res. 2021, 14, 254–264. [Google Scholar] [CrossRef]
- Guldhe, A.; Ansari, F.A.; Singh, P.; Bux, F. Heterotrophic Cultivation of Microalgae Using Aquaculture Wastewater: A Biorefinery Concept for Biomass Production and Nutrient Remediation. Ecol. Eng. 2017, 99, 47–53. [Google Scholar] [CrossRef]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal Cultivation Using Aquaculture Wastewater: Integrated Biomass Generation and Nutrient Remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Kothari, R.; Prasad, R.; Kumar, V.; Singh, D.P. Production of Biodiesel from Microalgae Chlamydomonas polypyrenoideum Grown on Dairy Industry Wastewater. Bioresour. Technol. 2013, 144, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, N.; Zamani, H. Biomass Production and Nutritional Properties of Chlorella sorokiniana Grown on Dairy Wastewater. J. Water Process Eng. 2022, 47, 102760. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S.; Chand, N.; Mutiyar, P.K. Phycoremediation of Milk Processing Wastewater and Lipid-Rich Biomass Production Using Chlorella vulgaris under Continuous Batch System. Sci. Total Environ. 2022, 833, 155110. [Google Scholar] [CrossRef]
- Gumbi, S.T.; Mutanda, T.; Olaniran, A.O. Nutrient Removal from Dairy and Poultry Wastewater with Simultaneous Biomass and Biodiesel Production by Chlorella sp. T4 Isolated from a Freshwater Stream in South Africa. Waste Biomass Valorization 2021, 12, 6931–6943. [Google Scholar] [CrossRef]
- Sudhanthiran, M.C.; Perumalsamy, M. Bioremediation of Dairy Industry Wastewater and Assessment of Nutrient Removal Potential of Chlorella vulgaris. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Campos, J.L.; Crutchik, D.; Franchi, Ó.; Pavissich, J.P.; Belmonte, M.; Pedrouso, A.; Mosquera-Corral, A.; Val del Río, Á. Nitrogen and Phosphorus Recovery From Anaerobically Pretreated Agro-Food Wastes: A Review. Front. Sustain. Food Syst. 2019, 2, 91. [Google Scholar] [CrossRef]
- Nunes Ferraz Junior, A.D.; Etchebehere, C.; Perecin, D.; Teixeira, S.; Woods, J. Advancing Anaerobic Digestion of Sugarcane Vinasse: Current Development, Struggles and Future Trends on Production and End-Uses of Biogas in Brazil. Renew. Sustain. Energy Rev. 2022, 157, 112045. [Google Scholar] [CrossRef]
- Chen, K.T.; Bai, M.D.; Yang, H.Y.; Chen, Y.C.; Lu, W.J.; Huang, C. Removal of Ammonia from Leachate by Using Thermophilic Microbial Fuel Cells Equipped with Membrane Electrode. Sustain. Environ. Res. 2020, 30, 5. [Google Scholar] [CrossRef]
- Li, X.; Shen, S.; Xu, Y.; Guo, T.; Dai, H.; Lu, X. Application of Membrane Separation Processes in Phosphorus Recovery: A Review. Sci. Total Environ. 2021, 767, 144346. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Srivastava, S.; Kumar, S. Development and Cost-Benefit Analysis of a Novel Process for Biofuel Production from Microalgae Using Pre-Treated High-Strength Fresh Cheese Whey Wastewater. Environ. Sci. Pollut. Res. 2020, 27, 23963–23980. [Google Scholar] [CrossRef]
- Debowski, M.; Zielinski, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Swica, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K. Attempts to Alleviate Inhibitory Factors of Anaerobic Digestate for Enhanced Microalgae Cultivation and Nutrients Removal: A Review. J. Environ. Manag. 2022, 304, 114266. [Google Scholar] [CrossRef]
- Yasir, S.; Siddiki, A.; Mofijur, M.; Kumar, P.S.; Forruque, S.; Chyuan, H.; Mahlia, T.M.I. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar]
- Chen, Y.D.; Ho, S.H.; Nagarajan, D.; Ren, N.Q.; Chang, J.S. Waste Biorefineries—Integrating Anaerobic Digestion and Microalgae Cultivation for Bioenergy Production. Curr. Opin. Biotechnol. 2018, 50, 101–110. [Google Scholar] [CrossRef]
- Álvarez, X.; Arévalo, O.; Salvador, M.; Mercado, I.; Velázquez-Martí, B. Cyanobacterial Biomass Produced in the Wastewater of the Dairy Industry and Its Evaluation in Anaerobic Co-Digestion with Cattle Manure for Enhanced Methane Production. Processes 2020, 8, 1290. [Google Scholar] [CrossRef]
- Zafisah, N.S.; Ang, W.L.; Mohammad, A.W. Cake Filtration for Suspended Solids Removal in Digestate from Anaerobic Digested Palm Oil Mill Effluent (POME). Water Conserv. Manag. 2018, 2, 5–9. [Google Scholar] [CrossRef]
- Poh, P.E.; Chong, M.F. Development of Anaerobic Digestion Methods for Palm Oil Mill Effluent (POME) Treatment. Bioresour. Technol. 2009, 100, 1–9. [Google Scholar] [CrossRef]
- Vieira, S.; Schneider, J.; Martinez-Burgos, W.J.; Magalhães, A.; Medeiros, A.B.P.; Carvalho, J.C.; Vandenberghe, L.P.D.S.; Soccol, C.R.; Soccol, C.R. Pretreatments of Solid Wastes for Anaerobic Digestion and Its Importance for the Circular Economy. In Handbook of Solid Waste Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–27. ISBN 9789811575259. [Google Scholar]
- Letti, L.A.; Woiciechowski, A.L.; Medeiros, A.B.P.; Rodrigues, C.; Carvalho, J.C.D.; Vandenberghe, L.P.; Karp, S.G.; Torres, L.A.Z.; Guarnizo, A.F.C.; Soccol, C.R. Valorization of Solid and Liquid Wastes from Palm Oil Industry. In Waste Biorefinery Value Addition Through Resource Utilization; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–265. ISBN 9780128218792. [Google Scholar]
- Silva, A.F.R.; Brasil, Y.L.; Koch, K.; Amaral, M.C.S. Resource Recovery from Sugarcane Vinasse by Anaerobic Digestion—A Review. J. Environ. Manag. 2021, 295, 113137. [Google Scholar] [CrossRef]
- Charalambous, P.; Shin, J.; Shin, S.G.; Vyrides, I. Anaerobic Digestion of Industrial Dairy Wastewater and Cheese Whey: Performance of Internal Circulation Bioreactor and Laboratory Batch Test at PH 5–6. Renew. Energy 2020, 147, 1–10. [Google Scholar] [CrossRef]
- Sousa, S.P.; Lovato, G.; Albanez, R.; Ratusznei, S.M.; Rodrigues, J.A.D. Improvement of Sugarcane Stillage (Vinasse) Anaerobic Digestion with Cheese Whey as Its Co-Substrate: Achieving High Methane Productivity and Yield. Appl. Biochem. Biotechnol. 2019, 189, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Weiser Meier, T.R.; Cremonez, P.A.; Maniglia, T.C.; Sampaio, S.C.; Teleken, J.G.; da Silva, E.A. Production of Biohydrogen by an Anaerobic Digestion Process Using the Residual Glycerol from Biodiesel Production as Additive to Cassava Wastewater. J. Clean. Prod. 2020, 258, 120833. [Google Scholar] [CrossRef]
- Agabo-García, C.; Solera, R.; Perez, M. Anaerobic Sequential Batch Reactor for CO-DIGESTION of Slaughterhouse Residues: Wastewater and Activated Sludge. Energy 2022, 255, 124575. [Google Scholar] [CrossRef]
- Brooms, T.; Apollo, S.; Otieno, B.; Onyango, M.S.; Kabuba, J.; Ochieng, A. Integrated Anaerobic Digestion and Photodegradation of Slaughterhouse Wastewater: Energy Analysis and Degradation of Aromatic Compounds. J. Mater. Cycles Waste Manag. 2020, 22, 1227–1236. [Google Scholar] [CrossRef]
- Venugopal, V.; Sasidharan, A. Seafood Industry Effluents: Environmental Hazards, Treatment and Resource Recovery. J. Environ. Chem. Eng. 2021, 9, 104758. [Google Scholar] [CrossRef]
- Choudhury, A.; Lepine, C.; Witarsa, F.; Good, C. Anaerobic Digestion Challenges and Resource Recovery Opportunities from Land-Based Aquaculture Waste and Seafood Processing Byproducts: A Review. Bioresour. Technol. 2022, 354, 127144. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.P.L.; Joyce, A.; Wuertz, S.; Jijakli, M.H.; Gross, A.; Eding, E.H.; Bläser, I.; Reuter, M.; Keizer, L.C.P.; et al. Nutrient Mineralization and Organic Matter Reduction Performance of RAS-Based Sludge in Sequential UASB-EGSB Reactors. Aquac. Eng. 2018, 83, 10–19. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Bento, H.B.S.; Alves, T.M.; Carvalho, A.; Castro, H. Vinasse Treatment within the Sugarcane-Ethanol Industry Using Ozone Combined with Anaerobic and Aerobic Microbial Processes. Environments 2019, 6, 5. [Google Scholar] [CrossRef]
- Moraes, B.S.; Petersen, S.O.; Zaiat, M.; Sommer, S.G.; Triolo, J.M. Reduction in Greenhouse Gas Emissions from Vinasse through Anaerobic Digestion. Appl. Energy 2020, 189, 21–30. [Google Scholar] [CrossRef]
- Zafisah, N.S.; Ang, W.L.; Johnson, D.J.; Mohammad, A.W.; Hilal, N. Effect of Different Filter Aids Used in Cake Filtration Process on the Removal of Suspended Solids in Anaerobically Digested Palm Oil Mill Effluent (POME). Desalination Water Treat. 2018, 110, 362–370. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; Sanglier, M.; de Medeiros Dantas, J.M.; Lavoie, J.-M. CO2 Capture and Inorganic Carbon Assimilation of Gaseous Fermentation Effluents Using Parachlorella kessleri Microalgae. J. CO2 Util. 2021, 50, 101581. [Google Scholar] [CrossRef]
- Iamtham, S.; Sornchai, P. Biofixation of CO2 from a Power Plant through Large-Scale Cultivation of Spirulina maxima. S. Afr. J. Bot. 2022, 147, 840–851. [Google Scholar] [CrossRef]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.A.; Schenk, P.M. Selection and Adaptation of Microalgae to Growth in 100% Unfiltered Coal-Fired Flue Gas. Bioresour. Technol. 2017, 233, 271–283. [Google Scholar] [CrossRef]
- Kim, B.; Praveenkumar, R.; Choi, E.; Lee, K.; Jeon, S.; Oh, Y.-K. Prospecting for Oleaginous and Robust Chlorella Spp. for Coal-Fired Flue-Gas-Mediated Biodiesel Production. Energies 2018, 11, 2026. [Google Scholar] [CrossRef]
- Moheimani, N.R. Tetraselmis suecica Culture for CO2 Bioremediation of Untreated Flue Gas from a Coal-Fired Power Station. J. Appl. Phycol. 2016, 28, 2139–2146. [Google Scholar] [CrossRef]
- Praveenkumar, R.; Kim, B.; Choi, E.; Lee, K.; Park, J.-Y.; Lee, J.-S.; Lee, Y.-C.; Oh, Y.-K. Improved Biomass and Lipid Production in a Mixotrophic Culture of Chlorella sp. KR-1 with Addition of Coal-Fired Flue-Gas. Bioresour. Technol. 2014, 171, 500–505. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Xie, T.; Seib, M.; Tale, V.P.; Zitomer, D. Anaerobic Digester Biogas Upgrading Using Microalgae. In Integrated Wastewater Management and Valorization Using Algal Cultures; Elsevier: Amsterdam, The Netherlands, 2022; pp. 183–214. [Google Scholar]
- Yadav, G.; Mathimani, T.; Sekar, M.; Sindhu, R.; Pugazhendhi, A. Strategic Evaluation of Limiting Factors Affecting Algal Growth—An Approach to Waste Mitigation and Carbon Dioxide Sequestration. Sci. Total Environ. 2021, 796, 149049. [Google Scholar] [CrossRef]
- de Godos, I.; Mendoza, J.L.; Acién, F.G.; Molina, E.; Banks, C.J.; Heaven, S.; Rogalla, F. Evaluation of Carbon Dioxide Mass Transfer in Raceway Reactors for Microalgae Culture Using Flue Gases. Bioresour. Technol. 2014, 153, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, D.; Yu, Y.; Li, D.; Yadav, R.S.; Feng, Y. Application of a Microalga, Scenedesmus Obliquus PF3, for the Biological Removal of Nitric Oxide (NO) and Carbon Dioxide. Environ. Pollut. 2019, 252, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, P.; Desi, S.; Vurimindi, H. Mixotrophic Cultivation of Microalgae Using Industrial Flue Gases for Biodiesel Production. Environ. Sci. Pollut. Res. 2016, 23, 9345–9354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.K.; Krishna, S.V.; Verma, K.; Pooja, K.; Bhagawan, D.; Himabindu, V. Phycoremediation of Sewage Wastewater and Industrial Flue Gases for Biomass Generation from Microalgae. S. Afr. J. Chem. Eng. 2018, 25, 133–146. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, Z.; Zhou, J.; Cen, K. Improving the CO2 Fixation Rate by Increasing Flow Rate of the Flue Gas from Microalgae in a Raceway Pond. Korean J. Chem. Eng. 2018, 35, 498–502. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Carbohydrate Biosynthesis in Plants and Bacteria. In Principles of Biochemistry; Lehninger, A.L., Nelson, D.L., Cox, M.M., Eds.; McGraw-Hill Book: New York, NY, USA, 2004; pp. 751–786. [Google Scholar]
- Kuo, C.-M.; Jian, J.-F.; Lin, T.-H.; Chang, Y.-B.; Wan, X.-H.; Lai, J.-T.; Chang, J.-S.; Lin, C.-S. Simultaneous Microalgal Biomass Production and CO2 Fixation by Cultivating Chlorella sp. GD with Aquaculture Wastewater and Boiler Flue Gas. Bioresour. Technol. 2016, 221, 241–250. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Sahoo, L.; Mohanty, K. Maximize Microalgal Carbon Dioxide Utilization and Lipid Productivity by Using Toxic Flue Gas Compounds as Nutrient Source. Bioresour. Technol. 2022, 348, 126784. [Google Scholar] [CrossRef]
- Duarte, J.H.; de Morais, E.G.; Radmann, E.M.; Costa, J.A.V. Biological CO2 Mitigation from Coal Power Plant by Chlorella fusca and Spirulina sp. Bioresour. Technol. 2017, 234, 472–475. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Chen, T.-Y.; Chang, Y.-B.; Chiu, T.-W.; Lin, H.-Y.; Chen, C.-D.; Chang, J.-S.; Lin, C.-S. Utilization of Carbon Dioxide in Industrial Flue Gases for the Cultivation of Microalga chlorella sp. Bioresour. Technol. 2014, 166, 485–493. [Google Scholar] [CrossRef]
- Yadav, G.; Dash, S.K.; Sen, R. A Biorefinery for Valorization of Industrial Waste-Water and Flue Gas by Microalgae for Waste Mitigation, Carbon-Dioxide Sequestration and Algal Biomass Production. Sci. Total Environ. 2019, 688, 129–135. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Mohd Yasin, N.H.; Ba-Abbad, M.M.; Mohd Hakimi, N.I.N. Potential of the Microalgae-Based Integrated Wastewater Treatment and CO2 Fixation System to Treat Palm Oil Mill Effluent (POME) by Indigenous Microalgae; Scenedesmus sp. and Chlorella sp. J. Water Process Eng. 2019, 32, 100907. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-H.; Zhang, J.-T.; Chi, Z.-Y.; Kong, F.-T.; Zhang, Q. The Long Overlooked Microalgal Nitrous Oxide Emission: Characteristics, Mechanisms, and Influencing Factors in Microalgae-Based Wastewater Treatment Scenarios. Sci. Total Environ. 2023, 856, 159153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, J.; Zhang, X.; Chen, L.; Liu, J. Metabolic Pathways of Chlorella sp. Cells Induced by Exogenous Spermidine against Nitric Oxide Damage from Coal-Fired Flue Gas. Bioresour. Technol. 2021, 328, 124827. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Banerjee, D.; Das, D. Carbon Dioxide Sequestration from Industrial Flue Gas by Chlorella sorokiniana. Bioresour. Technol. 2014, 152, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cheng, J.; Miao, Y.; Guo, W.; Zhou, J. SO2 Impurity in Simulated Flue Gas with 15% CO2 Affects Dynamic Bubble Dissolution and Arthrospira Photosynthetic Growth. ACS Sustain. Chem. Eng. 2021, 9, 5580–5589. [Google Scholar] [CrossRef]
- Matito-Martos, I.; Sepúlveda, C.; Gómez, C.; Acién, G.; Perez-Carbajo, J.; Delgado, J.A.; Águeda, V.I.; Ania, C.; Parra, J.B.; Calero, S.; et al. Potential of CO2 Capture from Flue Gases by Physicochemical and Biological Methods: A Comparative Study. Chem. Eng. J. 2021, 417, 128020. [Google Scholar] [CrossRef]
- Hong, M.E.; Chang, W.S.; Patel, A.K.; Oh, M.S.; Lee, J.J.; Sim, S.J. Microalgal-Based Carbon Sequestration by Converting LNG-Fired Waste CO2 into Red Gold Astaxanthin: The Potential Applicability. Energies 2019, 12, 1718. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ravindran, B.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Techno-Economic Estimation of Wastewater Phycoremediation and Environmental Benefits Using Scenedesmus Obliquus Microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef]
- Yadav, G.; Panda, S.P.; Sen, R. Strategies for the Effective Solid, Liquid and Gaseous Waste Valorization by Microalgae: A Circular Bioeconomy Perspective. J. Environ. Chem. Eng. 2020, 8, 104518. [Google Scholar] [CrossRef]
- Llamas, B.; Suárez-Rodríguez, M.C.; González-López, C.V.; Mora, P.; Acién, F.G. Techno-Economic Analysis of Microalgae Related Processes for CO2 Bio-Fixation. Algal Res. 2021, 57, 102339. [Google Scholar] [CrossRef]
- Song, C.; Hu, X.; Liu, Z.; Li, S.; Kitamura, Y. Combination of Brewery Wastewater Purification and CO2 Fixation with Potential Value-Added Ingredients Production via Different Microalgae Strains Cultivation. J. Clean. Prod. 2020, 268, 122332. [Google Scholar] [CrossRef]
- Rezvani, S.; Moheimani, N.R.; Bahri, P.A. Techno-Economic Assessment of CO2 Bio-Fixation Using Microalgae in Connection with Three Different State-of-the-Art Power Plants. Comput. Chem. Eng. 2016, 84, 290–301. [Google Scholar] [CrossRef]
- Hu, X.; Song, C.; Mu, H.; Liu, Z.; Kitamura, Y. Optimization of Simultaneous Soybean Processing Wastewater Treatment and Flue Gas CO2 Fixation via Chlorella sp. L166 Cultivation. J. Environ. Chem. Eng. 2020, 8, 103960. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Uma, V.S.; Mathimani, T.; Deviram, G.; Arul Ananth, D.; Prabaharan, D.; Uma, L. On-Site Concurrent Carbon Dioxide Sequestration from Flue Gas and Calcite Formation in Ossein Effluent by a Marine Cyanobacterium Phormidium valderianum BDU 20041. Energy Convers. Manag. 2017, 141, 315–324. [Google Scholar] [CrossRef]
- Bose, A.; O’Shea, R.; Lin, R.; Murphy, J.D. Optimisation and Performance Prediction of Photosynthetic Biogas Upgrading Using a Bubble Column. Chem. Eng. J. 2022, 437, 134988. [Google Scholar] [CrossRef]
- Wu, K.-K.; Zhao, L.; Sun, Z.-F.; Wang, Z.-H.; Chen, C.; Ren, H.-Y.; Yang, S.-S.; Ren, N.-Q. Synergistic Effect of Hydrogen and Nanoscale Zero-Valent Iron on Ex-Situ Biogas Upgrading and Acetate Recovery. Sci. Total Environ. 2023, 856, 159100. [Google Scholar] [CrossRef]
- Franco-Morgado, M.; Tabaco-Angoa, T.; Ramírez-García, M.A.; González-Sánchez, A. Strategies for Decreasing the O2 Content in the Upgraded Biogas Purified via Microalgae-Based Technology. J. Environ. Manag. 2021, 279, 111813. [Google Scholar] [CrossRef] [PubMed]
- Meier, L.; Stará, D.; Bartacek, J.; Jeison, D. Removal of H2S by a Continuous Microalgae-Based Photosynthetic Biogas Upgrading Process. Process Saf. Environ. Prot. 2018, 119, 65–68. [Google Scholar] [CrossRef]
- Rodero, M.D.R.; Lebrero, R.; Serrano, E.; Lara, E.; Arbib, Z.; García-Encina, P.A.; Muñoz, R. Technology Validation of Photosynthetic Biogas Upgrading in a Semi-Industrial Scale Algal-Bacterial Photobioreactor. Bioresour. Technol. 2019, 279, 43–49. [Google Scholar] [CrossRef]
- Bose, A.; Lin, R.; Rajendran, K.; O’Shea, R.; Xia, A.; Murphy, J.D. How to Optimise Photosynthetic Biogas Upgrading: A Perspective on System Design and Microalgae Selection. Biotechnol. Adv. 2019, 37, 107444. [Google Scholar] [CrossRef]
- Prandini, J.M.; da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Michelon, W.; Soares, H.M. Enhancement of Nutrient Removal from Swine Wastewater Digestate Coupled to Biogas Purification by Microalgae scenedesmus Spp. Bioresour. Technol. 2016, 202, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Rodero, M.D.R.; Carvajal, A.; Arbib, Z.; Lara, E.; de Prada, C.; Lebrero, R.; Muñoz, R. Performance Evaluation of a Control Strategy for Photosynthetic Biogas Upgrading in a Semi-Industrial Scale Photobioreactor. Bioresour. Technol. 2020, 307, 123207. [Google Scholar] [CrossRef] [PubMed]
- Rocher-Rivas, R.; González-Sánchez, A.; Ulloa-Mercado, G.; Muñoz, R.; Quijano, G. Biogas Desulfurization and Calorific Value Enhancement in Compact H2S/CO2 Absorption Units Coupled to a Photobioreactor. J. Environ. Chem. Eng. 2022, 10, 108336. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Integration of Anaerobic Digestion and Microalgal Cultivation for Digestate Bioremediation and Biogas Upgrading. Bioresour. Technol. 2019, 290, 121804. [Google Scholar] [CrossRef] [PubMed]
- Marín, D.; Méndez, L.; Suero, I.; Díaz, I.; Blanco, S.; Fdz-Polanco, M.; Muñoz, R. Anaerobic Digestion of Food Waste Coupled with Biogas Upgrading in an Outdoors Algal-Bacterial Photobioreactor at Pilot Scale. Fuel 2022, 324, 124554. [Google Scholar] [CrossRef]
- Méndez, L.; García, D.; Perez, E.; Blanco, S.; Muñoz, R. Photosynthetic Upgrading of Biogas from Anaerobic Digestion of Mixed Sludge in an Outdoors Algal-Bacterial Photobioreactor at Pilot Scale. J. Water Process Eng. 2022, 48, 102891. [Google Scholar] [CrossRef]
- Ángeles, R.; Vega-Quiel, M.J.; Batista, A.; Fernández-Ramos, O.; Lebrero, R.; Muñoz, R. Influence of Biogas Supply Regime on Photosynthetic Biogas Upgrading Performance in an Enclosed Algal-Bacterial Photobioreactor. Algal Res. 2021, 57, 102350. [Google Scholar] [CrossRef]
- González-Sánchez, A.; Posten, C. Fate of H2S during the Cultivation of Chlorella sp. Deployed for Biogas Upgrading. J. Environ. Manag. 2017, 191, 252–257. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Serejo, M.L.; Blanco, S.; Pérez, R.; Lebrero, R.; Muñoz, R. Photosynthetic Biogas Upgrading to Bio-Methane: Boosting Nutrient Recovery via Biomass Productivity Control. Algal Res. 2016, 17, 46–52. [Google Scholar] [CrossRef]
- Xu, M.; Xue, Z.; Sun, S.; Zhao, C.; Liu, J.; Liu, J.; Zhao, Y. Co-Culturing Microalgae with Endophytic Bacteria Increases Nutrient Removal Efficiency for Biogas Purification. Bioresour. Technol. 2020, 314, 123766. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, B.; Zhao, C.; Liu, J.; Zhao, Y.; Sun, S.; Wei, J. Simultaneous Biogas Upgrading and Biogas Slurry Treatment by Different Microalgae-Based Technologies under Various Strigolactone Analog (GR24) Concentrations. Bioresour. Technol. 2022, 351, 127033. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; O’Shea, R.; Lin, R.; Murphy, J.D. Design, Commissioning, and Performance Assessment of a Lab-Scale Bubble Column Reactor for Photosynthetic Biogas Upgrading with Spirulina platensis. Ind Eng Chem Res 2021, 60, 5688–5704. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Ishika, T.; Nwoba, E.G.; Tait, S.; Ward, A.; Moheimani, N.R. Biomass Production of Marine Microalga Tetraselmis suecica Using Biogas and Wastewater as Nutrients. Biomass Bioenergy 2021, 145, 105945. [Google Scholar] [CrossRef]
- Franco-Morgado, M.; Alcántara, C.; Noyola, A.; Muñoz, R.; González-Sánchez, A. A Study of Photosynthetic Biogas Upgrading Based on a High Rate Algal Pond under Alkaline Conditions: Influence of the Illumination Regime. Sci. Total Environ. 2017, 592, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Browne, J.D.; Murphy, J.D. What Is the Level of Incentivisation Required for Biomethane Upgrading Technologies with Carbon Capture and Reuse? Renew. Energy 2019, 133, 951–963. [Google Scholar] [CrossRef]
- Franco-Morgado, M.; Toledo-Cervantes, A.; González-Sánchez, A.; Lebrero, R.; Muñoz, R. Integral (VOCs, CO2, Mercaptans and H2S) Photosynthetic Biogas Upgrading Using Innovative Biogas and Digestate Supply Strategies. Chem. Eng. J. 2018, 354, 363–369. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Liu, J.; Zhao, C.; Sun, S.; Zhao, Y. Biogas Slurry Nutrient Removal and Biogas Upgrade in Co-Cultivated Microalgae and Fungi by Induction with Strigolactone. Algal Res. 2021, 59, 102467. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, C.; Liu, J.; Sun, S.; Zhao, Y.; Wei, J. Effects of Exogenous GR24 on Biogas Upgrading and Nutrient Removal by Co-Culturing Microalgae with Fungi under Mixed LED Light Wavelengths. Chemosphere 2021, 281, 130791. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, G.; Sun, S.; Hu, C.; Liu, J. Co-Pelletization of Microalgae and Fungi for Efficient Nutrient Purification and Biogas Upgrading. Bioresour. Technol. 2019, 289, 121656. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Toledo-Cervantes, A.; de Godos, I.; Gouveia, L.; Munõz, R. Life Cycle Assessment of Pilot and Real Scale Photosynthetic Biogas Upgrading Units. Algal Res. 2019, 44, 101668. [Google Scholar] [CrossRef]
- Bose, A.; O’Shea, R.; Lin, R.; Long, A.; Rajendran, K.; Wall, D.; De, S.; Murphy, J.D. The Marginal Abatement Cost of Co-Producing Biomethane, Food and Biofertiliser in a Circular Economy System. Renew. Sustain. Energy Rev. 2022, 169, 112946. [Google Scholar] [CrossRef]
- Morais, E.G.D.; Amaro Marques, J.C.; Cerqueira, P.R.; Dimas, C.; Sousa, V.S.; Gomes, N.; Ribau Teixeira, M.; Nunes, L.M.; Varela, J.; Barreira, L. Tertiary Urban Wastewater Treatment with Microalgae Natural Consortia in Novel Pilot Photobioreactors. J. Clean. Prod. 2022, 378, 134521. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Acién-Fernández, F.G.; Gómez-Serrano, C.; González-López, C.V. Annual Production of Microalgae in Wastewater Using Pilot-Scale Thin-Layer Cascade Photobioreactors. J. Appl. Phycol. 2021, 33, 3861–3871. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential Cultivation of Microalgae in Raw and Recycled Dairy Wastewater: Microalgal Growth, Wastewater Treatment and Biochemical Composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Malibari, R.; Sayegh, F.; Elazzazy, A.M.; Baeshen, M.N.; Dourou, M.; Aggelis, G. Reuse of Shrimp Farm Wastewater as Growth Medium for Marine Microalgae Isolated from Red Sea—Jeddah. J. Clean. Prod. 2018, 198, 160–169. [Google Scholar] [CrossRef]
- Morais, M.G.; Santos, T.D.; Moraes, L.; Vaz, B.S.; Morais, E.G.; Costa, J.A.V. Exopolysaccharides from Microalgae: Production in a Biorefinery Framework and Potential Applications. Bioresour. Technol. Rep. 2022, 18, 101006. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Swaminathan, M.K.; Boelen, P.; Buma, A.G.J. Sulfated Exopolysaccharide Production and Nutrient Removal by the Marine Diatom Phaeodactylum tricornutum Growing on Palm Oil Mill Effluent. J. Appl. Phycol. 2019, 31, 2335–2348. [Google Scholar] [CrossRef]
- Jung, S.-H.; Zell, N.; Boßle, F.; Teipel, U.; Rauh, C.; McHardy, C.; Lindenberger, C. Influence of Process Operation on the Production of Exopolysaccharides in Arthrospira platensis and Chlamydomonas asymmetrica. Front. Sustain. Food Syst. 2022, 6, 883069. [Google Scholar] [CrossRef]
- ONU United Nations Organization. The Millennium Development Goals Report; ONU United Nations Organization: New York, NY, USA, 2015. [Google Scholar]
- Rempel, A.; Gutkoski, J.P.; Nazari, M.T.; Biolchi, G.N.; Cavanhi, V.A.F.; Treichel, H.; Colla, L.M. Current Advances in Microalgae-Based Bioremediation and Other Technologies for Emerging Contaminants Treatment. Sci. Total Environ. 2021, 772, 144918. [Google Scholar] [CrossRef]
- Pacheco, D.; Rocha, A.C.; Pereira, L.; Verdelhos, T. Microalgae Water Bioremediation: Trends and Hot Topics. Appl. Sci. 2020, 10, 1886. [Google Scholar] [CrossRef]
- Xiong, J.; Kurade, M.B.; Jeon, B. Can Microalgae Remove Pharmaceutical Contaminants from Water ? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ying, G.; Yang, B.; Liu, S.; Lai, H.; Liu, Y.; Chen, Z.; Zhou, G. Biotransformation of Progesterone and Norgestrel by Two Freshwater Microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): Transformation Kinetics and Products Identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kurade, M.B.; Abou-shanab, R.A.I.; Ji, M.; Choi, J.; Oh, J.; Jeon, B. Biodegradation of Carbamazepine Using Freshwater Microalgae Chlamydomonas Mexicana and Scenedesmus Obliquus and the Determination of Its Metabolic Fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sforza, E.; Pastore, M.; Santeufemia Sanchez, S.; Bertucco, A. Bioaugmentation as a Strategy to Enhance Nutrient Removal: Symbiosis between Chlorella protothecoides and Brevundimonas diminuta. Bioresour. Technol. Rep. 2018, 4, 153–158. [Google Scholar] [CrossRef]
- Mehariya, S.; Kumar, R.; Parthiba, O.; Verma, P. Chemosphere Microalgae for High-Value Products: A Way towards Green Nutraceutical and Pharmaceutical Compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef]
- Mendonça, H.V.D.; Assemany, P.; Abreu, M.; Couto, E.; Maciel, A.M.; Duarte, R.L.; Santos, M.G.B.D.; Reis, A. Microalgae in a Global World: New Solutions for Old Problems? Renew. Energy 2021, 165, 842–862. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Ho, S.H. Microalgae as a Solution of Third World Energy Crisis for Biofuels Production from Wastewater toward Carbon Neutrality: An Updated Review. Chemosphere 2022, 291, 132863. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as Source of Polyhydroxyalkanoates (PHAs)—A Review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.D.S.; Oliveira, P.Z.D.; Bittencourt, G.A.; Mello, A.F.M.D.; Sarmiento, Z.; Vásquez; Karp, S.G.; Soccol, C.R. The 2G and 3G Bioplastics: An Overview. Biotechnol. Res. Innov. 2021, 5, e2021004. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent Advances in the Sustainable Design and Applications of Biodegradable Polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Zeller, M.A.; Hunt, R.; Jones, A.; Sharma, S. Bioplastics and Their Thermoplastic Blends from Spirulina and Chlorella microalgae. J. Appl. Polym. Sci. 2013, 130, 3263–3275. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Panchamoorthy Gopinath, K.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A Critical Review on Production of Biopolymers from Algae Biomass and Their Applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Zia, K.M.; Zuber, M.; Ali, M.; Mujahid, M. A Critical Review of Algal Biomass: A Versatile Platform of Bio-Based Polyesters from Renewable Resources. Int. J. Biol. Macromol. 2016, 86, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Kaparapu, J. Polyhydroxyalkanoate (PHA) Production by Genetically Engineered Microalgae: A Review. J. New Biol. Rep. 2018, 7, 68–73. [Google Scholar]
- Pérez, J.P.; Munoz, A.A.; Figuero, C.P.; Agurto-Munoz, C. Current Analytical Techniques for the Characterization of Lipophilic Bioactive Compounds from Microalgae Extracts. Biomass Bioenergy 2021, 149, 106078. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I. Progress in Physicochemical Parameters of Microalgae Cultivation for Biofuel Production. Crit. Rev. Biotechnol. 2019, 39, 835–859. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgaebiofuelsasanalternativetofossilfuelforpowergeneration. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Di Chen, Y.; Liu, F.; Ren, N.Q.; Ho, S.H. Revolutions in Algal Biochar for Different Applications: State-of-the-Art Techniques and Future Scenarios. Chin. Chem. Lett. 2020, 31, 2591–2602. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological Remediation of Acid Mine Drainage: Review of Past Trends and Current Outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of Biofuels from Microalgae—A Review on Cultivation, Harvesting, Lipid Extraction, and Numerous Applications of Microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Narindri Rara Winayu, B.; Tung Lai, K.; Ta Hsueh, H.; Chu, H. Production of Phycobiliprotein and Carotenoid by Efficient Extraction from Thermosynechococcus sp. CL-1 Cultivation in Swine Wastewater. Bioresour. Technol. 2021, 319, 124125. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae Biotechnology for Industrial Wastewater Treatment, Bioenergy Production, and High-Value Bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Thangam, K.R.; Santhiya, A.; Sri, S.R.A.; MubarakAli, D.; Karthikumar, S.; Kumar, R.S.; Thajuddin, N.; Soosai, M.R.; Varalakshmi, P.; Moorthy, I.G.; et al. Bio-Refinery Approaches Based Concomitant Microalgal Biofuel Production and Wastewater Treatment. Sci. Total Environ. 2021, 785, 147267. [Google Scholar] [CrossRef]
- Baldev, E.; Mubarak, D.; Pugazhendhi, A.; Thajuddin, N. Wastewater as an Economical and Ecofriendly Green Medium for Microalgal Biofuel Production. Fuel 2021, 294, 120484. [Google Scholar] [CrossRef]
- Vieira, H.; Mendonça, D.; Henrique, M.; Marchão, L.; Lomeu, A.; Salvador, D.; Souza, D.; Reis, A. Science of the Total Environment Biofuel Recovery from Microalgae Biomass Grown in Dairy Wastewater Treated with Activated Sludge: The next Step in Sustainable Production. Sci. Total Environ. 2022, 824, 153838. [Google Scholar]
- Cardoso, L.G.; Duarte, J.H.; Andrade, B.B.; Lemos, P.V.F.; Costa, J.A.V.; Druzian, J.I.; Chinalia, F.A. Spirulina sp. LEB 18 Cultivation in Outdoor Pilot Scale Using Aquaculture Wastewater: High Biomass, Carotenoid, Lipid and Carbohydrate Production. Aquaculture 2020, 525, 735272. [Google Scholar] [CrossRef]
- Bhuyar, P.; Sundararaju, S.; Rahim, M.H.A.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Microalgae Cultivation Using Palm Oil Mill Effluent as Growth Medium for Lipid Production with the Effect of CO2 Supply and Light Intensity. Biomass Convers. Biorefin. 2021, 11, 1555–1563. [Google Scholar] [CrossRef]
- Kavitha, G.; Kurinjimalar, C.; Sivakumar, K.; Kaarthik, M.; Aravind, R.; Palani, P.; Rengasamy, R. Optimization of Polyhydroxybutyrate Production Utilizing Waste Water as Nutrient Source by Botryococcus braunii Kütz Using Response Surface Methodology. Int. J. Biol. Macromol. 2016, 93, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The Role of Microalgae in the Bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Banu, R.J.; Preethi; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae Based Biorefinery Promoting Circular Bioeconomy-Techno Economic and Life-Cycle Analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar] [CrossRef]
- Monari, C.; Righi, S.; Olsen, S.I. Greenhouse Gas Emissions and Energy Balance of Biodiesel Production from Microalgae Cultivated in Photobioreactors in Denmark: A Life-Cycle Modeling. J. Clean. Prod. 2016, 112, 4084–4092. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-Economic Analysis of Autotrophic Microalgae for Fuel Production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Thomassen, G.; Egiguren Vila, U.; Van Dael, M.; Lemmens, B.; Van Passel, S. A Techno-Economic Assessment of an Algal-Based Biorefinery. Clean Technol. Environ. Policy 2016, 18, 1849–1862. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Silva-Gálvez, A.L.; Aguilar-Juárez, Ó.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-Based Livestock Wastewater Treatment (MbWT) as a Circular Bioeconomy Approach: Enhancement of Biomass Productivity, Pollutant Removal and High-Value Compound Production. J. Environ. Manag. 2022, 308, 114612. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; García-Perez, J.S.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Castillo-Zacarías, C.; Afewerki, S.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Phyco-Remediation of Swine Wastewater as a Sustainable Model Based on Circular Economy. J. Environ. Manag. 2021, 278, 111534. [Google Scholar] [CrossRef]
- Ciardi, M.; Gómez-Serrano, C.; Lafarga, T.; González-Céspedes, A.; Acién, G.; López-Segura, J.G.; Fernández-Sevilla, J.M. Pilot-Scale Annual Production of Scenedesmus almeriensis Using Diluted Pig Slurry as the Nutrient Source: Reduction of Water Losses in Thin-Layer Cascade Reactors. J. Clean. Prod. 2022, 359, 132076. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Gutiérrez, R.; Uggetti, E.; Matamoros, V.; García, J.; Ferrer, I. Use of Full-Scale Hybrid Horizontal Tubular Photobioreactors to Process Agricultural Runoff. Biosyst. Eng. 2018, 166, 138–149. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-Based Biorefineries for Sustainable Resource Recovery from Wastewater. J. Water Process Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).