Photosynthetic Carbon Uptake Correlates with Cell Protein Content during Lipid Accumulation in the Microalga Chlorella vulgaris NIES 227
Abstract
1. Introduction
2. Materials and Methods
2.1. Micro-Organisms and Culture Conditions
2.2. Laboratory Analysis
2.2.1. Sampling
2.2.2. Biomass Growth Monitoring
2.2.3. Biomass Metabolic Status
Proteins
Carbohydrates
Lipids
Functional Compartment
2.2.4. Biomass Productivity Status
2.3. Data Analysis
2.4. Complementary Measurements
- -
- Sunlight intensity measured throughout the study duration. Sunlight intensity was measured in the greenhouse in the vicinity of the photobioreactor using the continuous data logging (data logger 2648A Hydra Series III, Fluke™, Everett, WA, USA) of a pyranometer CM21 (Kipp & Zonen™, Delft, The Netherlands). No data could be recovered for the period preceding the 9 March 2021, or the period ranging from 19 April 2021 to 27 April 2021).
- -
- Total nitrogen in the dissolved phase measured from the filtration of the centrifugation supernatant of the culture (filters Puradisc 0.2 µm, Whatman™, Maidstone, UK). Total nitrogen in the dissolved phase was determined using the total organic carbon analyzer TOC-L (Shimadzu™, Kyoto, Japan).
3. Results
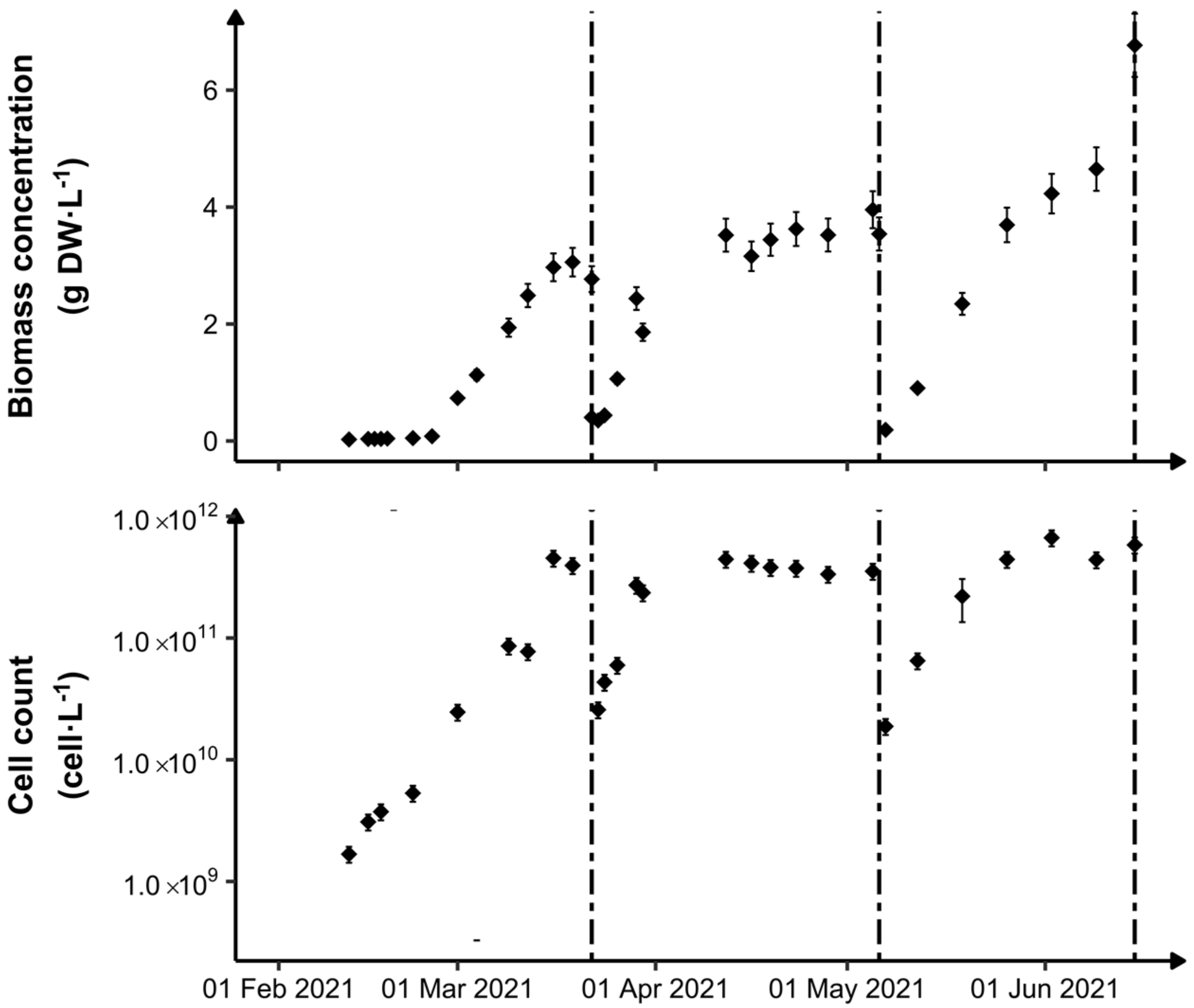
3.1. Photobioreactor Monitoring
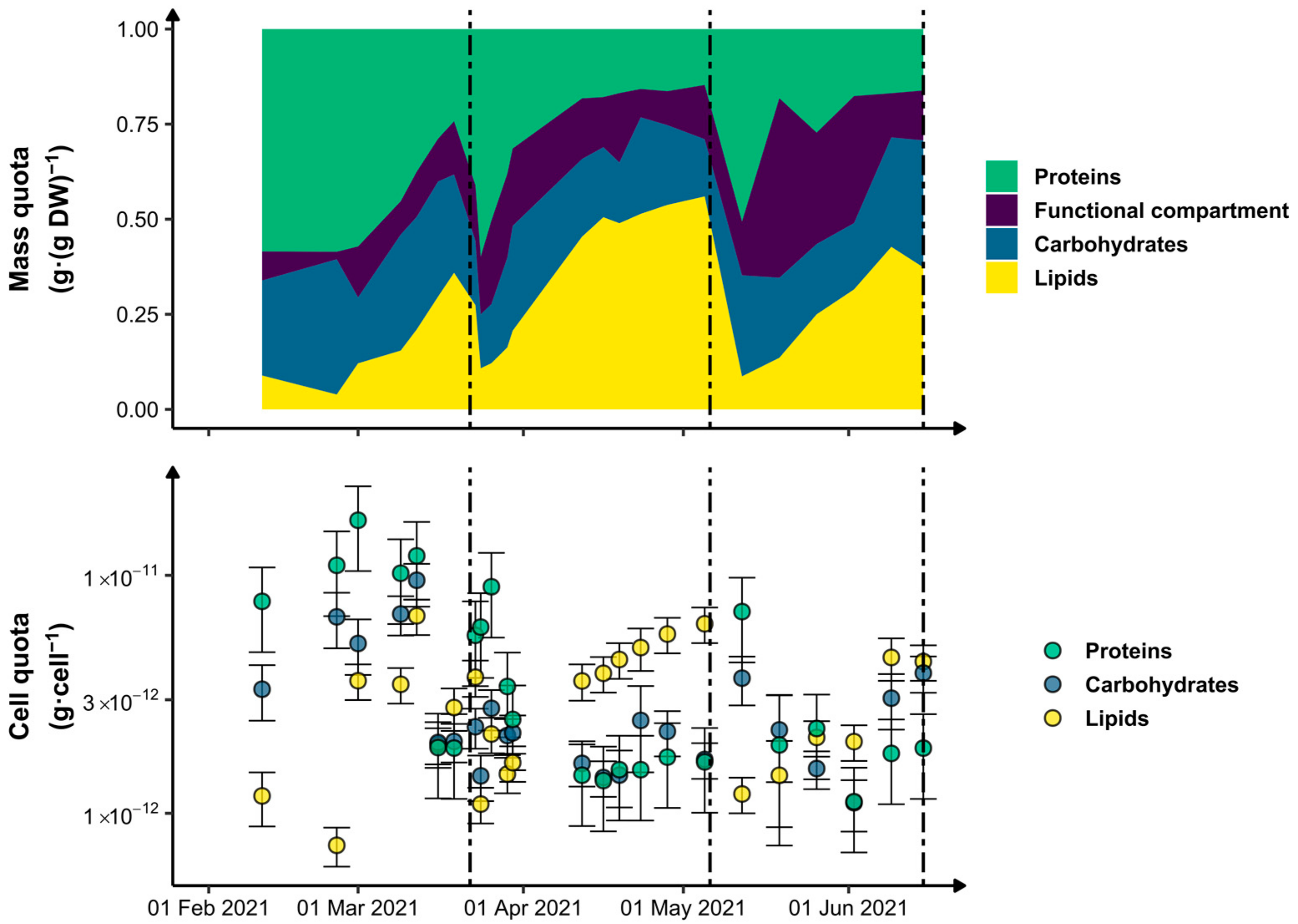
3.2. Biomass Metabolic Status
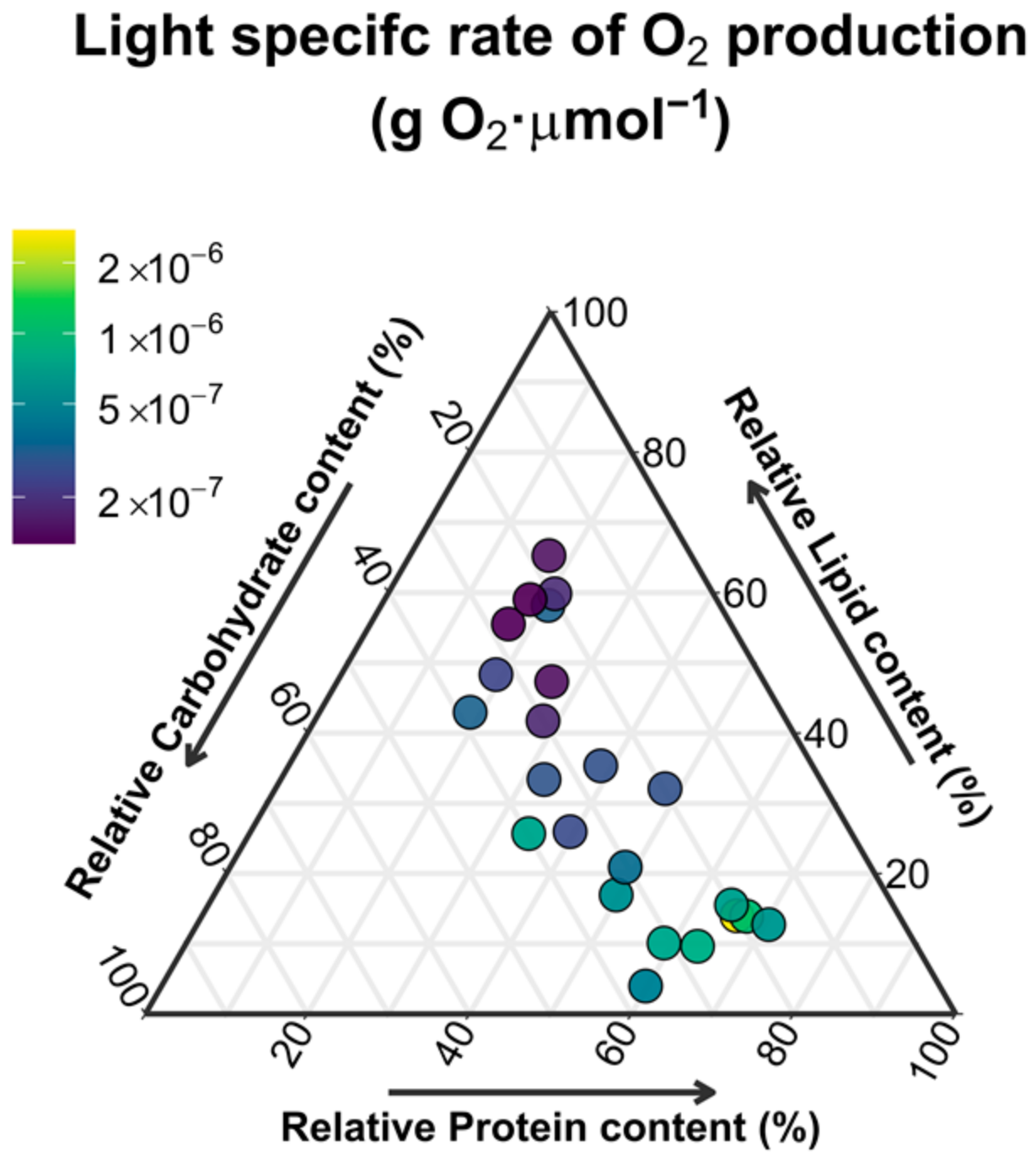
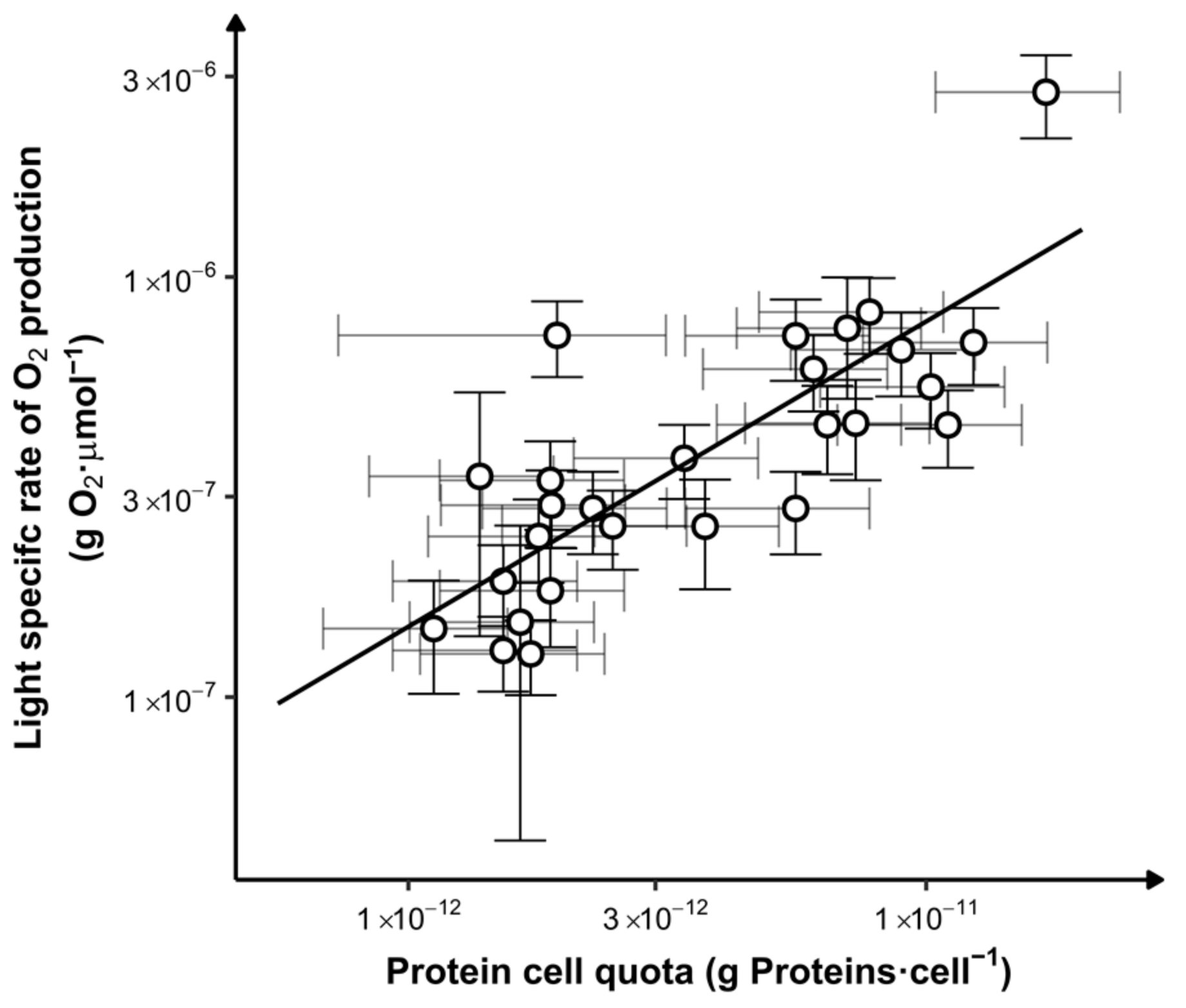
3.3. Biomass Photosynthetic Productivity
4. Discussion
4.1. Light versus Nutrient Limitations
4.2. Consequences for the Prediction of Lipid Accumulation
- I.
- The microalgae perform photosynthesis with an efficiency dependent on the current physiological state of the microalgae (i.e., partition between the cell content in carbohydrates, proteins, and lipids) and light availability, as evidenced by this study. This photosynthetic activity delivers a pool of electrons converted into carbohydrates [21].
- II.
- The carbohydrates are stored and can subsequently be reduced to generate reducing power or be used as a carbon source for the following processes:
- a.
- Basic maintenance of the cell [34].
- b.
- Assimilation of nitrogen and protein formation: this study evidenced that the average light received by the cells likely modulates the quantity of nitrogen assimilated.
- c.
- Accumulation of lipids when excess carbon is absorbed in parallel with limited nitrogen assimilation.
4.3. Future Studies
4.3.1. Need for Short-Term Studies of the Evolution of Photosynthesis Performance
4.3.2. Nitrate Utilization Study
4.3.3. Other Research Needs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- IEA. Liquid biofuel production by feedstock and technology in the Net Zero Scenario, 2021 and 2030. Available online: https://www.iea.org/data-and-statistics/charts/liquid-biofuel-production-by-feedstock-and-technology-in-the-net-zero-scenario-2021-and-2030 (accessed on 27 September 2022).
- Calderon, C.; Greelen, J.; Jossart, J.-M. Biomass Supply 2014; Bioenergy Europe, Bruxelles. 2021. Available online: https://bioenergyeurope.org/article/240-statistical-report-2021.html (accessed on 4 January 2022).
- Lam, M.K.; Lee, K.T. Microalgae Biofuels: A Critical Review of Issues, Problems and the Way Forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative Energy Life-Cycle Analyses of Microalgal Biomass Production in Open Ponds and Photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef]
- Guieysse, B.; Béchet, Q.; Shilton, A. Variability and Uncertainty in Water Demand and Water Footprint Assessments of Fresh Algae Cultivation Based on Case Studies from Five Climatic Regions. Bioresour. Technol. 2013, 128, 317–323. [Google Scholar] [CrossRef]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal Cultivation Using Aquaculture Wastewater: Integrated Biomass Generation and Nutrient Remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Nagi, M.; He, M.; Li, D.; Gebreluel, T.; Cheng, B.; Wang, C. Utilization of Tannery Wastewater for Biofuel Production: New Insights on Microalgae Growth and Biomass Production. Sci. Rep. 2020, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Cabanelas, I.T.D.; Ruiz, J.; Arbib, Z.; Chinalia, F.A.; Garrido-Pérez, C.; Rogalla, F.; Nascimento, I.A.; Perales, J.A. Comparing the Use of Different Domestic Wastewaters for Coupling Microalgal Production and Nutrient Removal. Bioresour. Technol. 2013, 131, 429–436. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Resource Recovery through Bioremediation of Wastewaters and Waste Carbon by Microalgae: A Circular Bioeconomy Approach. Environ. Sci. Pollut. Res. 2021, 28, 58837–58856. [Google Scholar] [CrossRef]
- Xia, L.; Song, S.; He, Q.; Yang, H.; Hu, C. Selection of Microalgae for Biodiesel Production in a Scalable Outdoor Photobioreactor in North China. Bioresour. Technol. 2014, 174, 274–280. [Google Scholar] [CrossRef]
- Wen, X.; Du, K.; Wang, Z.; Peng, X.; Luo, L.; Tao, H.; Xu, Y.; Zhang, D.; Geng, Y.; Li, Y. Effective Cultivation of Microalgae for Biofuel Production: A Pilot-Scale Evaluation of a Novel Oleaginous Microalga Graesiella Sp. WBG-1. Biotechnol. Biofuels 2016, 9, 123. [Google Scholar] [CrossRef]
- Moody, J.W.; McGinty, C.M.; Quinn, J.C. Global Evaluation of Biofuel Potential from Microalgae. Proc. Natl. Acad. Sci. USA 2014, 111, 8691–8696. [Google Scholar] [CrossRef] [PubMed]
- Roessler, P.G. Environmental Control of Glycerolipid Metabolism in Microalgae Commercial Implicationsand Future Research Directions. J. Phycol. 1990, 26, 393–399. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal Lipids: A Review of Lipids Potential and Quantification for 95 Phytoplankton Species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Béchet, Q.; Shilton, A.; Guieysse, B. Modeling the Effects of Light and Temperature on Algae Growth: State of the Art and Critical Assessment for Productivity Prediction during Outdoor Cultivation. Biotechnol. Adv. 2013, 31, 1648–1663. [Google Scholar] [CrossRef]
- Bekirogullari, M.; Figueroa-Torres, G.M.; Pittman, J.K.; Theodoropoulos, C. Models of Microalgal Cultivation for Added-Value Products—A Review. Biotechnol. Adv. 2020, 44, 107609. [Google Scholar] [CrossRef]
- Ramírez-Romero, A.; Da Costa Magalhães, B.; Dimitriades-Lemaire, A.; Sassi, J.-F.; Delrue, F.; Steyer, J.-P. Chlorellaceae Feedstock Selection under Balanced Nutrient Limitation. Fermentation 2022, 8, 554. [Google Scholar] [CrossRef]
- Hutner, S.H.; Provasoli, L.; Schatz, A.; Haskins, C.P. Some Approaches to the Study of the Role of Metals in the Metabolism of Microorganisms. Proc. Am. Philos. Soc. 1950, 94, 152–170. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Béchet, Q.; Chambonnière, P.; Shilton, A.; Guizard, G.; Guieysse, B. Algal Productivity Modeling: A Step toward Accurate Assessments of Full-Scale Algal Cultivation. Biotechnol. Bioeng. 2015, 112, 987–996. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Dempster, T.A.; Jones, H.D.T.; Wolfrum, E.J.; Van Wychen, S.; McAllister, J.S.P.; Rencenberger, M.; Parchert, K.J.; Gloe, L.M. Algal Biomass Constituent Analysis: Method Uncertainties and Investigation of the Underlying Measuring Chemistries. Anal. Chem. 2012, 84, 1879–1887. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lanfer Marquez, U.M.; Aidar, E. Distribution of Intracellular Nitrogen in Marine Microalgae: Basis for the Calculation of Specific Nitrogen-to-Protein Conversion Factors. J. Phycol. 1998, 34, 798–811. [Google Scholar] [CrossRef]
- Templeton, D.W.; Laurens, L.M.L. Nitrogen-to-Protein Conversion Factors Revisited for Applications of Microalgal Biomass Conversion to Food, Feed and Fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef]
- Li, X.; Rao, N.R.H.; Linge, K.L.; Joll, C.A.; Khan, S.; Henderson, R.K. An Evaluation of Measurement Techniques for Algal-Derived Organic Nitrogen. Water Res. 2019, 165, 114998. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ryu, K.H.; Sung, M.; Kim, B.; Heo, S.; Chang, Y.K.; Lee, J.H. A Mathematical Model of Intracellular Behavior of Microalgae for Predicting Growth and Intracellular Components Syntheses under Nutrient-replete and -deplete Conditions. Biotechnol. Bioeng. 2018, 115, 2441–2455. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Chambonniere, P.; Ramírez-Romero, A.; Dimitriades, A.; Sassi, J.-F.; Delrue, F. Photosynthetic Carbon Uptake Correlates with Cell Protein Content during Lipid Accumulation in the Microalgae Chlorella Vulgaris NIES 227. Figshare. Collect. 2022. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L.; Campbell, W.H.; Redinbaugh, M.G. Apparent Dependence of the Light Activation of Nitrate Reductase and Sucrose-Phosphate Synthase Activities in Spinach Leaves on Protein Synthesis. Plant Cell Physiol. 1992, 33, 639–646. [Google Scholar] [CrossRef]
- Packard, T.T. The Light Dependence of Nitrate Reductase in Marine Phytoplankton. Limnol. Oceanogr. 1973, 18, 466–469. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M. Brock Biology of Microorganisms, 11th ed.; Pearson Education, Inc.: London, UK, 2006. [Google Scholar]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding Nitrate Assimilation and Its Regulation in Microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef]
- Manhaeghe, D.; Michels, S.; Rousseau, D.P.L.; Van Hulle, S.W.H. A Semi-Mechanistic Model Describing the Influence of Light and Temperature on the Respiration and Photosynthetic Growth of Chlorella Vulgaris. Bioresour. Technol. 2019, 274, 361–370. [Google Scholar] [CrossRef]
- Geider, R.J.; MacIntyre, H.L.; Kana, T.M. A Dynamic Model of Photoadaptation in Phytoplankton. Limnol. Oceanogr. 1996, 41, 1–15. [Google Scholar] [CrossRef]
- Kiefer, D.A.; Mitchell, B.G. A Simple, Steady State Description of Phytoplankton Growth Based on Absorption Cross Section and Quantum Efficiency. Limnol. Oceanogr. 1983, 28, 770–776. [Google Scholar] [CrossRef]
- Droop, M.R. Vitamin b 12 and Marine Ecology. Iv. The Kinetics of Uptake, Growth and Inhibition in Monochrysis Lutheri. J. Mar. Biol. Assoc. United Kingd. 1968, 48, 689–733. [Google Scholar] [CrossRef]
- Mairet, F.; Bernard, O.; Masci, P.; Lacour, T.; Sciandra, A. Modelling Neutral Lipid Production by the Microalga Isochrysis Aff. Galbana under Nitrogen Limitation. Bioresour. Technol. 2011, 102, 142–149. [Google Scholar] [CrossRef]
- Deschênes, J.S.; Wouwer, A. Vande. Parameter Identification of a Dynamic Model of Cultures of Microalgae Scenedesmus Obliquus- An Experimental Study. IFAC-PapersOnLine 2016, 49, 1050–1055. [Google Scholar] [CrossRef]
- Figueroa-Torres, G.M.; Pittman, J.K.; Theodoropoulos, C. Kinetic Modelling of Starch and Lipid Formation during Mixotrophic, Nutrient-Limited Microalgal Growth. Bioresour. Technol. 2017, 241, 868–878. [Google Scholar] [CrossRef]
- Bekirogullari, M.; Pittman, J.K.; Theodoropoulos, C. Multi-Factor Kinetic Modelling of Microalgal Biomass Cultivation for Optimised Lipid Production. Bioresour. Technol. 2018, 269, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Kim, J.H.; Lee, J.M. Dynamic Modelling of Mixotrophic Microalgal Photobioreactor Systems with Time-Varying Yield Coefficient for the Lipid Consumption. Bioresour. Technol. 2014, 162, 228–235. [Google Scholar] [CrossRef]
- Adesanya, V.O.; Davey, M.P.; Scott, S.A.; Smith, A.G. Kinetic Modelling of Growth and Storage Molecule Production in Microalgae under Mixotrophic and Autotrophic Conditions. Bioresour. Technol. 2014, 157, 293–304. [Google Scholar] [CrossRef]
- Cardol, P.; Forti, G.; Finazzi, G. Regulation of Electron Transport in Microalgae. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 912–918. [Google Scholar] [CrossRef]
- Béchet, Q.; Shilton, A.; Guieysse, B. Full-Scale Validation of a Model of Algal Productivity. Environ. Sci. Technol. 2014, 48, 13826–13833. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambonniere, P.; Ramírez-Romero, A.; Dimitriades-Lemaire, A.; Sassi, J.-F.; Delrue, F. Photosynthetic Carbon Uptake Correlates with Cell Protein Content during Lipid Accumulation in the Microalga Chlorella vulgaris NIES 227. Fermentation 2022, 8, 614. https://doi.org/10.3390/fermentation8110614
Chambonniere P, Ramírez-Romero A, Dimitriades-Lemaire A, Sassi J-F, Delrue F. Photosynthetic Carbon Uptake Correlates with Cell Protein Content during Lipid Accumulation in the Microalga Chlorella vulgaris NIES 227. Fermentation. 2022; 8(11):614. https://doi.org/10.3390/fermentation8110614
Chicago/Turabian StyleChambonniere, Paul, Adriana Ramírez-Romero, Alexandra Dimitriades-Lemaire, Jean-François Sassi, and Florian Delrue. 2022. "Photosynthetic Carbon Uptake Correlates with Cell Protein Content during Lipid Accumulation in the Microalga Chlorella vulgaris NIES 227" Fermentation 8, no. 11: 614. https://doi.org/10.3390/fermentation8110614
APA StyleChambonniere, P., Ramírez-Romero, A., Dimitriades-Lemaire, A., Sassi, J.-F., & Delrue, F. (2022). Photosynthetic Carbon Uptake Correlates with Cell Protein Content during Lipid Accumulation in the Microalga Chlorella vulgaris NIES 227. Fermentation, 8(11), 614. https://doi.org/10.3390/fermentation8110614





