Use of Yarrowia lipolytica to Obtain Fish Waste Functional Hydrolysates Rich in Flavoring Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Screening for Enzymatic Properties
2.2.1. Proteolytic Property
2.2.2. Lipolytic Property
2.2.3. Protease Activity
2.3. Production of Fish Waste Hydrolysates
2.3.1. Fish Waste
2.3.2. Fermentation Process
2.4. Microbiological Analyses
2.5. Protein Analyses
Protein Concentration Assay, Peptide Content and Degree of Hydrolysis (DH)
2.6. Antioxidant Activity
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.7. Volatile Molecule Profile
2.8. Statistical Analysis
3. Results
3.1. Screening for Enzymatic Properties
3.2. Fermentation of Fish Waste
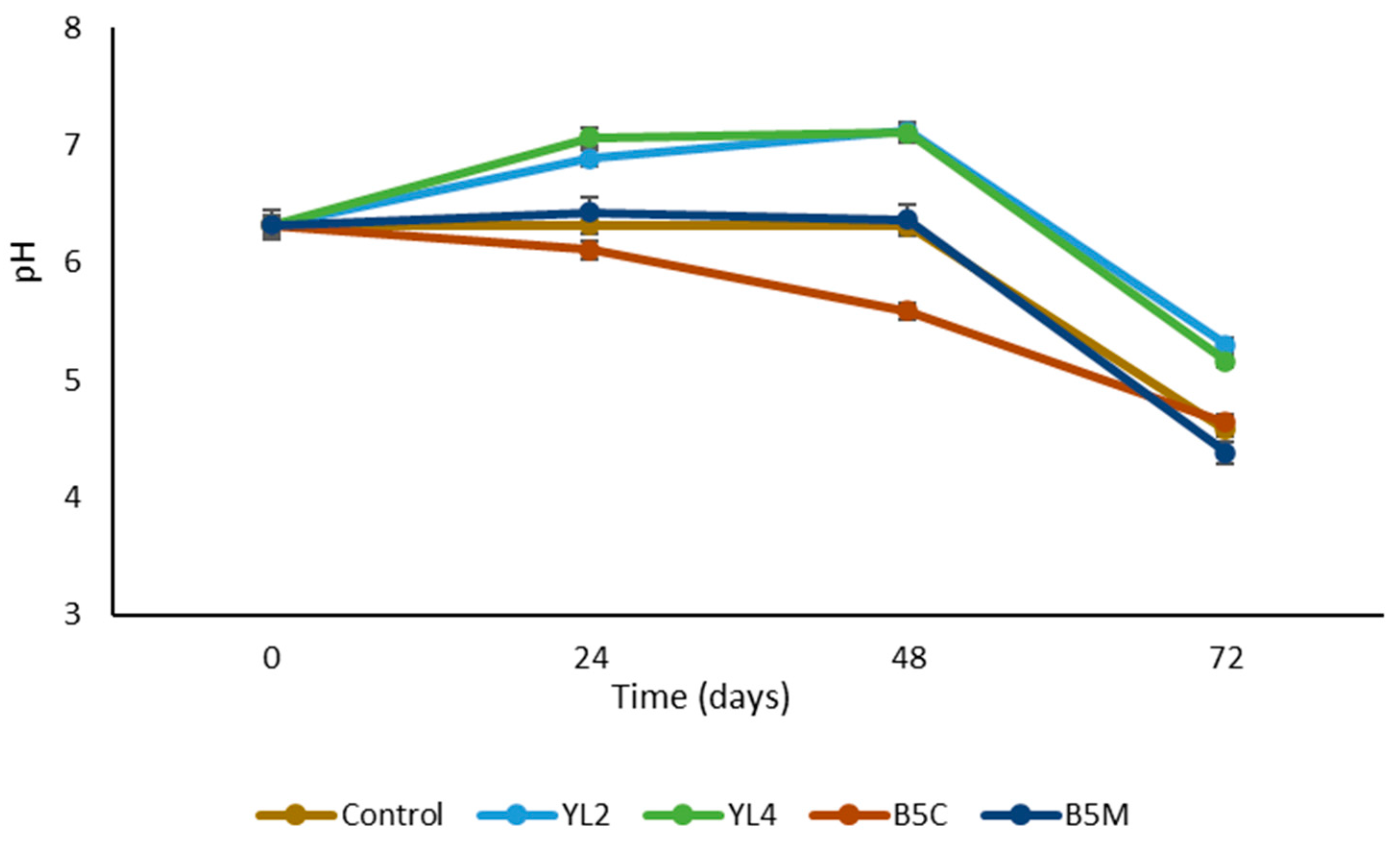
3.2.1. Microbial Characterization and pH
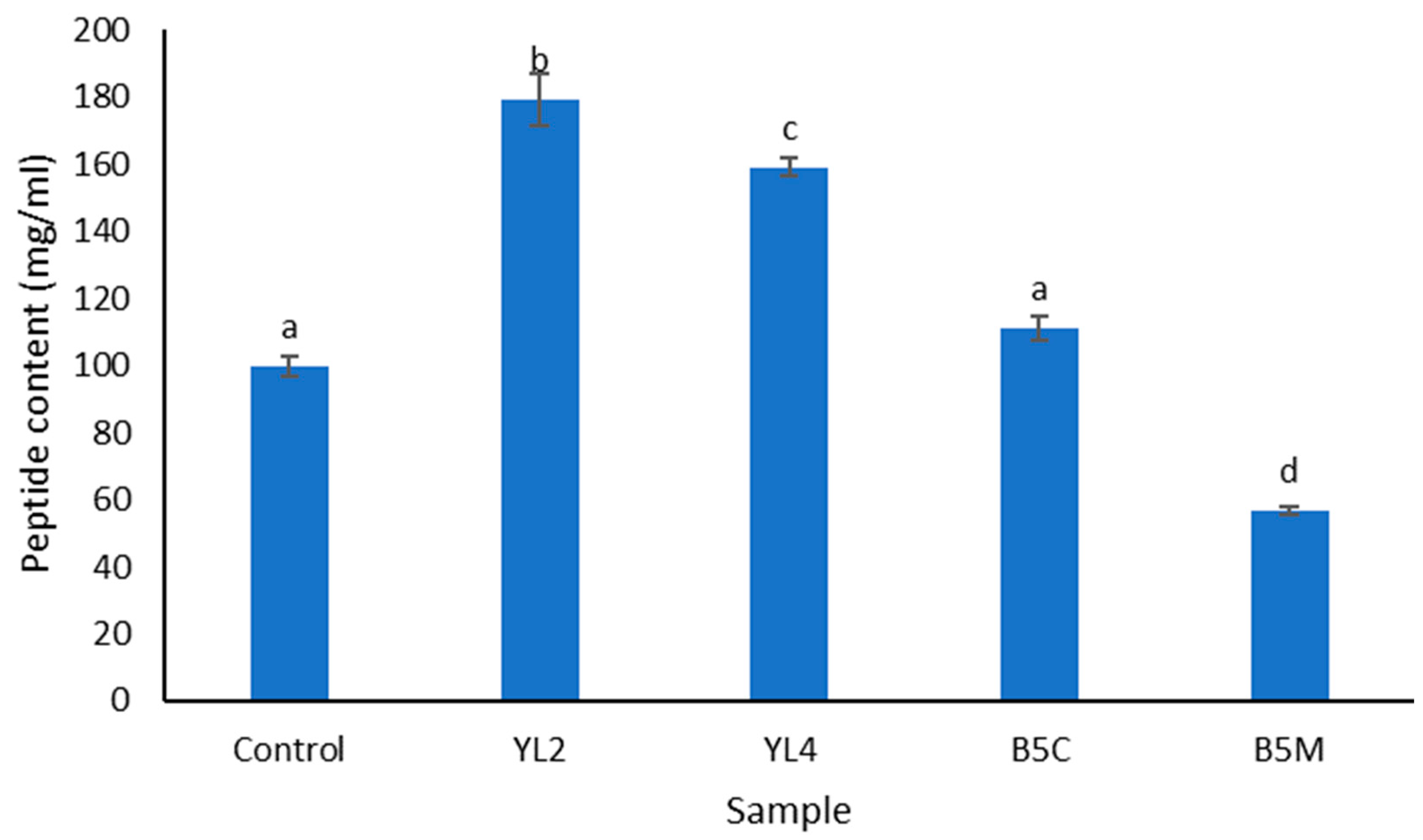
3.2.2. Degree of Hydrolysis (DH) and Peptide Content
3.2.3. Antioxidant Activity
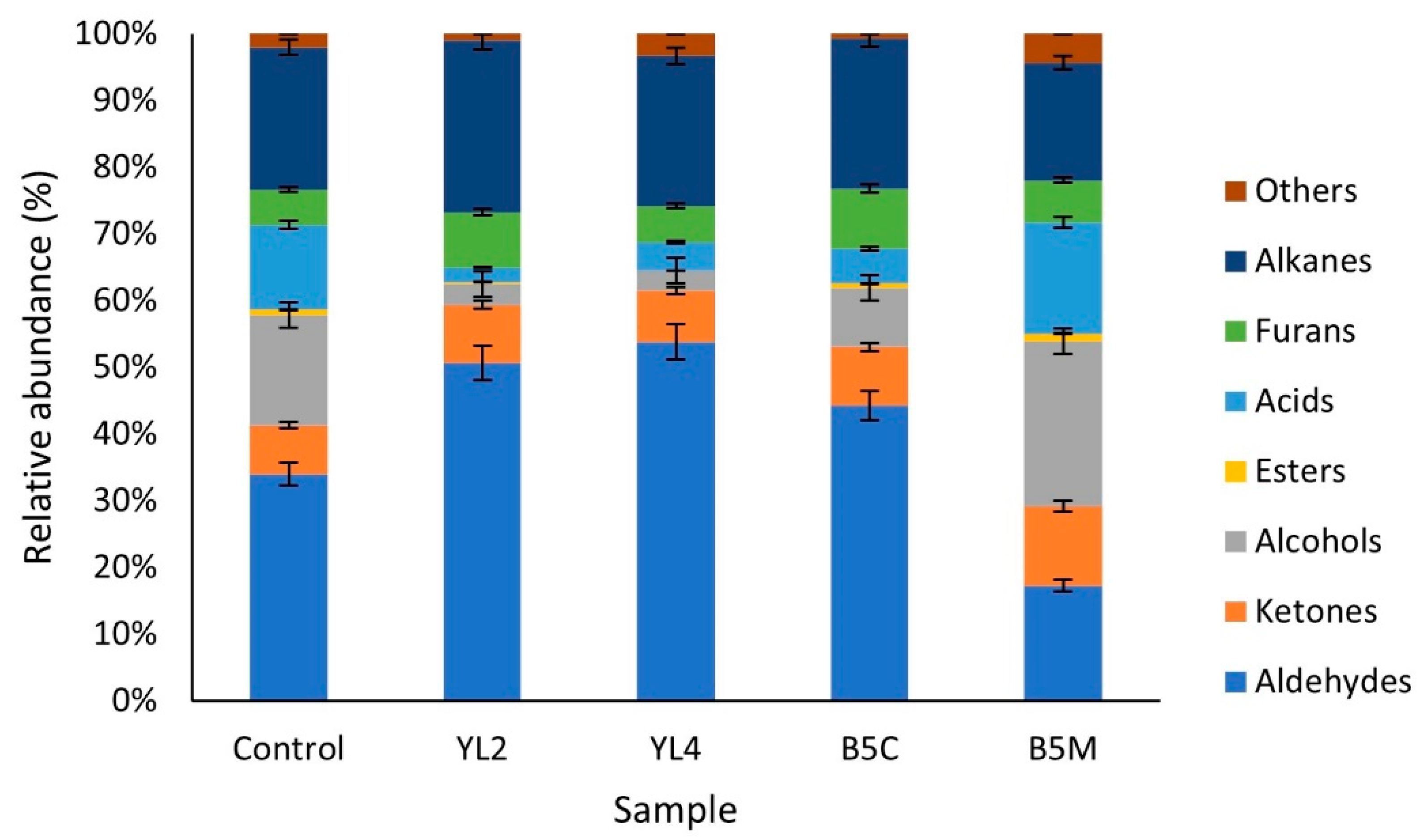
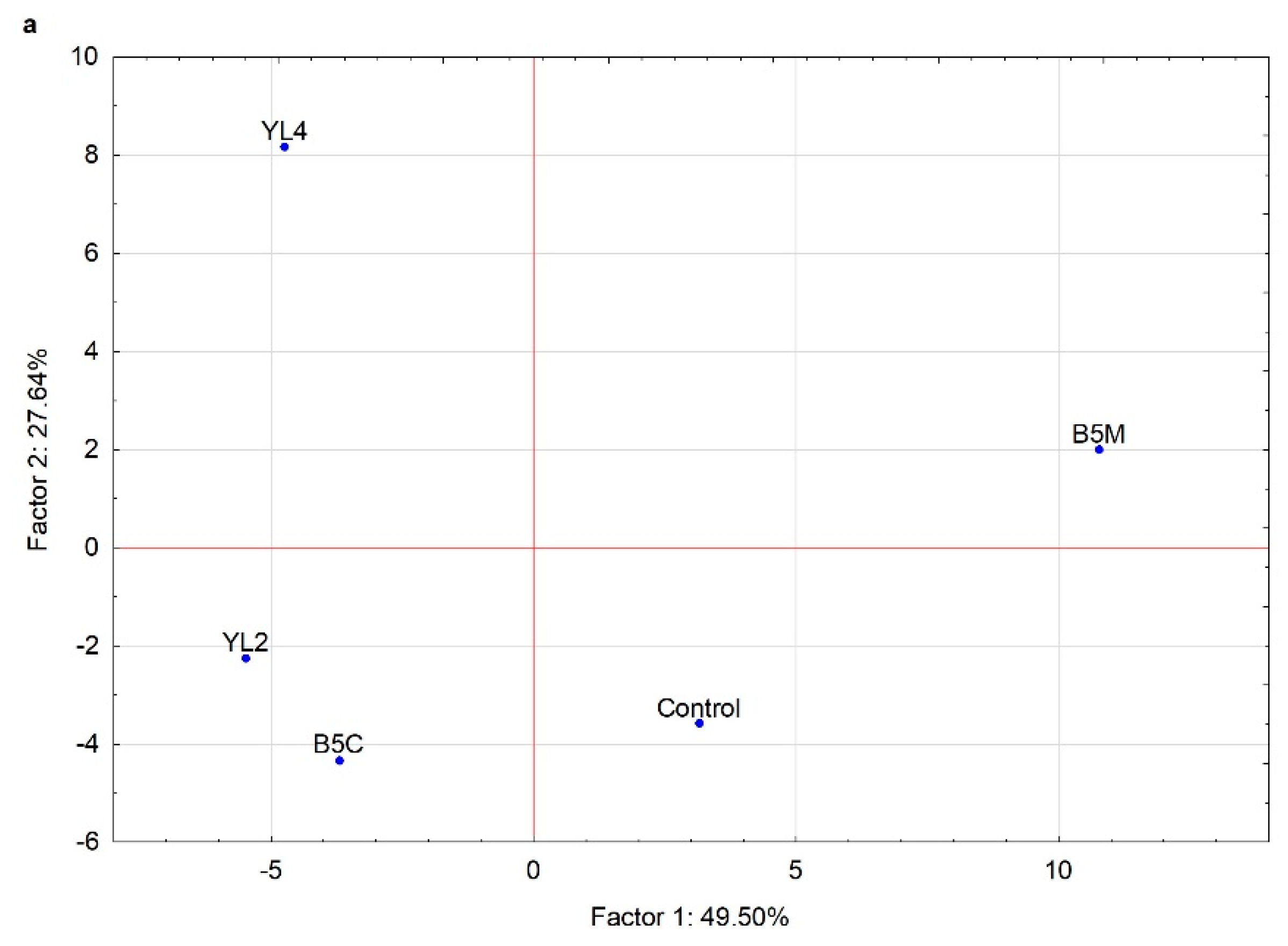
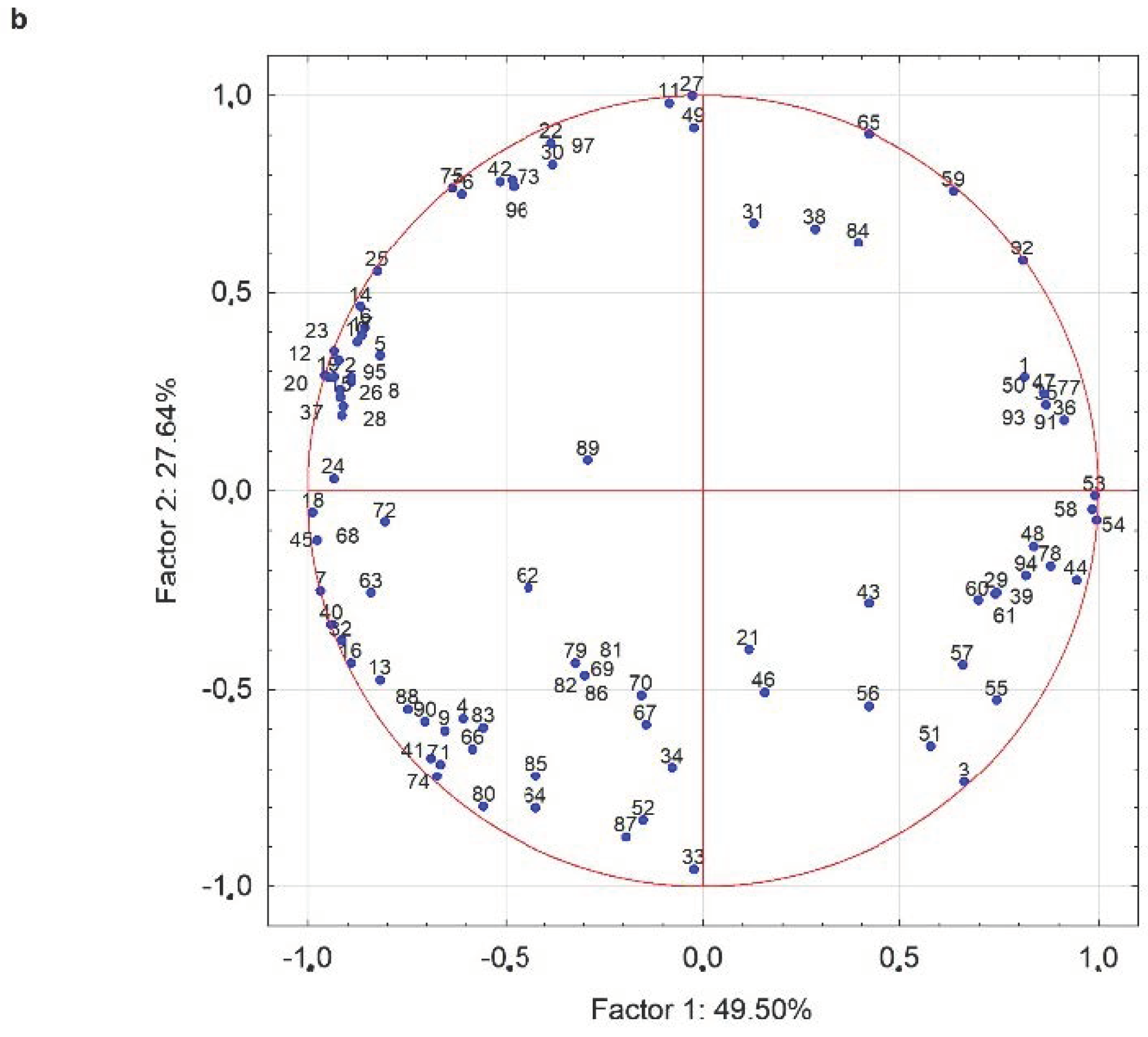
3.2.4. Volatile Molecule Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchi, M.; Chopin, F.; Farme, T.; Franz, N.; Fuentevilla, C.; Garibaldi, L.; Laurenti, A. FAO: The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 1–230. [Google Scholar]
- Ishak, N.; Sarbon, N. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Durán, A.I.; Menduíña, A.; Nogueira, M. Biotechnological valorization of food marine wastes: Microbial productions on peptones obtained from aquaculture by-products. Biomolecules 2020, 10, 1184. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Oikawa, H.; Satomi, M. Reduction of lipids in fish meal prepared from fish waste by a yeast Yarrowia lipolytica. Int. J. Food Microbiol. 2008, 121, 302–307. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Miled, N. Fish processing wastes for microbial enzyme production: A review. 3 Biotech 2013, 3, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mayta-Apaza, A.C.; García-Cano, I.; Dabrowski, K.; Jiménez-Flores, R. Bacterial Diversity Analysis and Evaluation Proteins Hydrolysis During the Acid Whey and Fish Waste Fermentation. Microorganisms 2021, 9, 100. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Wu, Y.; Xiang, H.; Zhao, Y.; Chen, S.; Qi, B.; Li, L. Insights into lipid oxidation and free fatty acid profiles to the development of volatile organic compounds in traditional fermented golden pomfret based on multivariate analysis. LWT 2022, 171, 114112. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Tornos, A.; Príncep, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.-J.; Barba, F.J. Impact of Fermentation on the Recovery of Antioxidant Bioactive Compounds from Sea Bass Byproducts. Antioxidants 2020, 9, 239. [Google Scholar] [CrossRef]
- Feng, L.; Tang, N.; Liu, R.; Gong, M.; Wang, Z.; Guo, Y.; Wang, Y.; Zhang, Y.; Chang, M. The relationship between flavor formation, lipid metabolism, and microorganisms in fermented fish products. Food Funct. 2021, 12, 5685–5702. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, K.; Tokur, B. Optimization of hydrolysis conditions for the production of protein hydrolysates from fish wastes using response surface methodology. Food Biosci. 2021, 45, 101312. [Google Scholar] [CrossRef]
- Zou, Y.; Robbens, J.; Heyndrickx, M.; Debode, J.; Raes, K. Quantification of Extracellular Proteases and Chitinases from Marine Bacteria. Curr. Microbiol. 2020, 77, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- Kusumaningtyas, E.; Nurilmala, M.; Sibarani, D. Antioxidant and antifungal activities of collagen hydrolysates from skin of milkfish (Chanos chanos) hydrolyzed using various bacillus proteases. In Proceedings of the 3rd EMBRIO International Workshop on Marine Biodiversity: Understanding, Utilization, Conservation, Bogor, Indonesia, 9–10 October 2018. [Google Scholar]
- Mhina, C.F.; Jung, H.Y.; Kim, J.K. Recovery of antioxidant and antimicrobial peptides through the reutilization of Nile perch wastewater by biodegradation using two Bacillus species. Chemosphere 2020, 253, 126728. [Google Scholar] [CrossRef]
- da Silva Bernardo, B.; Kopplin, B.W.; Daroit, D.J. Bioconversion of Fish Scales and Feather Wastes by Bacillus sp. CL18 to Obtain Protease and Bioactive Hydrolysates. Waste Biomass Valorization 2022, 1–12. [Google Scholar] [CrossRef]
- Gicana, R.G.; Yeh, F.-I.; Hsiao, T.-H.; Chiang, Y.-R.; Yan, J.-S.; Wang, P.-H. Valorization of fish waste and sugarcane bagasse for Alcalase production by Bacillus megaterium via a circular bioeconomy model. J. Taiwan Inst. Chem. Eng. 2022, 135, 104358. [Google Scholar] [CrossRef]
- Bhagwat, P.K.; Bhise, K.K.; Bhuimbar, M.V.; Dandge, P.B. Use of statistical experimental methods for optimization of collagenolytic protease production by Bacillus cereus strain SUK grown on fish scales. Environ. Sci. Pollut. Res. 2018, 25, 28226–28236. [Google Scholar] [CrossRef]
- Umesh, M.; Suresh, S.; Sarojini, S.; Santosh, A.S. A sustainable approach for fish waste valorization through polyhydroxyalkanoate production by Bacillus megaterium NCDC0679 and its optimization studies. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Hu, J.; Luo, J.; Zhu, Z.; Chen, B.; Ye, X.; Zhu, P.; Zhang, B. Multi-Scale Biosurfactant Production by Bacillus subtilis Using Tuna Fish Waste as Substrate. Catalysts 2021, 11, 456. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Vannini, L.; Patrignani, F.; Lanciotti, R. Recovery and valorization of agri-food wastes and by-products using the non-conventional yeast Yarrowia lipolytica. Trends Food Sci. Technol. 2021, 115, 74–86. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zieniuk, B.; Kozłowska, M.; Mazurczak-Zieniuk, P.M.; Wołoszynowska, M.; Misiukiewicz-Stępień, P.; Nowak, D. Studies on Upgradation of Waste Fish Oil to Lipid-Rich Yeast Biomass in Yarrowia lipolytica Batch Cultures. Foods 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and Stirring in Yarrowia lipolytica Lipase Biosynthesis during Batch Cultures with Waste Fish Oil as a Carbon Source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization—Challenges and opportunities. Crit. Rev. Biotechnol. 2021, 42, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Ozturkoglu-Budak, S.; Wiebenga, A.; Bron, P.A.; de Vries, R.P. Protease and lipase activities of fungal and bacterial strains derived from an artisanal raw ewe’s milk cheese. Int. J. Food Microbiol. 2016, 237, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cupp-Enyard, C. Sigma’s non-specific protease activity assay-casein as a substrate. J. Vis. Exp. 2008, e899. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using o-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Hong, P.K.; Gottardi, D.; Ndagijimana, M.; Betti, M. Glycation and transglutaminase mediated glycosylation of fish gelatin peptides with glucosamine enhance bioactivity. Food Chem. 2014, 142, 285–293. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Contesini, F.J.; de Melo, R.R.; Sato, H.H. An overview of Bacillus proteases: From production to application. Crit. Rev. Biotechnol. 2018, 38, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Guncheva, M.; Zhiryakova, D. Catalytic properties and potential applications of Bacillus lipases. J. Mol. Catal. B Enzym. 2011, 68, 1–21. [Google Scholar] [CrossRef]
- Olmos, J.; Paniagua-Michel, J. Bacillus subtilis a potential probiotic bacterium to formulate functional feeds for aquaculture. J. Microb. Biochem. Technol. 2014, 6, 361–365. [Google Scholar] [CrossRef]
- Devaraj, K.; Aathika, S.; Periyasamy, K.; Periyaraman, P.M.; Palaniyandi, S.; Subramanian, S. Production of thermostable multiple enzymes from Bacillus amyloliquefaciens KUB29. Nat. Prod. Res. 2018, 33, 1674–1677. [Google Scholar] [CrossRef]
- Patrignani, F.; Iucci, L.; Vallicelli, M.; Guerzoni, M.E.; Gardini, F.; Lanciotti, R. Role of surface-inoculated Debaryomyces hansenii and Yarrowia lipolytica strains in dried fermented sausage manufacture. Part 1: Evaluation of their effects on microbial evolution, lipolytic and proteolytic patterns. Meat Sci. 2007, 75, 676–686. [Google Scholar] [CrossRef]
- Patrignani, F.; Vannini, L.; Gardini, F.; Guerzoni, M.E.; Lanciotti, R. Variability of the lipolytic activity and volatile molecules production by a strain of Yarrowia lipolytica in pork fat and its dependence on environmental conditions. Meat Sci. 2011, 89, 21–26. [Google Scholar] [CrossRef]
- Suzzi, G.; Lanorte, M.; Galgano, F.; Andrighetto, C.; Lombardi, A.; Lanciotti, R.; Guerzoni, M. Proteolytic, lipolytic and molecular characterisation of Yarrowia lipolytica isolated from cheese. Int. J. Food Microbiol. 2001, 69, 69–77. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, N.F.; Allergens, F.; Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef]
- Hamdani, S.; Asstiyani, N.; Astriany, D.; Singgih, M.; Ibrahim, S. Isolation and identification of proteolytic bacteria from pig sludge and protease activity determination. IOP Conf. Series Earth Environ. Sci. 2019, 230, 012095. [Google Scholar] [CrossRef]
- Suberu, Y.; Akande, I.; Samuel, T.; Lawal, A.; Olaniran, A. Optimization of protease production in indigenous Bacillus species isolated from soil samples in Lagos, Nigeria using response surface methodology. Biocatal. Agric. Biotechnol. 2019, 18, 101011. [Google Scholar] [CrossRef]
- Zou, Y.; Tortorella, E.; Robbens, J.; Heyndrickx, M.; Debode, J.; De Pascale, D.; Raes, K. Bioactivity Screening of Hydrolysates From Brown Crab Processing Side Streams Fermented by Marine Pseudoalteromonas Strains. Waste Biomass Valorization 2021, 12, 2459–2468. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.-Y.; Kasim, N.S.; Diem, Q.-D.; Huynh, L.-H.; Ho, Q.-P.; Truong, C.-T.; Ju, Y.-H. Oil Production from Yarrowia lipolytica Po1g Using Rice Bran Hydrolysate. J. Biomed. Biotechnol. 2012, 2012, 378384. [Google Scholar] [CrossRef] [PubMed]
- Kommoji, S.; Gopinath, M.; Sagar, P.S.; Yuvaraj, D.; Iyyappan, J.; Varsha, A.J.; Sunil, V. Lipid bioproduction from delignified native grass (Cyperus distans) hydrolysate by Yarrowia lipolytica. Bioresour. Technol. 2021, 324, 124659. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Parrotta, L.; Del Duca, S.; Rosa, M.D.; Patrignani, F.; Schluter, O.; Lanciotti, R. Effect of Yarrowia lipolytica RO25 cricket-based hydrolysates on sourdough quality parameters. LWT 2021, 148, 111760. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Bajzert, J.; Babij, K.; Szołtysik, M.; Stefaniak, T.; Willak-Janc, E.; Chrzanowska, J. Reduced IgE and IgG antigenic response to milk proteins hydrolysates obtained with the use of non-commercial serine protease from Yarrowia lipolytica. Food Chem. 2020, 302, 125350. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Influence of the degree of hydrolysis (DH) on antioxidant properties and radical-scavenging activities of peanut peptides prepared from fermented peanut meal. Eur. Food Res. Technol. 2011, 232, 941–950. [Google Scholar] [CrossRef]
- Shahi, Z.; Sayyed-Alangi, S.Z.; Najafian, L. Effects of enzyme type and process time on hydrolysis degree, electrophoresis bands and antioxidant properties of hydrolyzed proteins derived from defatted Bunium persicum Bioss. press cake. Heliyon 2020, 6, e03365. [Google Scholar] [CrossRef]
- Godinho, I.; Pires, C.; Pedro, S.; Teixeira, B.; Mendes, R.; Nunes, M.L.; Batista, I. Antioxidant Properties of Fish Protein Hydrolysates Prepared from Cod Protein Hydrolysate by Bacillus sp. Appl. Biochem. Biotechnol. 2016, 178, 1095–1112. [Google Scholar] [CrossRef]
- Manni, L.; Ghorbel-Bellaaj, O.; Jellouli, K.; Younes, I.; Nasri, M. Extraction and Characterization of Chitin, Chitosan, and Protein Hydrolysates Prepared from Shrimp Waste by Treatment with Crude Protease from Bacillus cereus SV1. Appl. Biochem. Biotechnol. 2010, 162, 345–357. [Google Scholar] [CrossRef]
- Xu, X.; Mo, H.; Yan, M.; Zhu, Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. J. Sci. Food Agric. 2007, 87, 1502–1504. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Q.; Xu, Y.; Xia, W. Effect of mixed kojis on physiochemical and sensory properties of rapid-fermented fish sauce made with freshwater fish by-products. Int. J. Food Sci. Technol. 2017, 52, 2088–2096. [Google Scholar] [CrossRef]
- Varlet, V.; Prost, C.; Serot, T. Volatile aldehydes in smoked fish: Analysis methods, occurence and mechanisms of formation. Food Chem. 2007, 105, 1536–1556. [Google Scholar] [CrossRef]
- Misharina, T.A.; Andreenkov, V.A.; Vashchuk, E.A. Changes in the Composition of Volatile Compounds during Aging of Dry-cured Sausages. Appl. Biochem. Microbiol. 2001, 37, 413–418. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhao, Y.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Cen, J.; Yang, S.; Yang, D. Novel insight into the formation mechanism of volatile flavor in Chinese fish sauce (Yu-lu) based on molecular sensory and metagenomics analyses. Food Chem. 2020, 323, 126839. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.K.; Yeo, J.; Jang, E.Y.; Kim, M.J.; Lee, J. Aldehydes from Oxidized Lipids Can React with 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radicals in Isooctane Systems. J. Am. Oil Chem. Soc. 2012, 89, 1831–1838. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Fabiszewska, A. Use of Non-Conventional Yeast Yarrowia lipolytica in Treatment or Upgradation of Hydrophobic Industry Wastes. Waste Biomass Valorization 2021, 13, 757–779. [Google Scholar] [CrossRef]

| Genera and Species | Strain | Origin | Proteolytic Clearing Zone in Skim Milk (mm) | Gelatinase Activity | Lipolytic Clearing Zone (mm) |
|---|---|---|---|---|---|
| B. subtilis | B5C | Plant origin | 8 ± 1.0 | +++ | 14 ± 0.1 |
| B12C | Plant origin | 6 ± 0.4 | +++ | 10 ± 0.3 | |
| B15C | Plant origin | 5 ± 0.3 | +++ | 12 ± 0.5 | |
| B28C | Plant origin | - | +++ | - | |
| B46C | Plant origin | - | + | - | |
| B47C | Plant origin | - | ++ | - | |
| B. licheniformis | B1M | Wine lees | 8 ± 0.2 | - | 10 ± 0.3 |
| B21M | Wine lees | - | - | - | |
| B. amyloliquefaciens | B5M | Wine lees | 9 ± 0.3 | +++ | 14 ± 1.1 |
| B. stratosphericus | B18M | Wine lees | - | + | 9 ± 0.2 |
| Y. lipolytica | YL1 | River | 9 ± 0.1 | +++ | 14 ± 0.3 |
| YL2 | River | 11 ± 0.3 | +++ | 15 ± 0.2 | |
| YL3 | River | 10 ± 0.1 | +++ | 13 ± 0.5 | |
| YL4 | River | 11 ± 0.2 | +++ | 15 ± 0.1 | |
| YL5 | Dairy product | - | + | - | |
| YL6 | Dairy product | - | +++ | - | |
| YL7 | Meat | - | +++ | - | |
| YL8 | Smoked ham | - | +++ | - |
| Fish Waste Hydrolysate | DPPH (Radical Scavenging Activity % of Fresh Sample) | DPPH (mg of Ascorbic Acid Equivalent/mg of Lyophilized Samples) | ABTS (Radical Scavenging Activity % of Fresh Sample) | ABTS (Trolox Equivalent μmol/g) |
|---|---|---|---|---|
| Control 72 h | 51.2 ± 15.0 a | 17.0 ± 3.1 a | 12.7 ± 1.2 a | 135.8 ± 22.1 a |
| YL2 | 86.4 ± 9.4 b | 34.5 ± 1.5 c | 34.9 ± 2.5 c | 129.7 ± 12.7 a |
| YL4 | 65.5 ± 2.7 a | 26.9 ± 2.2 b | 45.0 ± 2.3 b | 237.2 ± 33.4 b |
| B5C | 72.5 ± 3.7 c | 35.1 ± 2.1 c | 39.1 ± 3.3 b, c | 52.5 ± 10.1 c |
| B5M | 15.6 ± 2.1 d | 22.8 ± 2.6 a, b | 0.9 ± 1.1 d | 30.0 ± 11.2 c |
| Class of Compounds | Volatile Compound | Control | YL2 | YL4 | B5C | B5M |
|---|---|---|---|---|---|---|
| Aldehydes | Acetaldehyde | 0.04 | 0.06 | 0.09 | 0.08 | 0.37 |
| Propanal | 0.1 | 0.83 | 0.75 | 0.39 | - | |
| 2-propenal | 0.17 | 0.1 | - | 0.11 | 0.15 | |
| Butanal | - | 0.08 | - | 0.07 | - | |
| 3-methylbutanal | - | 0.2 | 0.17 | 0.06 | - | |
| Pentanal | 0.49 | 0.7 | 0.75 | 0.51 | 0.37 | |
| 2-butenal | 0.3 | 0.48 | 0.38 | 0.44 | 0.11 | |
| 2-pentenal | 2.56 | 2.81 | 3.53 | 2.68 | 1.38 | |
| Hexanal | 0.99 | 4.44 | 0.58 | 3.58 | 0.23 | |
| Heptanal | 1.08 | 1.71 | 1.88 | 1.14 | 0.63 | |
| 2-hexenal | 1.48 | 1.39 | 5.04 | 0.93 | 2.45 | |
| Octanal | 1.38 | 2.11 | 2.22 | 1.46 | 0.63 | |
| 2-heptenal | 1.06 | 1.14 | 0.83 | 1.17 | 0.05 | |
| Nonanal | 1.54 | 3.36 | 4.16 | 2.1 | 0.73 | |
| 2,4-hexadienal | 1.24 | 2.27 | 3.62 | 3.07 | 0.12 | |
| 2-octenal | 1.6 | 3.35 | 1.74 | 2.93 | - | |
| Decanal | 0.31 | 0.7 | 0.73 | 0.39 | 0.19 | |
| 2,4-heptadienal | 7.7 | 12.13 | 11.66 | 12.38 | 4.7 | |
| 2-nonenal | 1.55 | 2.44 | 2.77 | 1.96 | 0.78 | |
| Benzaldehyde | 2.79 | 4.76 | 5.82 | 4.24 | 1.2 | |
| 2,6-nonadienal | 3.84 | 1.36 | 1.06 | 0.9 | 0.73 | |
| Undecanal | - | - | 0.08 | - | - | |
| 2-decenal | 1.06 | 1.99 | 2.62 | 1.74 | 0.37 | |
| 2,4-nonadienal | 0.1 | 0.13 | 0.18 | 0.18 | - | |
| 2-undecenal | 0.36 | 0.67 | 1.12 | 0.58 | 0.16 | |
| 2,4-decadienal | 0.33 | 0.75 | 0.77 | 0.58 | 0.25 | |
| Tetradecanal | 0.46 | 0.53 | 1.14 | 0.41 | 0.76 | |
| 3-ethylbenzaldehyde | - | 0.14 | 0.16 | 0.15 | - | |
| 5-hydroxymethyl-2-Furaldehyde | 1.48 | - | - | - | 0.86 | |
| Ketones | Acetone | - | 0.24 | 0.37 | - | 0.13 |
| Methyl isobutyl ketone | 0.17 | 0.09 | 0.2 | 0.11 | 0.14 | |
| 1-penten-3-one | 1.72 | 2.2 | 1.73 | 2.04 | 0.83 | |
| 2,4-pentanedione | 0.12 | 0.11 | 0.07 | 0.14 | 0.09 | |
| 4-methyl-2-hexanone | 0.04 | - | - | 0.04 | - | |
| 3-octanone | - | - | - | - | 0.38 | |
| 3-hydroxy-2 butanone | 0.86 | - | - | - | 8.15 | |
| 1-octen-3-one | 0.52 | 1.12 | 1.75 | 1.62 | - | |
| 2,5-octanedione | 0.26 | 0.35 | 0.36 | 0.3 | 0.4 | |
| 1-hydroxy-2-propnaone | 0.26 | - | - | - | 0.15 | |
| 2-nonanone | 1.21 | 1.7 | 1.35 | 1.64 | 0.68 | |
| 3,5-octadien-2-one | 1.41 | 2.69 | 0.91 | 2.41 | 0.52 | |
| 2-undecanone | - | 0.25 | 0.71 | 0.33 | 0.21 | |
| 2,6-dimethyl-4-heptanone | 0.77 | 0.05 | 0.19 | 0.2 | 0.25 | |
| Alcohols | Ethanol | 12.16 | 1.54 | 2.25 | 7.82 | 16.46 |
| 1-penten-3-ol | 0.13 | 0.19 | 0.18 | 0.2 | 0.09 | |
| 1-pentanol | 1.01 | 0.18 | - | 0.1 | - | |
| 3-methyl-1-butanol | - | - | 0.09 | - | 3.16 | |
| 1-pentanol | 0.07 | 0.09 | - | 0.07 | 0.25 | |
| 2-penten-1-ol | 0.1 | - | 0.34 | - | 0.11 | |
| 1-hexanol | - | - | - | - | 0.28 | |
| 1-octen-3-ol | 0.77 | 0.83 | - | 0.51 | 0.93 | |
| Benzyl alcohol | 0.1 | 0.05 | - | 0.07 | - | |
| Phenyl ethanol | 2.11 | 0.19 | 0.14 | 0.11 | 3.46 | |
| Esters | Ethyl acetate | 0.22 | - | - | 0.06 | 0.4 |
| Acetic acid ethenyl ester | 0.4 | 0.29 | - | 0.16 | 0.42 | |
| Tetradecanoic acid ethyl ester | 0.11 | - | - | 0.21 | 0.14 | |
| Hexanoic acid ethylester | 0.13 | - | - | 0.2 | 0.2 | |
| Acids | Acetic acid | 11.84 | 2.16 | 4 | 5.23 | 15.92 |
| Propanoic acid | 0.13 | - | 0.24 | - | 0.28 | |
| Octanoic acid | 0.32 | - | - | - | 0.16 | |
| Decanoic acid | 0.41 | - | - | - | 0.24 | |
| Furans | 2-methyl furan | 0.15 | 1.26 | - | 1.48 | - |
| 2 -ethyl furan | 2.16 | 2.15 | 2.16 | 2.19 | 2 | |
| Butyl furan | 0.04 | 0.05 | - | 0.03 | - | |
| 2-methyl furan | 0.15 | 1.20 | 0.00 | 1.48 | 0.00 | |
| 2-pentyl furan | 0.24 | - | 0.94 | - | 0.84 | |
| 2-(2-pentenyl) furan | 1.77 | 3.66 | 1.53 | 4.31 | 3.06 | |
| 2 methoxy furan | 0.17 | 0.38 | 0.28 | 0.28 | - | |
| 3-pentyl-furan | - | - | - | 0.05 | - | |
| 2-(2-propenyl) furan | 0.17 | 0.17 | - | - | - | |
| 4-methyl-2propyl furan | 0.62 | 0.62 | 0.54 | 0.65 | 0.46 | |
| Alkanes, alkenes, alkynes | hexadecane | 0.55 | 0.78 | 0.64 | 0.64 | 0.54 |
| 2,6,10,14 tetramethyl pentadecane | 0.64 | 0.9 | 1.01 | 0.71 | 0.77 | |
| 1,3-cyclooctadiene | 0.86 | 1.07 | 0.72 | 1.07 | 0.63 | |
| Heptadecane | 2.86 | 3.95 | 5.26 | 3.2 | 2.96 | |
| 1-pentadecene | 0.1 | 0.18 | 0.59 | 0.12 | - | |
| 2,4-dimethyl-1-heptene | - | - | - | - | 0.09 | |
| Decane | 0.06 | 0.06 | 0.04 | 0.05 | 0.08 | |
| 1,3-cis-5-cis-octatriene | 0.11 | 0.22 | - | - | - | |
| 1,3-trans-5-cis-octatriene | 0.07 | 0.15 | - | 0.16 | - | |
| 1-ethyl-1,4-cyclohexadiene | - | - | - | 0.11 | - | |
| 3-ethyl-2-methyl-1-pentene | - | - | - | 0.03 | - | |
| Dodecane | - | 0.15 | - | 0.24 | - | |
| Tridecane | 0.47 | 0.46 | 0.5 | 0.32 | 0.51 | |
| Tetradecane | 0.11 | 0.17 | - | 0.07 | - | |
| 2,4-dimethyl-1,3-pentadiene | - | - | - | 0.04 | - | |
| 3,5,5-trimethyl-2-hexene | 0.9 | 1.02 | 0.21 | 0.72 | 0.43 | |
| Pentadecane | 14.35 | 16.6 | 13.23 | 14.7 | 11.48 | |
| Cyclooctane | 0.26 | 0.13 | 0.29 | 0.32 | 0.16 | |
| Others | 2.02 | 0.87 | 3.09 | 0.74 | 3.71 | |
| Total area 1 | 1894 | 2555 | 1818 | 2668 | 1759 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottardi, D.; Ciccone, M.; Siroli, L.; Lanciotti, R.; Patrignani, F. Use of Yarrowia lipolytica to Obtain Fish Waste Functional Hydrolysates Rich in Flavoring Compounds. Fermentation 2022, 8, 708. https://doi.org/10.3390/fermentation8120708
Gottardi D, Ciccone M, Siroli L, Lanciotti R, Patrignani F. Use of Yarrowia lipolytica to Obtain Fish Waste Functional Hydrolysates Rich in Flavoring Compounds. Fermentation. 2022; 8(12):708. https://doi.org/10.3390/fermentation8120708
Chicago/Turabian StyleGottardi, Davide, Marianna Ciccone, Lorenzo Siroli, Rosalba Lanciotti, and Francesca Patrignani. 2022. "Use of Yarrowia lipolytica to Obtain Fish Waste Functional Hydrolysates Rich in Flavoring Compounds" Fermentation 8, no. 12: 708. https://doi.org/10.3390/fermentation8120708
APA StyleGottardi, D., Ciccone, M., Siroli, L., Lanciotti, R., & Patrignani, F. (2022). Use of Yarrowia lipolytica to Obtain Fish Waste Functional Hydrolysates Rich in Flavoring Compounds. Fermentation, 8(12), 708. https://doi.org/10.3390/fermentation8120708






