Effect of Fibrolytic Enzymes, Cellulolytic Fungi and Lactic Acid Bacteria on Fermentation Characteristics, Structural Carbohydrate Composition and In Vitro Digestibility of Rice Straw Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Additives and Silage Preparation
2.2. Analysis of Extract and Solid Samples
2.3. In Vitro Incubation of 60-Day Silage
2.4. Statistical Analyses
3. Results
3.1. Chemical Compositions of Raw Rice Straw
3.2. Fermentation Characteristics of Rice Straw Silage
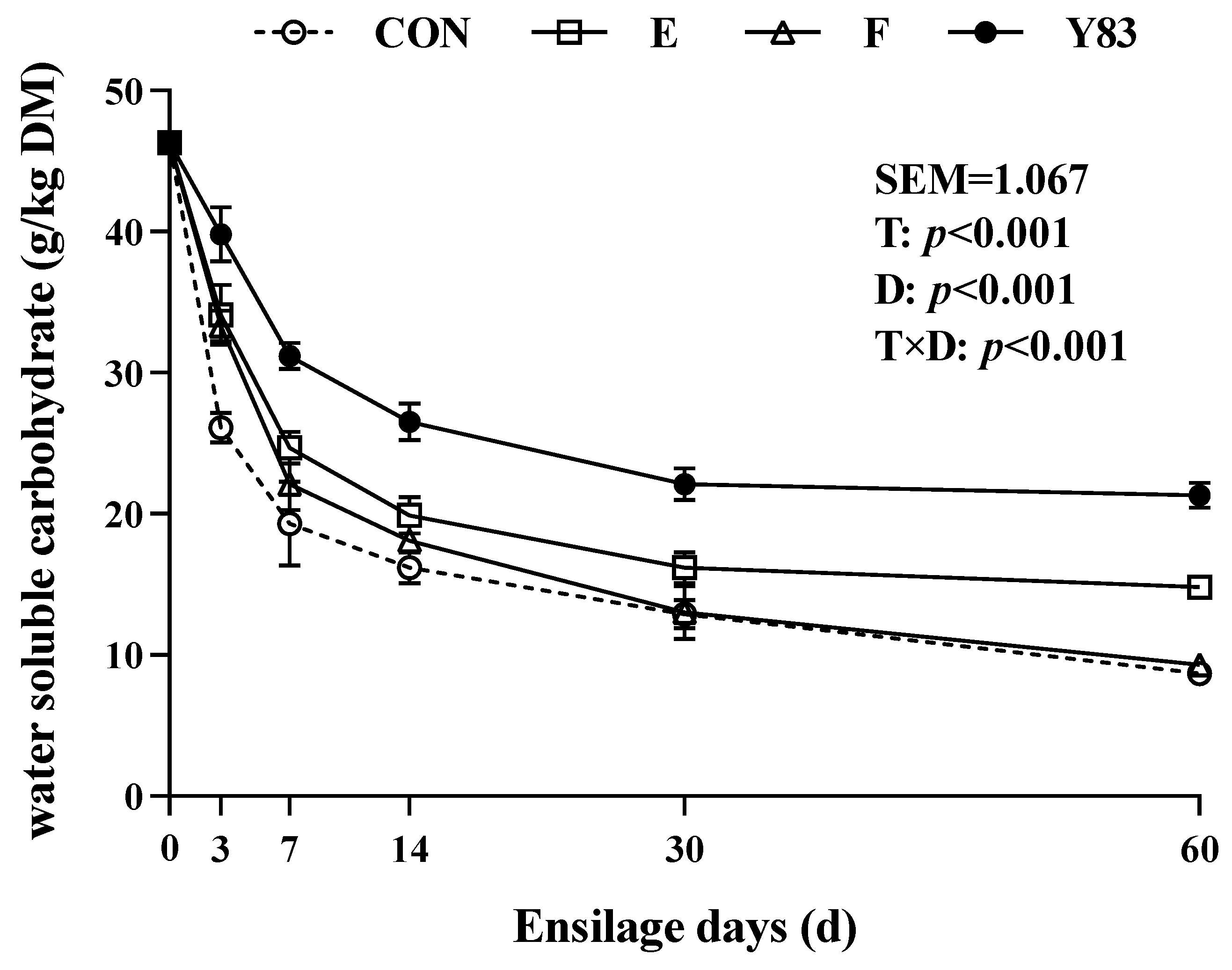
3.3. Carbohydrate Compositions of Rice Straw Silage
3.4. In Vitro Gas Production and Digestibility
4. Discussion
4.1. Fermentation Characteristics of Rice Straw Silage
4.2. Carbohydrate Compositions of Rice Straw Silage
4.3. In Vitro Gas Production and Digestibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Srivastava, M.; Shukla, A. Environmental sustainability of bioethanol production from rice straw in India: A review. Renew. Sustain. Energy Rev. 2016, 54, 202–216. [Google Scholar] [CrossRef]
- Gao, L.; Yang, H.; Wang, X.; Huang, Z.; Masaharu, I.; Yasuo, I.; Cui, Z. Rice straw fermentation using lactic acid bacteria. Bioresour. Technol. 2008, 99, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, Z.; Li, J.; Chen, L.; Shao, T. Pediococcus acidilacticistrains as silage inoculants for improving the fermentation quality, nutritive value and in vitro ruminal digestibility in different forages. J. Appl. Microbiol. 2018, 126, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Chen, L.; Han, L. The effect of an inoculant and enzymes on fermentation and nutritive value of sorghum straw silages. Bioresour. Technol. 2009, 100, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.B.; Adesogan, A.T.; Krueger, N.; Littell, R.C. Effect of fibrolytic enzymes on the fermentation characteristics, aerobic stability, and digestibility of bermudagrass silage. J. Dairy Sci. 2005, 88, 994–1003. [Google Scholar] [CrossRef]
- Gandra, J.R.; Miranda, G.A.; Goes, R.H.T.B.; Takiya, C.S.; Valle, T.A.D.; Oliveira, E.R.; Junior, J.E.F.; Gandra, E.R.S.; Araki, H.M.C.; Santos, A.L.A.V. Fibrolytic enzyme supplementation through ruminal bolus on eating behavior, nutrient digestibility and ruminal fermentation in Jersey heifers fed either corn silage- or sugarcane silage-based diets. Anim. Feed Sci. Tech. 2017, 231, 29–37. [Google Scholar] [CrossRef]
- Vicini, J.L.; Bateman, H.G.; Bhat, M.K.; Clark, J.H.; Erdman, R.A.; Phipps, R.H.; Amburgh, M.E.V.; Hartnell, G.F.; Hintz, R.L.; Hard, D.L. Effect of feeding supplemental fibrolytic enzymes or soluble sugars with malic acid on milk production. J. Dairy Sci. 2003, 86, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.L.; Roche, C.D. Effects of strain and fermentation conditions on production of cellulase by trichoderma reesei. Biotechnol. Bioeng. 1989, 33, 650–656. [Google Scholar] [CrossRef]
- Ren, H.; Richard, T.L.; Moore, K.J. The impact of enzyme characteristics on corn stover fiber degradation and acid production during ensiled storage. Appl. Biochem. Biotech. 2007, 137, 221–238. [Google Scholar]
- Li, J.; Yuan, X.; Desta, S.T.; Dong, Z.; Mugabe, W.; Shao, T. Characterization of Enterococcus faecalis JF85 and Enterococcus faecium Y83 isolated from Tibetan yak (Bos grunniens) for ensiling Pennisetum sinese. Bioresour. Technol. 2018, 257, 76–83. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, C.; Cheng, Y.; Zhang, R.; Jenkins, B.; VanderGheynst, J.S. Effects of ensilage on storage and enzymatic degradability of sugar beet pulp. Bioresour. Technol. 2010, 102, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and In Vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Han, Z.; Wang, S.; Dai, T.; Dong, D.; Zong, C.; Yin, X.; Jia, Y.; Shao, T. Soy sauce residue in total mixed ration silage: Fermentation characteristics, chemical compositions, in vitro digestibility and gas production. Ital. J. Anim. Sci. 2022, 21, 1058–1066. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, W.; Huang, L.; Ding, C.; Dai, C. The effect of lactic acid bacterial starter culture and chemical additives on wilted rice straw silage. Anim. Sci. J. 2015, 87, 525–535. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Wang, S.; Zhao, J.; Dong, Z.; Zhao, Q.; Xu, Y.; Pan, X.; Shao, T. Effect of sorbic acid, ethanol, molasses, previously fermented juice and combined additives on ensiling characteristics and nutritive value of napier grass (Pennisetum purpureum) silage. Fermentation 2022, 8, 528. [Google Scholar] [CrossRef]
- Wang, S.; Guo, G.; Li, J.; Chen, L.; Dong, Z.; Shao, T. Improvement of fermentation profile and structural carbohydrate compositions in mixed silages ensiled with fibrolytic enzymes, molasses and Lactobacillus plantarum MTD-1. Ital. J. Anim. Sci. 2018, 18, 328–335. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Ensiling as pretreatment of rice straw: The effect of hemicellulase and Lactobacillus plantarum on hemicellulose degradation and cellulose conversion. Bioresour. Technol. 2018, 266, 158–165. [Google Scholar] [CrossRef]
- Mcdonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Cambrian Printers Ltd.: Wales, UK, 1991; pp. 184–236. [Google Scholar]
- Li, J.; Yuan, X.; Dong, Z.; Mugabe, W.; Shao, T. The effects of fibrolytic enzymes, cellulolytic fungi and bacteria on the fermentation characteristics, structural carbohydrates degradation, and enzymatic conversion yields of Pennisetum sinese silage. Bioresour. Technol. 2018, 264, 123–130. [Google Scholar] [CrossRef]
- Reich, L.J.; Kung, L. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Dewar, W.A.; McDonald, P.; Whittenbury, R. The hydrolysis of grass hemicelluloses during ensilage. J. Sci. Food Agric. 1963, 14, 411–417. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Yu, C.; Li, J.; Tao, X.; Chen, S.; Shao, T. Nutritional evaluation of wet brewers’ grains as substitute for common vetch in ensiled total mixed ration. Ital. J. Anim. Sci. 2020, 19, 1015–1025. [Google Scholar] [CrossRef]
- Li, J.; Ding, H.; Zhao, J.; Wang, S.; Dong, Z.; Shao, T. Characterization and identification of a novel microbial consortium M2 and its effect on fermentation quality and enzymatic hydrolysis of sterile rice straw. J. Appl. Microbiol. 2021, 132, 1687–1699. [Google Scholar] [CrossRef]
- Lee, S.M.; Guan, L.L.; Eun, J.-S.; Kim, C.-H.; Lee, S.J.; Kim, E.T.; Lee, S.S. The effect of anaerobic fungal inoculation on the fermentation characteristics of rice straw silages. J. Appl. Microbiol. 2015, 118, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2008, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yuan, X.; Desta, S.T.; Zhang, J.; Shao, T. Effects of Lactobacillus plantarum and fibrolytic enzyme on the fermentation quality and in vitro digestibility of total mixed rations silage including rape straw. J. Integr. Agric. 2016, 15, 2087–2096. [Google Scholar] [CrossRef]
- Tao, L.; Yu, Z.; Guo, X.; Zhou, H. Ensiling and in vitro digestibility characteristics of Ceratoides arborescens treated with lactic acid bacteria inoculants and cellulase. Afr. J. Biotechnol. 2011, 10, 14947–14953. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Effects of sugar sources and doses on fermentation dynamics, carbohydrates changes, in vitro digestibility and gas production of rice straw silage. Ital. J. Anim. Sci. 2019, 18, 1345–1355. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, C.; Zhang, L.; Ning, G.; Shi, W.; Zhang, X.; Yang, Z. Ligninolytic enzyme involved in removal of high molecular weight polycyclic aromatic hydrocarbons by Fusarium strain ZH-H2. Environ. Sci. Pollut. R. 2020, 27, 42969–42978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Effects of lactic acid bacteria and molasses on fermentation dynamics, structural and nonstructural carbohydrate composition and in vitro ruminal fermentation of rice straw silage. Asian-Australas J. Anim. Sci. 2019, 32, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cai, Y.; Takahashi, T.; Yoshida, N.; Tohno, M.; Uegaki, R.; Nonaka, K.; Terada, F. Effect of lactic acid bacteria inoculant and beet pulp addition on fermentation characteristics and in vitro ruminal digestion of vegetable residue silage. J. Dairy Sci. 2011, 94, 3902–3912. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Weisbjerg, M.R.; Hvelplund, T.; Kristensen, N.B. Effect of enzyme addition to forage at ensiling on silage chemical composition and NDF degradation characteristics. Livest. Sci. 2012, 150, 51–58. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Dong, Z.; Chen, L.; Shao, T. Inclusion of alfalfa improves nutritive value and in vitro digestibility of various straw-grass mixed silages in Tibet. Grass Forage Sci. 2018, 73, 694–704. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A.; Rode, L.M.; Beauchemin, K.A.; Morgavi, D.P.; Nsereko, V.L.; Iwaasa, A.D.; Yang, W. Effects of an exogenous enzyme preparation on microbial protein synthesis, enzyme activity and attachment to feed in the Rumen Simulation Technique (Rusitec). Brit. J. Nutr. 2001, 85, 325–332. [Google Scholar] [CrossRef] [PubMed]

| Items | Rice Straw |
|---|---|
| Dry matter (g/kg FW) | 411 ± 3.61 |
| Crude protein | 61.2 ± 2.74 |
| Water soluble carbohydrate | 46.3 ± 1.25 |
| Neutral detergent fibre | 715 ± 6.24 |
| Acid detergent fibre | 432 ± 5.29 |
| Acid detergent lignin | 65.8 ± 0.70 |
| Cellulose | 366 ± 5.29 |
| Hemicellulose | 283 ± 5.20 |
| Items | Treatments 1 | Ensiling Days (d) | SEM | p-Value 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 30 | 60 | T | D | T × D | |||
| Dry matter (g/kg FW) | CON | 397 | 400 | 397 | 391 | 380 | 1.60 | ns | <0.001 | ns |
| E | 408 | 405 | 402 | 392 | 384 | |||||
| F | 409 | 404 | 398 | 396 | 386 | |||||
| Y83 | 410 | 406 | 403 | 397 | 391 | |||||
| pH | CON | 4.56 | 4.52 a | 4.55 a | 4.43 a | 4.41 a | 0.025 | <0.001 | ns | ns |
| E | 4.51 | 4.49 a | 4.46 a | 4.38 ab | 4.36 a | |||||
| F | 4.56 | 4.49 a | 4.43 a | 4.35 ab | 4.34 a | |||||
| Y83 | 4.31 | 4.20 b | 4.18 b | 4.16 b | 4.15 b | |||||
| Lactic acid | CON | 20.3 | 22.8 b | 27.2 b | 24.3 d | 19.6 c | 1.67 | <0.001 | <0.001 | <0.001 |
| E | 21.0 | 23.6 b | 25.4 b | 34.1 c | 29.8 bc | |||||
| F | 21.3 | 25.5 b | 27.1 b | 37.6 b | 32.2 b | |||||
| Y83 | 26.0 | 41.3 a | 56.3 a | 61.6 a | 51.7 a | |||||
| Acetic acid | CON | 10.5 a | 11.9 | 12.6 | 16.5 a | 21.2 a | 0.502 | <0.001 | <0.001 | ns |
| E | 8.66 ab | 9.99 | 11.2 | 12.5 ab | 17.4 ab | |||||
| F | 7.61 ab | 9.45 | 11.3 | 11.5 ab | 13.2 b | |||||
| Y83 | 6.43 b | 8.85 | 9.01 | 10.1 b | 12.7 b | |||||
| Propionic acid | CON | 0.50 | 0.61 | 0.72 | 0.77 | 1.13 | 0.054 | <0.001 | <0.001 | <0.001 |
| E | 0.55 | 0.72 | 0.90 | 0.97 | 1.02 | |||||
| F | ND | ND | 0.68 | 0.79 | 1.02 | |||||
| Y83 | ND | ND | ND | ND | ND | |||||
| Butyric acid | CON | 0.42 | 0.55 | 0.73 | 0.86 | 1.21 | 0.150 | <0.001 | <0.001 | <0.001 |
| E | 0.49 | 0.78 | 0.87 | 0.85 | 1.16 | |||||
| F | ND | ND | ND | 0.60 | 0.91 | |||||
| Y83 | ND | ND | ND | 0.56 | 0.86 | |||||
| NH3-N (g/kg TN) | CON | 60.1 a | 74.6 a | 78.3 a | 84.3 a | 93.4 a | 1.93 | <0.001 | <0.001 | ns |
| E | 57.7 a | 61.1 ab | 64.6 ab | 66.8 b | 77.3 ab | |||||
| F | 39.7 b | 56.3 b | 54.3 b | 60.6 b | 69.9 b | |||||
| Y83 | 29.7 c | 39.3 c | 59.3 b | 65.0 b | 69.8 b | |||||
| Items | Treatments 1 | Ensiling Days (d) | SEM | p-Value 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 30 | 60 | T | D | T × D | |||
| NDF | CON | 702 a | 698 a | 693 a | 691 a | 687 a | 1.42 | <0.001 | <0.001 | ns |
| E | 691 b | 685 b | 678 b | 675 b | 670 b | |||||
| F | 688 b | 683 b | 676 b | 673 b | 669 b | |||||
| Y83 | 688 b | 685 b | 682 b | 676 b | 660 c | |||||
| ADF | CON | 425 | 423 a | 421 a | 420 a | 414 a | 1.18 | <0.001 | <0.001 | ns |
| E | 419 | 412 b | 415 b | 408 b | 400 b | |||||
| F | 424 | 417 ab | 412 b | 408 b | 401 b | |||||
| Y83 | 419 | 419 ab | 417 ab | 412 b | 397 b | |||||
| ADL | CON | 64.2 | 63.6 | 63.1 | 63.6 a | 64.5 a | 0.29 | <0.001 | ns | ns |
| E | 62.5 | 61.4 | 61.7 | 61.7 ab | 61.2 a | |||||
| F | 61.7 | 61.8 | 61.6 | 60.4 b | 60.5 b | |||||
| Y83 | 61.0 | 59.9 | 59.4 | 59.8 b | 58.1 b | |||||
| Hemicellulose | CON | 277 | 274 | 272 | 271 | 272 | 1.04 | 0.011 | ns | ns |
| E | 272 | 273 | 263 | 266 | 269 | |||||
| F | 264 | 266 | 264 | 265 | 267 | |||||
| Y83 | 268 | 266 | 264 | 264 | 263 | |||||
| Cellulose | CON | 361 | 360 | 357 | 356 a | 350 a | 1.12 | 0.012 | <0.001 | ns |
| E | 356 | 351 | 353 | 346 b | 339 b | |||||
| F | 362 | 355 | 350 | 347 b | 341 b | |||||
| Y83 | 358 | 359 | 358 | 352 ab | 338 b | |||||
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | E | F | Y83 | |||
| Potential gas production, b (mL) | 45.6 c | 61.2 a | 54.5 b | 64.0 a | 2.26 | <0.001 |
| Gas production rate constant, c (mL/h) | 0.076 | 0.056 | 0.063 | 0.054 | 0.0037 | ns |
| GP24 (mL) | 35.3 b | 41.7 ab | 39.7 ab | 43.3 a | 1.12 | 0.034 |
| In vitro dry matter degradability (%) | 49.4 b | 58.5 a | 48.4 b | 58.8 a | 1.70 | 0.007 |
| In vitro neutral detergent fibre (%) | 44.6 b | 53.4 a | 45.1 b | 55.5 a | 1.56 | <0.001 |
| In vitro acid detergent fibre (%) | 38.5 b | 49.3 a | 40.7 b | 50.1 a | 1.87 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Han, Z.; Li, J.; Li, X.; Dong, Z.; Zhao, J.; Wang, S.; Shao, T. Effect of Fibrolytic Enzymes, Cellulolytic Fungi and Lactic Acid Bacteria on Fermentation Characteristics, Structural Carbohydrate Composition and In Vitro Digestibility of Rice Straw Silage. Fermentation 2022, 8, 709. https://doi.org/10.3390/fermentation8120709
Ding H, Han Z, Li J, Li X, Dong Z, Zhao J, Wang S, Shao T. Effect of Fibrolytic Enzymes, Cellulolytic Fungi and Lactic Acid Bacteria on Fermentation Characteristics, Structural Carbohydrate Composition and In Vitro Digestibility of Rice Straw Silage. Fermentation. 2022; 8(12):709. https://doi.org/10.3390/fermentation8120709
Chicago/Turabian StyleDing, Hao, Zhe Han, Junfeng Li, Xinbao Li, Zhihao Dong, Jie Zhao, Siran Wang, and Tao Shao. 2022. "Effect of Fibrolytic Enzymes, Cellulolytic Fungi and Lactic Acid Bacteria on Fermentation Characteristics, Structural Carbohydrate Composition and In Vitro Digestibility of Rice Straw Silage" Fermentation 8, no. 12: 709. https://doi.org/10.3390/fermentation8120709
APA StyleDing, H., Han, Z., Li, J., Li, X., Dong, Z., Zhao, J., Wang, S., & Shao, T. (2022). Effect of Fibrolytic Enzymes, Cellulolytic Fungi and Lactic Acid Bacteria on Fermentation Characteristics, Structural Carbohydrate Composition and In Vitro Digestibility of Rice Straw Silage. Fermentation, 8(12), 709. https://doi.org/10.3390/fermentation8120709








