Enhancing Dark Fermentative Hydrogen Production from Problematic Substrates via the Co-Fermentation Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Experimental Setup and Operating Conditions
2.3. Analytical Methods
3. Results
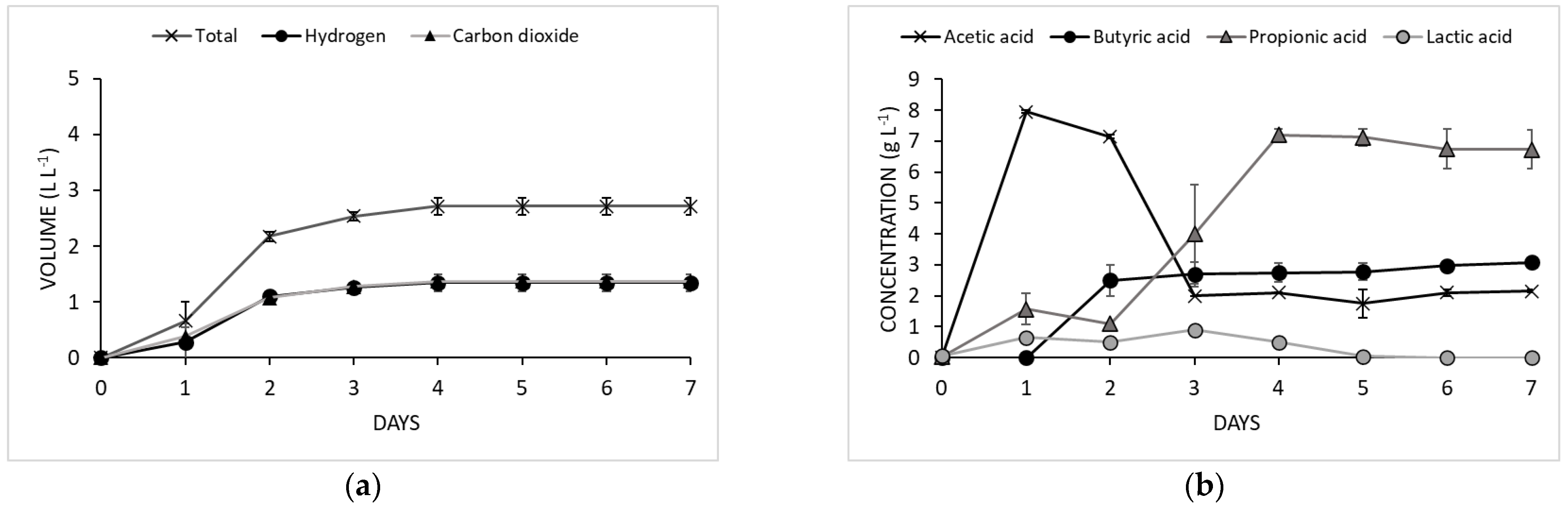
3.1. Effect of Olive Mill Wastewater and Cheese Whey on Dark Fermentation
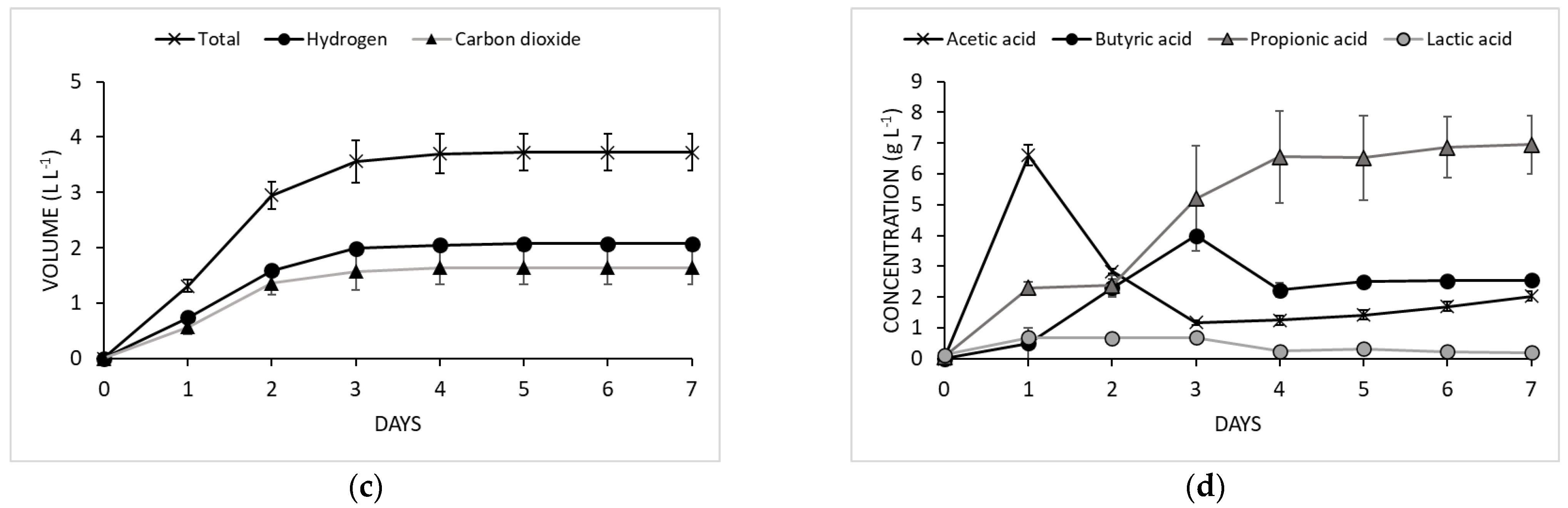
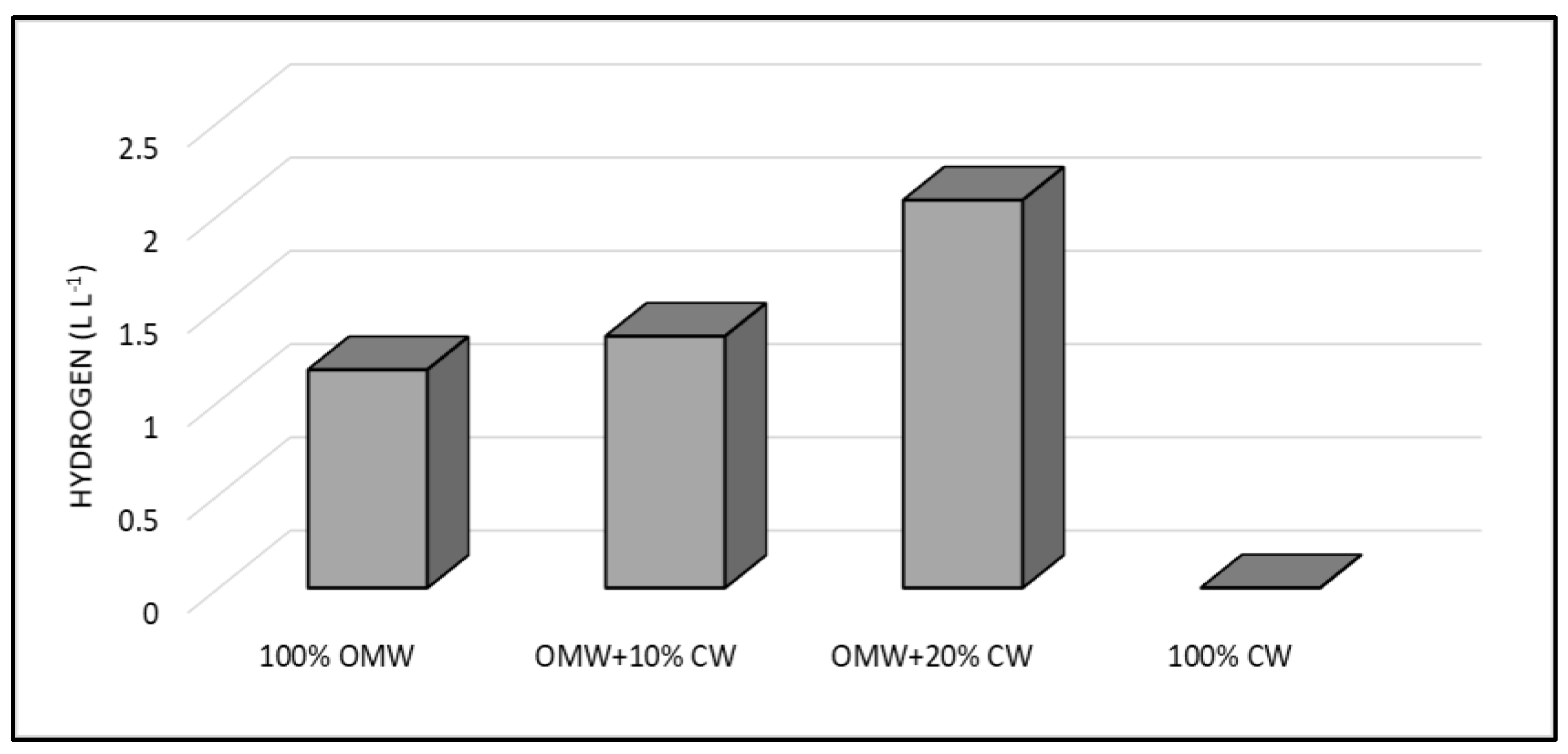
3.2. Co-Fermentation Strategy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Carbon Catabolite Repression Occurrence in Photo Fermentation of Ethanol-Rich Substrates. J. Environ. Manage. 2021, 297, 113371. [Google Scholar] [CrossRef] [PubMed]
- Perna, V.; Castelló, E.; Wenzel, J.; Zampol, C.; Fontes Lima, D.M.; Borzacconi, L.; Varesche, M.B.; Zaiat, M.; Etchebehere, C. Hydrogen Production in an Upflow Anaerobic Packed Bed Reactor Used to Treat Cheese Whey. Int. J. Hydrogen Energy 2013, 38, 54–62. [Google Scholar] [CrossRef]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of Operational Parameters on Dark Fermentative Hydrogen Production from Biodegradable Complex Waste Biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen Production from Biomass Using Dark Fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for Olive Mill Wastewater (OMW) Treatment: A Review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Ibarruri, J.; Hernández, I. Valorization of Cheese Whey and Orange Molasses for Fungal Biomass Production by Submerged Fermentation with Rhizopus Sp. Bioprocess Biosyst. Eng. 2019, 42, 1285–1300. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-Fermentative Hydrogen Production from Cheese Whey: Engineering of a Mixed Culture Process in a Semi-Continuous, Tubular Photo-Bioreactor. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Dessì, P.; Asunis, F.; Ravishankar, H.; Cocco, F.G.; De Gioannis, G.; Muntoni, A.; Lens, P.N.L. Fermentative Hydrogen Production from Cheese Whey with In-Line, Concentration Gradient-Driven Butyric Acid Extraction. Int. J. Hydrogen Energy 2020, 45, 24453–24466. [Google Scholar] [CrossRef]
- Ramos, L.R.; Silva, E.L. Continuous Hydrogen Production from Cofermentation of Sugarcane Vinasse and Cheese Whey in a Thermophilic Anaerobic Fluidized Bed Reactor. Int. J. Hydrogen Energy 2018, 43, 13081–13089. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, R.; El-Mashad, H.M.; Sun, H.; Ying, Y. Effect of Food to Microorganism Ratio on Biohydrogen Production from Food Waste via Anaerobic Fermentation. Int. J. Hydrogen Energy 2008, 33, 6968–6975. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Green Hydrogen and Platform Chemicals Production from Acidogenic Conversion of Brewery Spent Grains Co-Fermented with Cheese Whey Wastewater: Adding Value to Acidogenic CO2. Sustain. Energy Fuels 2022, 6, 778–790. [Google Scholar] [CrossRef]
- Basak, B.; Fatima, A.; Jeon, B.H.; Ganguly, A.; Chatterjee, P.K.; Dey, A. Process Kinetic Studies of Biohydrogen Production by Co-Fermentation of Fruit-Vegetable Wastes and Cottage Cheese Whey. Energy Sustain. Dev. 2018, 47, 39–52. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Continuous Biohydrogen Production by Thermophilic Dark Fermentation of Cheese Whey: Use of Buffalo Manure as Buffering Agent. Int. J. Hydrogen Energy 2017, 42, 4861–4869. [Google Scholar] [CrossRef]
- Goberna, M.; Schoen, M.A.; Sperl, D.; Wett, B.; Insam, H. Mesophilic and Thermophilic Co-Fermentation of Cattle Excreta and Olive Mill Wastes in Pilot Anaerobic Digesters. Biomass and Bioenergy 2010, 34, 340–346. [Google Scholar] [CrossRef]
- Marques, I.P. Anaerobic Digestion Treatment of Olive Mill Wastewater for Effluent Re-Use in Irrigation. Desalination 2001, 137, 233–239. [Google Scholar] [CrossRef]
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.; Fabbricino, M. Repeated-Batch Fermentation of Cheese Whey for Semi-Continuous Lactic Acid Production Using Mixed Cultures at Uncontrolled PH. Sustainability 2019, 11, 3330. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Clesceri, L.S. American Public Health Association (APHA) Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005; ISBN 0875532357. [Google Scholar]
- Liu, Y. Bioenergetic Interpretation on the S0/X0 Ratio in Substrate-Sufficient Batch Culture. Water Res. 1996, 30, 2766–2770. [Google Scholar] [CrossRef]
- Argun, H.; Dao, S. Bio-Hydrogen Production from Waste Peach Pulp by Dark Fermentation: Effect of Inoculum Addition. Int. J. Hydrogen Energy 2017, 42, 2569–2574. [Google Scholar] [CrossRef]
- Alsafadi, D.; Al-Mashaqbeh, O. A One-Stage Cultivation Process for the Production of Poly-3-(Hydroxybutyrate-Co-Hydroxyvalerate) from Olive Mill Wastewater by Haloferax Mediterranei. N. Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.H.; Li, L.; Sung, S. Biological Hydrogen Production: Effects of PH and Intermediate Products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Itoh, Y.; Tada, K.; Kanno, T.; Horiuchi, J.I. Selective Production of Lactic Acid in Continuous Anaerobic Acidogenesis by Extremely Low PH Operation. J. Biosci. Bioeng. 2012, 114, 537–539. [Google Scholar] [CrossRef]
- Choi, G.; Kim, J.; Lee, C. Effect of Low PH Start-up on Continuous Mixed-Culture Lactic Acid Fermentation of Dairy Effluent. Appl. Microbiol. Biotechnol. 2016, 100, 10179–10191. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Lamia, A.; Moktar, H. Fermentative Decolorization of Olive Mill Wastewater by Lactobacillus Plantarum. Process Biochem. 2003, 39, 59–65. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Cheese Whey Fermentation into Volatile Fatty Acids in an Anaerobic Sequencing Batch Reactor. Bioresour. Technol. 2020, 308, 123226. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Mugnai, G.; Borruso, L.; Mimmo, T.; Cesco, S.; Luongo, V.; Frunzo, L.; Fabbricino, M.; Pirozzi, F.; Cappitelli, F.; Villa, F. Dynamics of Bacterial Communities and Substrate Conversion during Olive-Mill Waste Dark Fermentation: Prediction of the Metabolic Routes for Hydrogen Production. Bioresour. Technol. 2021, 319, 124157. [Google Scholar] [CrossRef]
- Eroǧlu, E.; Eroǧlu, I.; Gündüz, U.; Yücel, M. Effect of Clay Pretreatment on Photofermentative Hydrogen Production from Olive Mill Wastewater. Bioresour. Technol. 2008, 99, 6799–6808. [Google Scholar] [CrossRef]
- Policastro, G.; Carraturo, F.; Compagnone, M.; Giugliano, M.; Guida, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. A Preliminary Study on a Novel Bioaugmentation Technique Enhancing Lactic Acid Production by Mixed Cultures Fermentation. Bioresour. Technol. 2021, 340, 125595. [Google Scholar] [CrossRef]


| Sample | TS 3 (gL−1) | VS 4 (gL−1) | COD (gCODL−1) |
|---|---|---|---|
| OMW 1 | 39 ± 2 | 32 ± 1 | 71 ± 3 |
| CW 2 | 52 ± 2 | 44 ± 3 | 72 ± 8 |
| Digestate | 67 ± 1 | 47 ± 1 | 69 ± 5 |
| SET | Test | F/M | Digestate 2 [L] | OMW 2 [L] | CW 2 [L] | Substrate | CODIN 1 [g L−1] |
|---|---|---|---|---|---|---|---|
| SETOMW | O1 | 1 | 0.16 | 0.24 | - | 100% OMW | 16.9 |
| O2.5 | 2.5 | 0.09 | 0.31 | - | 100% OMW | 22.3 | |
| O5 | 5 | 0.05 | 0.35 | - | 100% OMW | 25 | |
| SETCW | C1 | 1 | 0.19 | - | 0.21 | 100% CW | 14.9 |
| C2.5 | 2.5 | 0.11 | - | 0.29 | 100% CW | 20.9 | |
| C5 | 5 | 0.06 | - | 0.34 | 100% CW | 24.2 | |
| SETCO | CO10 | 2.5 | 0.09 | 0.28 | 0.03 | 90% OMW–10% CW | 22.17 |
| CO20 | 2.5 | 0.09 | 0.25 | 0.06 | 80% OMW–20% CW | 22 |
| Test | Vbiogas [L·L−1] | VH2 [L·L−1] | rH2 [mL·L−1·h−1] | YH2 [mL·gCOD−1] | OAs [g·L−1] | COD Conversion [%] 1 | pH Range [-] |
|---|---|---|---|---|---|---|---|
| O1 | 2.42 ± 0.3 | 1.04 ± 0.31 | 10.80 | 61.53 | 5 ± 1.2 | 35 | 5.6–5.3 |
| O2.5 | 2.34 ± 0.01 | 1.17 ± 0.01 | 11.21 | 52.46 | 13 ± 1.7 | 65 | 5.9–5.6 |
| O5 | 0.76 ± 0.26 | 0.36 ± 0.24 | 2.17 | 14.40 | 15 ± 2.4 | 64 | 6.0–5.4 |
| C1 | 1.61 ± 0.01 | <0.01 | - | - | 14 ± 1.3 | 86 | 4.5–3.9 |
| C2.5 | 3.05 ± 0.30 | <0.01 | - | - | 9 ± 1.3 | 45 | 4.5–3.8 |
| C5 | 1.17 ± 0.02 | <0.01 | - | - | 12 ±2.3 | 61 | 4.4–3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Policastro, G.; Lamboglia, R.; Fabbricino, M.; Pirozzi, F. Enhancing Dark Fermentative Hydrogen Production from Problematic Substrates via the Co-Fermentation Strategy. Fermentation 2022, 8, 706. https://doi.org/10.3390/fermentation8120706
Policastro G, Lamboglia R, Fabbricino M, Pirozzi F. Enhancing Dark Fermentative Hydrogen Production from Problematic Substrates via the Co-Fermentation Strategy. Fermentation. 2022; 8(12):706. https://doi.org/10.3390/fermentation8120706
Chicago/Turabian StylePolicastro, Grazia, Rosetta Lamboglia, Massimiliano Fabbricino, and Francesco Pirozzi. 2022. "Enhancing Dark Fermentative Hydrogen Production from Problematic Substrates via the Co-Fermentation Strategy" Fermentation 8, no. 12: 706. https://doi.org/10.3390/fermentation8120706
APA StylePolicastro, G., Lamboglia, R., Fabbricino, M., & Pirozzi, F. (2022). Enhancing Dark Fermentative Hydrogen Production from Problematic Substrates via the Co-Fermentation Strategy. Fermentation, 8(12), 706. https://doi.org/10.3390/fermentation8120706









