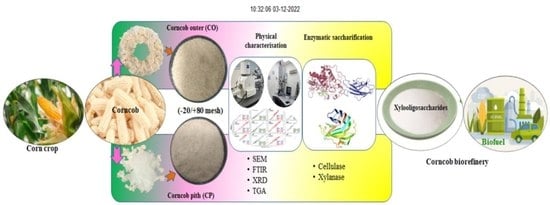
A New Insight into the Composition and Physical Characteristics of Corncob—Substantiating Its Potential for Tailored Biorefinery Objectives
Abstract
1. Introduction
2. Materials and Methods
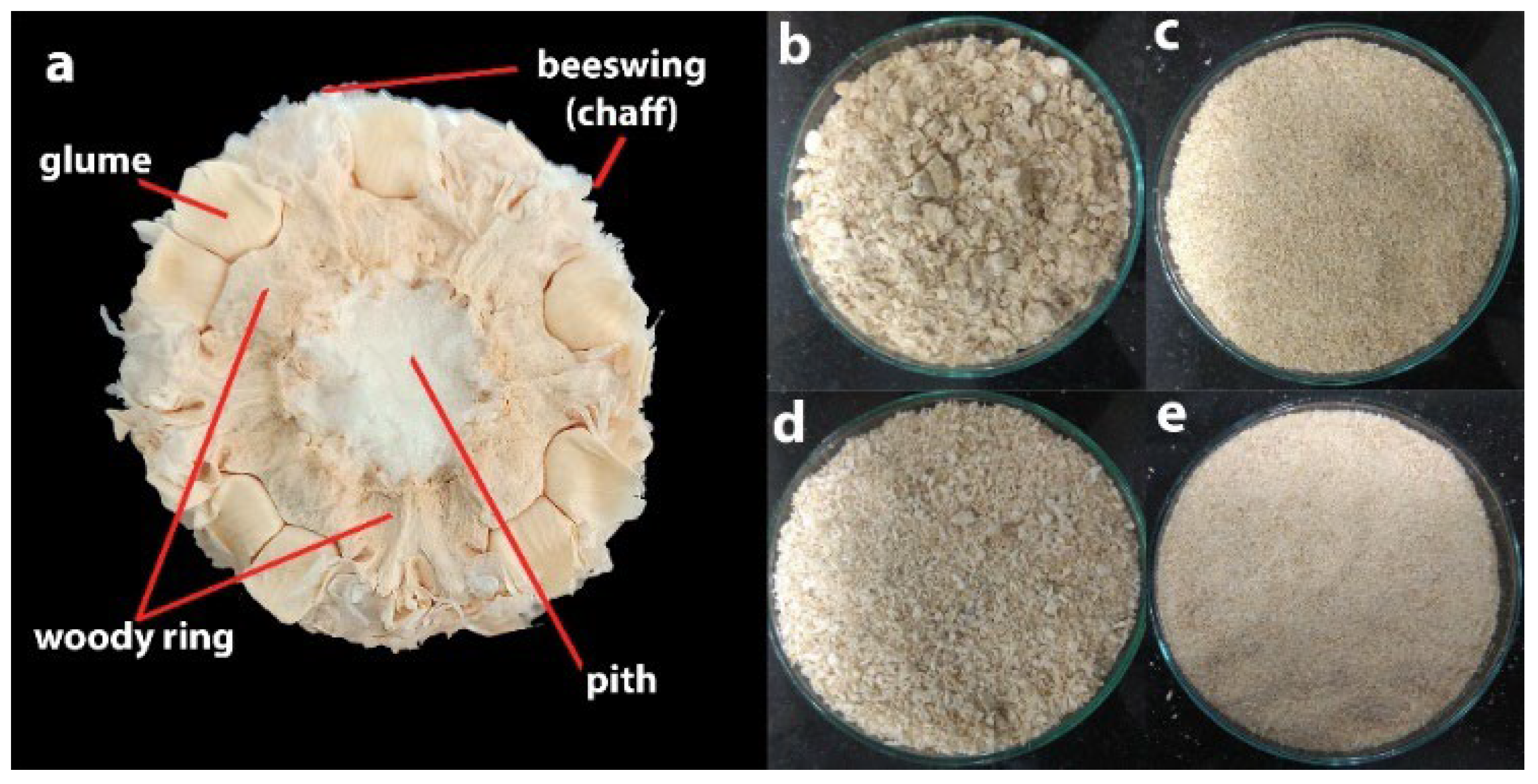
2.1. Sample Selection and Preparation
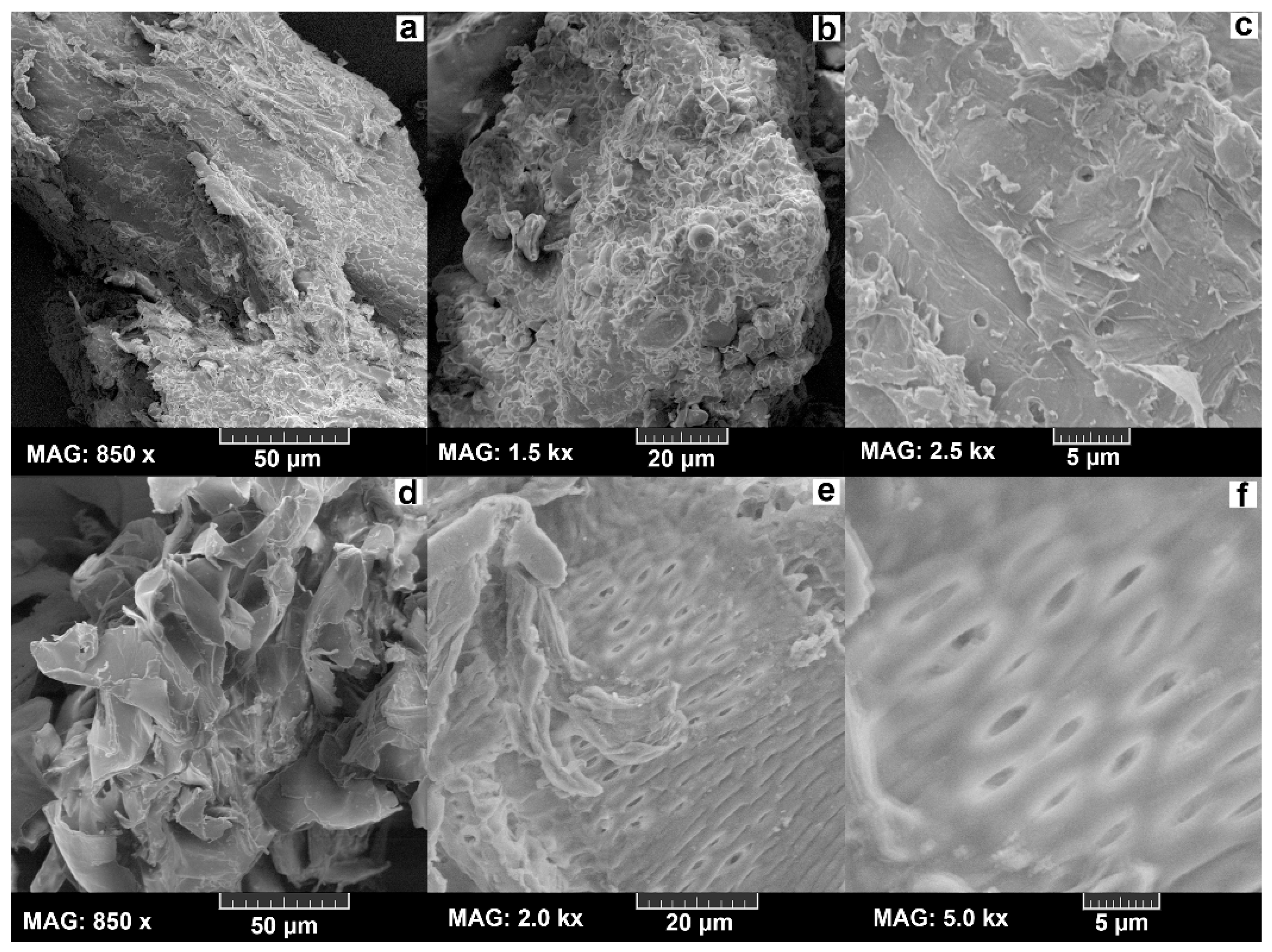
2.2. Scanning Electron Microscopy (SEM) Analysis
2.3. NREL Method for Biomass Composition Analysis
2.4. Van Soest Method for Fiber Analysis
2.5. NIR Spectroscopy Method for Rapid Biomass Composition Analysis
2.6. Thermogravimetric Analysis (TGA)
2.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.8. X-ray Diffraction (XRD) Analysis
2.9. Enzymatic Saccharification of Untreated Corncob Samples
3. Results and Discussion
3.1. SEM Analysis
3.2. NREL Method for Biomass Composition Analysis
3.3. Van Soest Method for Fiber Analysis
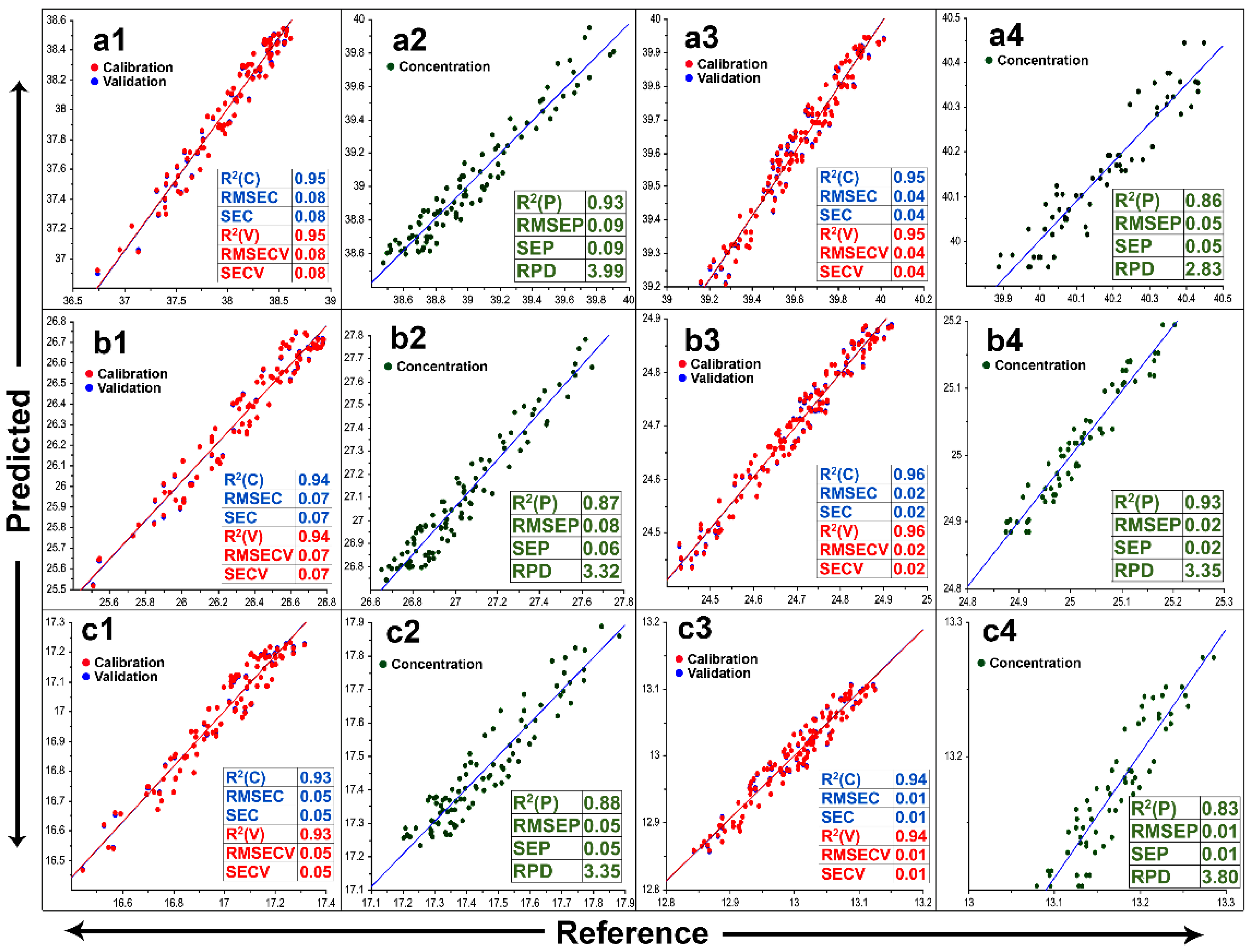
3.4. NIR Method for Rapid Biomass Composition Analysis
3.5. TGA Analysis
3.6. FTIR Analysis
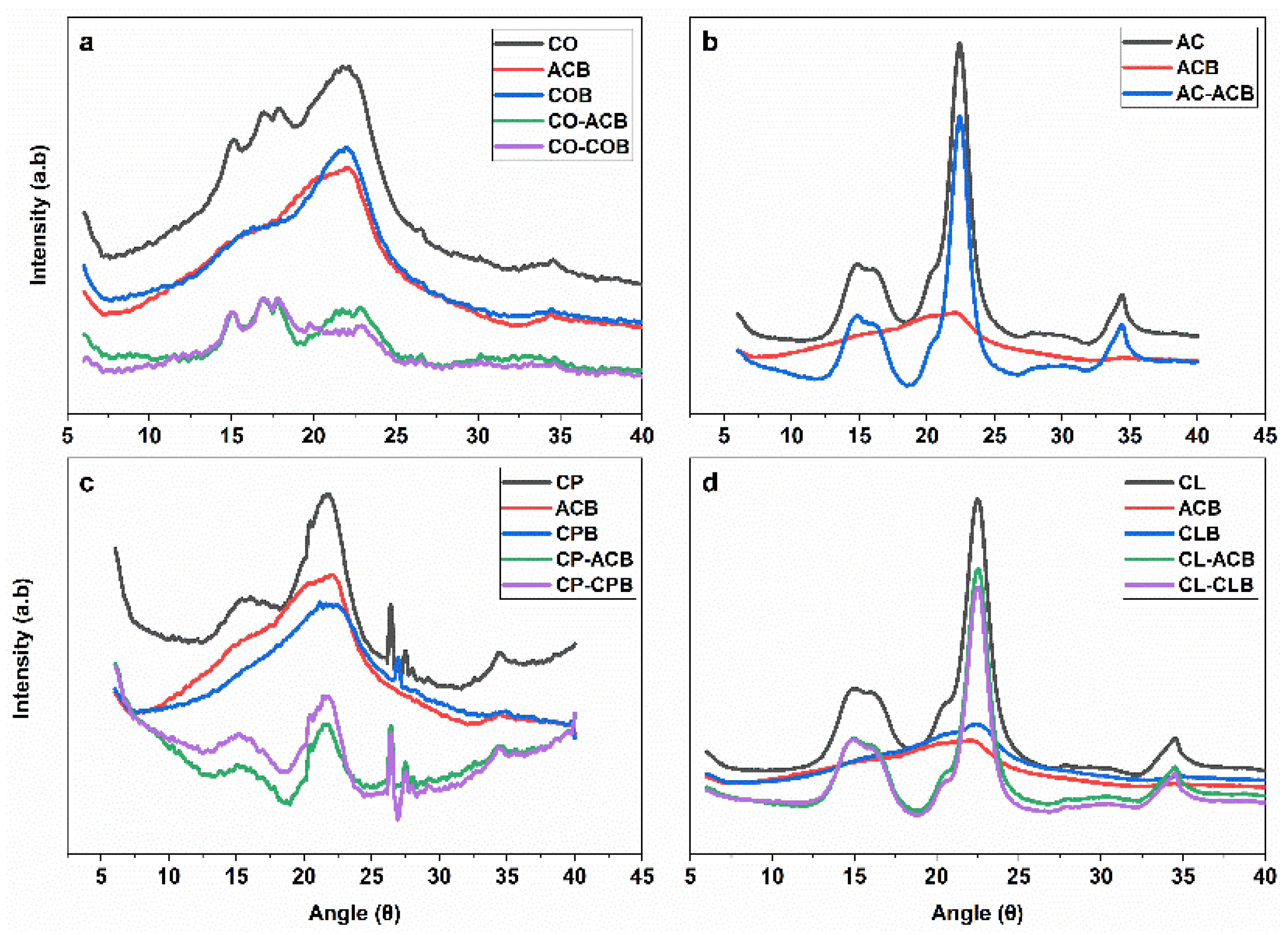
3.7. XRD Analysis
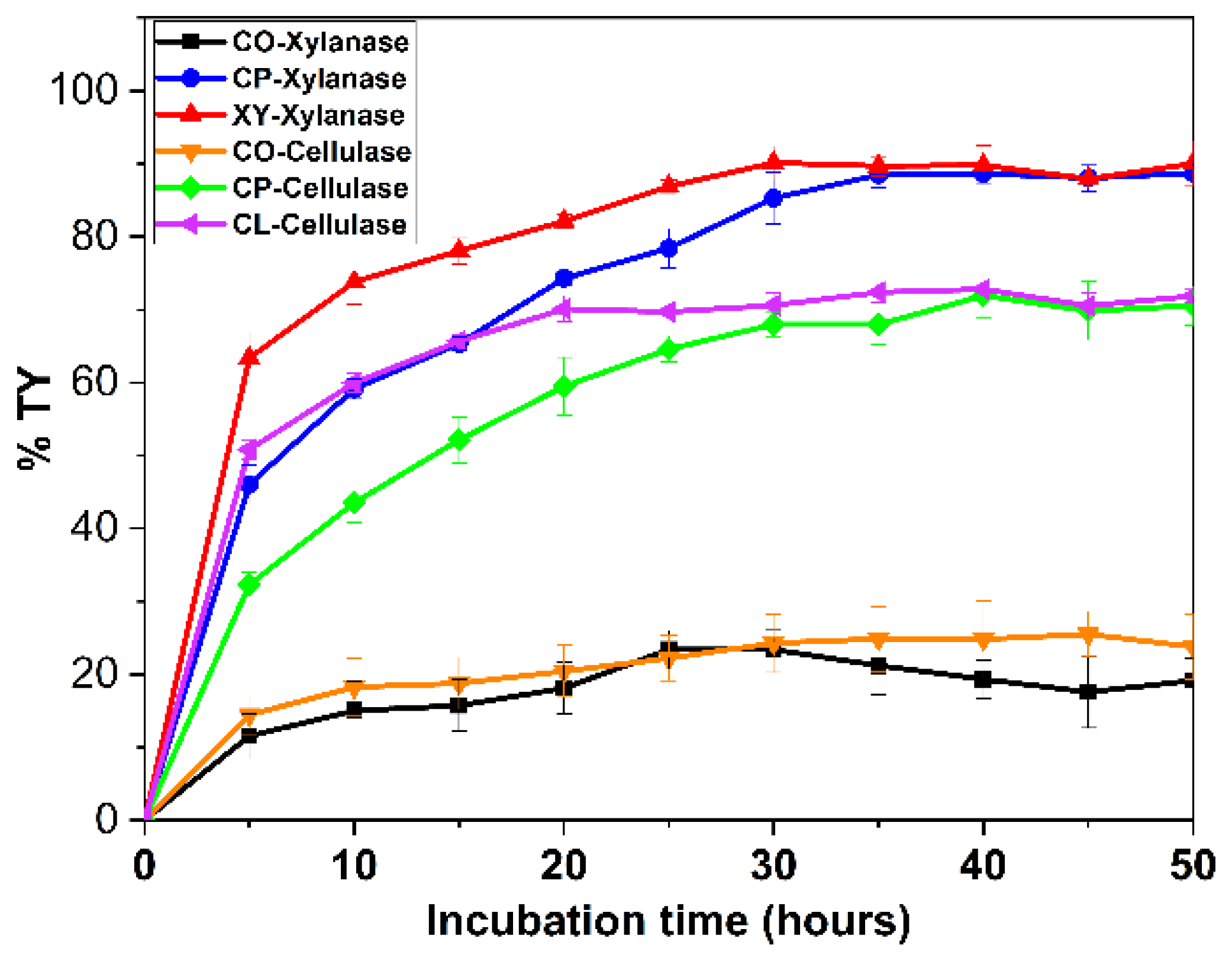
3.8. Enzymatic Saccharification of Untreated Corncob Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Baadhe, R.R.; Sharma, M.; Thakur, V.K.; Sharma, G.D.; Ranjitha, J.; Gupta, V.K. Second-generation bioethanol production from corncob—A comprehensive review on pretreatment and bioconversion strategies, including techno-economic and lifecycle perspective. Ind. Crops Prod. 2022, 186, 115245. [Google Scholar] [CrossRef]
- Bhatt, A.K.; Bhatia, R.K.; Thakur, S.; Rana, N.; Sharma, V.; Rathour, R.K. Fuel from Waste: A Review on Scientific Solution for Waste Management and Environment Conservation BT—Prospects of Alternative Transportation Fuels; Singh, A.P., Agarwal, R.A., Agarwal, A.K., Dhar, A., Shukla, M.K., Eds.; Springer: Singapore, 2018; pp. 205–233. [Google Scholar] [CrossRef]
- Dukhnytskyi, B. World agricultural production. Ekon. APK 2019, 7, 59–65. [Google Scholar] [CrossRef]
- Ioelovich, M. Plant Biomass as a Renewable Source of Biofuels and Biochemicals; LAP LAMBERT Academic Publishing: Sunnyvale, CA, USA, 2013; pp. 1–58. [Google Scholar]
- Qing, Q.; Huang, M.; He, Y.; Wang, L.; Zhang, Y. Dilute Oxalic Acid Pretreatment for High Total Sugar Recovery in Pretreatment and Subsequent Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2015, 177, 1493–1507. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Zhang, J.A.; Ling, H.Z.; Ping, W.X.; Huang, W.; Ge, J.P.; Xu, J.M. Optimization of pH and acetic acid concentration for bioconversion of hemicellulose from corncobs to xylitol by Candida tropicalis. Biochem. Eng. J. 2009, 43, 203–207. [Google Scholar] [CrossRef]
- Hoang, P.H.; Cuong, T.D.; Dien, L.Q. Ultrasound Assisted Conversion of Corncob-Derived Xylan to Furfural Under HSO3-ZSM-5 Zeolite Catalyst. Waste Biomass Valorization 2020, 12, 1955–1962. [Google Scholar] [CrossRef]
- Deng, A.; Ren, J.; Wang, W.; Li, H.; Lin, Q.; Yan, Y.; Sun, R.; Liu, G. Production of xylo-sugars from corncob by oxalic acid-assisted ball milling and microwave-induced hydrothermal treatments. Ind. Crops Prod. 2016, 79, 137–145. [Google Scholar] [CrossRef]
- Zhang, P.; Liao, X.; Ma, C.; Li, Q.; Li, A.; He, Y. Chemoenzymatic Conversion of Corncob to Furfurylamine via Tandem Catalysis with Tin-Based Solid Acid and Transaminase Biocatalyst. ACS Sustain. Chem. Eng. 2019, 7, 17636–17642. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Ma, C.L.; Shen, Y.F.; Sun, J.F.; Jiang, K.; Jiang, Z.B.; Dai, Y.J.; He, Y.C. Enhanced Biosynthesis of Furoic Acid via the Effective Pretreatment of Corncob into Furfural in the Biphasic Media. Catal. Lett. 2020, 150, 2220–2227. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, C.; Gao, C.; Xu, P. Efficient utilization of hemicellulose hydrolysate for propionic acid production using Propionibacterium acidipropionici. Bioresour. Technol. 2012, 114, 711–714. [Google Scholar] [CrossRef]
- Wang, C.; Yang, G.; Zhang, X.; Shao, L.; Lyu, G.; Mao, J.; Liu, S.; Xu, F. A kinetic study on the hydrolysis of corncob residues to levulinic acid in the FeCl3–NaCl system. Cellulose 2019, 26, 8313–8323. [Google Scholar] [CrossRef]
- Guo, W.; Jia, W.; Li, Y.; Chen, S. Performances of Lactobacillus brevis for producing lactic acid from hydrolysate of lignocellulosics. Appl. Biochem. Biotechnol. 2010, 161, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, X.; Zhu, L.; Huang, H.; Wang, T.; Jiang, L. Pretreatment with γ-Valerolactone/[Mmim]DMP and Enzymatic Hydrolysis on Corncob and Its Application in Immobilized Butyric Acid Fermentation. J. Agric. Food Chem. 2018, 66, 11709–11717. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wang, Y.; Tu, G.; Zan, Z.; Wu, X. Adaptation and transcriptome analysis of Aureobasidium pullulans in corncob hydrolysate for increased inhibitor tolerance to malic acid production. PLoS ONE 2015, 10, e0121416. [Google Scholar] [CrossRef]
- Brar, K.K.; Kaur, S.; Chadha, B.S. A novel staggered hybrid SSF approach for efficient conversion of cellulose/hemicellulosic fractions of corncob into ethanol. Renew. Energy 2016, 98, 16–22. [Google Scholar] [CrossRef]
- Marchal, R.; Ropars, M.; Pourquié, J.; Fayolle, F.; Vandecasteele, J.P. Large-scale enzymatic hydrolysis of agricultural lignocellulosic biomass. Part 2: Conversion into acetone-butanol. Bioresour. Technol. 1992, 42, 205–217. [Google Scholar] [CrossRef]
- Ma, L.; Ma, Q.; Guo, G.; Du, L.; Zhang, Y.; Cui, Y.; Xiao, D. Optimization of sodium percarbonate pretreatment for improving 2,3-butanediol production from corncob. Prep. Biochem. Biotechnol. 2018, 48, 218–225. [Google Scholar] [CrossRef]
- Shah, A.R.; Madamwar, D. Xylanase production under solid-state fermentation and its characterization by an isolated strain of Aspergillus foetidus in India. World J. Microbiol. Biotechnol. 2005, 21, 233–243. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Extrusion and enzymatic hydrolysis as pretreatments on corn cob for biogas production. Renew. Energy 2017, 107, 597–603. [Google Scholar] [CrossRef]
- Yang, H.; Guo, L.; Liu, F. Enhanced bio-hydrogen production from corncob by a two-step process: Dark- and photo-fermentation. Bioresour. Technol. 2010, 101, 2049–2052. [Google Scholar] [CrossRef]
- Mureed, F.; Nadeem, R.; Mehmood, A.; Siddique, M.; Bukhari, M. Biosorption of zinc by chemically modified biomass of corncob (Zea mays L.). Middle East J. Sci. Res. 2012, 11, 1226–1231. [Google Scholar] [CrossRef]
- Gupta, G.K.; Ram, M.; Bala, R.; Kapur, M.; Mondal, M.K. Pyrolysis of chemically treated corncob for biochar production and its application in Cr(VI) removal. Environ. Prog. Sustain. Energy 2018, 37, 1606–1617. [Google Scholar] [CrossRef]
- Zhou, D.; Li, D.; Li, A.; Qi, M.; Cui, D.; Wang, H.; Wei, H. Activated carbons prepared via reflux-microwave-assisted activation approach with high adsorption capability for methylene blue. J. Environ. Chem. Eng. 2021, 9, 104671. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Qi, P.; Guo, L. A new approach for economical pretreatment of corncobs. Appl. Sci. 2019, 9, 504. [Google Scholar] [CrossRef]
- Araújo, D.; Castro, M.C.R.; Figueiredo, A.; Vilarinho, M.; Machado, A. Green synthesis of cellulose acetate from corncob: Physicochemical properties and assessment of environmental impacts. J. Clean. Prod. 2020, 260, 120865. [Google Scholar] [CrossRef]
- Araújo, D.; Vilarinho, M.; Machado, A. Effect of combined dilute-alkaline and green pretreatments on corncob fractionation: Pretreated biomass characterization and regenerated cellulose film production. Ind. Crops Prod. 2019, 141, 111785. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Huang, Z.; Zhao, K.; Wei, G.; Wang, X.; He, F.; Li, H. Catalytic fast pyrolysis of biomass pretreated by torrefaction with varying severity. Energy Fuels 2014, 28, 5804–5811. [Google Scholar] [CrossRef]
- Gandam, P.K.; Chinta, M.L.; Prashanth, N.P.; Velidandi, A.; Sharma, M.; Kuhad, R.C.; Tabatabaei, M.; Aghbashlo, M.; Baadhe, R.R.; Gupta, V.K. Corncob based biorefinery: A comprehensive review of pretreatment methodologies, and biorefinery platforms. J. Energy Inst. 2022, 101, 290–308. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, M.H.; Wright, M.M. Lifecycle energy consumption and greenhouse gas emissions from corncob ethanol in China. Biofuels Bioprod. Biorefining. 2018, 12, 1037–1046. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Samuel, R.; Pu, Y.; Raman, B.; Ragauskas, A.J. Structural characterization and comparison of switchgrass ball-milled lignin before and after dilute acid pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.; Rathour, R.K.; Sharma, V.; Rana, N.; Bhatt, A.K.; Bhatia, R.K. Co-fermentation of forest pine needle waste biomass hydrolysate into bioethanol. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–13. [Google Scholar] [CrossRef]
- Li, X.; Ximenes, E.; Kim, Y.; Slininger, M.; Meilan, R.; Ladisch, M.; Chapple, C. Lignin monomer composition affects Arabidopsis cell-wall degradability after liquid hot water pretreatment. Biotechnol. Biofuels 2010, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, J.; Zhang, Y.; Guo, Y.; Xu, H.; Liang, C.; Wang, Z.; Xu, J. Lignin prepared from different alkaline pretreated sugarcane bagasse and its effect on enzymatic hydrolysis. Int. J. Biol. Macromol. 2019, 141, 484–492. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Suri, P.; Dwivedi, D.; Rathour, R.K.; Rana, N.; Sharma, V.; Bhatia, R.K.; Bhatt, A.K. Enhanced C-5 sugar production from pine needle waste biomass using Bacillus sp. XPB-11 mutant and its biotransformation to bioethanol, Biomass Convers. Biorefinery 2022, 12, 3663–3672. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and Enzyme Characteristics that Limit Cellulose Hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Zhao, J.; Wang, D. Effects of particle size on biomass pretreatment and hydrolysis performances in bioethanol conversion, Biomass Convers. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Liu, Z.H.; Qin, L.; Pang, F.; Jin, M.J.; Li, B.Z.; Kang, Y.; Dale, B.E.; Yuan, Y.J. Effects of biomass particle size on steam explosion pretreatment performance for improving the enzyme digestibility of corn stover. Ind. Crops Prod. 2013, 44, 176–184. [Google Scholar] [CrossRef]
- Wang, B.; Shen, P.; Zhu, W.; Pang, Z.; Dong, C. Effect of ground wood particle size on biomass fractionation using p-toluenesulfonic acid treatment. Cellulose 2020, 27, 4043–4052. [Google Scholar] [CrossRef]
- Peng, J.H.; Bi, H.T.; Sokhansanj, S.; Lim, J.C. A study of particle size effect on biomass torrefaction and densification. Energy Fuels 2012, 26, 3826–3839. [Google Scholar] [CrossRef]
- Silva, G.G.D.; Couturier, M.; Berrin, J.-G.; Buléon, A.; Rouau, X. Effects of grinding processes on enzymatic degradation of wheat straw. Bioresour. Technol. 2012, 103, 192–200. [Google Scholar] [CrossRef]
- Ishizawa, C.I.; Davis, M.F.; Schell, D.F.; Johnson, D.K. Porosity and Its Effect on the Digestibility of Dilute Sulfuric Acid Pretreated Corn Stover. J. Agric. Food Chem. 2007, 55, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lin, X.; Yue, J.; Li, X.; Fang, X.; Zhu, M.; Lin, J.; Qu, Y.; Xiao, L. High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour. Technol. 2010, 101, 4952–4958. [Google Scholar] [CrossRef] [PubMed]
- Pagala, B. Removal of Cadmium Metal using a Novel Material Corncob: Characteristics and Kinetics Study. J. Inst. Eng. Ser. D 2022, 1–10. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, J.W. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2008; Volume 9, pp. 1–6. Available online: https://www.nrel.gov/docs/gen/fy08/42621.pdf (accessed on 30 November 2022).
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, a.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis. Laboratory Analytical Procedure (LAP); Preparation of Samples for Compositional Analysis Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008; Volume 1617, pp. 65–71. [Google Scholar]
- Fan, X.; Cheng, G.; Zhang, H.; Li, M.; Wang, S.; Yuan, Q. Effects of acid impregnated steam explosion process on xylose recovery and enzymatic conversion of cellulose in corncob. Carbohydr. Polym. 2014, 114, 21–26. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, C.; Zhou, B.; He, Y. Determination of Hemicellulose, Cellulose and Lignin in Moso Bamboo by Near Infrared Spectroscopy. Sci. Rep. 2015, 5, 17210. [Google Scholar] [CrossRef]
- ASTM E1131; Standard Test Method for Compositional Analysis by Thermogravimetry. ASTM International: West Conshohocken, PA, USA, 2015; Volume 8, p. 6. Available online: https://compass.astm.org/EDIT/html_annot.cgi?E1131+20 (accessed on 10 August 2022).
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Di ff erent Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model. Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Rego, F.; Dias, A.P.S.; Casquilho, M.; Rosa, F.C.; Rodrigues, A. Fast determination of lignocellulosic composition of poplar biomass by thermogravimetry. Biomass Bioenergy 2019, 122, 375–380. [Google Scholar] [CrossRef]
- Gendreau, R.M.; Burton, R. The KBr Pellet: A Useful Technique for Obtaining Infrared Spectra of Inorganic Species. Appl. Spectrosc. 1979, 33, 581–584. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Fernand, G. Classification of Fine Structural Characteristics in Cellulose by Infrared Spectroscopy. Anal. Chem. 1960, 32, 177–181. [Google Scholar]
- Struszczyk, H. Modification of Lignins. 111. Reaction of Lignosulfonates with Chlorophosphazenes. J. Macromol. Sci. Part A—Chem. 1986, 23, 973–992. [Google Scholar] [CrossRef]
- Balogun, A.O.; Lasode, O.A.; Li, H.; McDonald, A.G. Fourier Transform Infrared (FTIR) Study and Thermal Decomposition Kinetics of Sorghum bicolour Glume and Albizia pedicellaris Residues. Waste Biomass Valorization 2015, 6, 109–116. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Chao, Y.; Nawawi, D.S.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Analysis of lignin aromatic structure in wood based on the IR spectrum. J. Wood Chem. Technol. 2012, 32, 294–303. [Google Scholar] [CrossRef]
- Mvondo, R.R.N.; Meukam, P.; Jeong, J.; Meneses, D.D.S.; Nkeng, E.G. Influence of water content on the mechanical and chemical properties of tropical wood species. Results Phys. 2017, 7, 2096–2103. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Rotaru, R.; Savin, M.; Tudorachi, N.; Peptu, C.; Samoila, P.; Sacarescu, L.; Harabagiu, V. Ferromagnetic iron oxide-cellulose nanocomposites prepared by ultrasonication. Polym. Chem. 2018, 9, 860–868. [Google Scholar] [CrossRef]
- Ruland, W. X-ray determination of crystallinity and diffuse disorder scattering. Acta Crystallogr. 1961, 14, 1180–1185. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grosse und der inneren Struktur von Kolloidterilchen mittels Rontgestrahlen, Nachrichten von Der Gesellschaft Der Wissenschaften Zu Göttingen, Math. Klasse. 1918, pp. 98–100. Available online: https://gdz.sub.uni-goettingen.de/id/PPN252457811_1918? (accessed on 30 November 2022).
- Bragg, W.H.; Bragg, W.L. The reflection of X-rays by crystals. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Asmarani, O.; Pertiwi, A.D.; Puspaningsih, N.N.T. Application of enzyme cocktails from Indonesian isolates to corncob (Zea mays) waste saccharification. Biocatal. Agric. Biotechnol. 2020, 24, 101537. [Google Scholar] [CrossRef]
- Mandels, M.; Sternberg, D. Recent advances in cellulase technology, Hakko Kogaku Zasshi. In Proceedings of the Conference: Annual Meeting of the Society of Fermentation Technology, Osaka, Japan, 30 October 1975; Volume 54, pp. 267–286. [Google Scholar]
- Alrumman, S.A. Enzymatic saccharification and fermentation of cellulosic date palm wastes to glucose and lactic acid. Braz. J. Microbiol. 2016, 47, 110–119. [Google Scholar] [CrossRef]
- Hu, H.; Liang, W.; Zhang, Y.; Wu, S.; Yang, Q.; Wang, Y.; Zhang, M.; Liu, Q. Multipurpose Use of a Corncob Biomass for the Production of Polysaccharides and the Fabrication of a Biosorbent. ACS Sustain. Chem. Eng. 2018, 6, 3830–3839. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Luo, Z.; Jiao, Y.; Ai, F.; Zhang, H.; Zhu, S.; Zhang, Q.; Zhang, Z. Surfactant assisted microwave irradiation pretreatment of corncob: Effect on hydrogen production capacity, energy consumption and physiochemical structure. Bioresour. Technol. 2022, 357, 127302. [Google Scholar] [CrossRef]
- Selvakumar, P.; Adane, A.A.; Zelalem, T.; Hunegnaw, B.M.; Karthik, V.; Kavitha, S.; Jayakumar, M.; Karmegam, N.; Govarthanan, M.; Kim, W. Optimization of binary acids pretreatment of corncob biomass for enhanced recovery of cellulose to produce bioethanol. Fuel 2022, 321, 124060. [Google Scholar] [CrossRef]
- Bu, C.Y.; Yan, Y.X.; Zou, L.H.; Ouyang, S.P.; Zheng, Z.J.; Ouyang, J. Comprehensive utilization of corncob for furfuryl alcohol production by chemo-enzymatic sequential catalysis in a biphasic system. Bioresour. Technol. 2021, 319, 124156. [Google Scholar] [CrossRef] [PubMed]
- Anioła, J.; Gawȩcki, J.; Czarnocińska, J.; Galiński, G. Corncobs as a source of dietary fiber. Polish J. Food Nutr. Sci. 2009, 59, 247–249. [Google Scholar]
- Yao, X.; Xu, K.; Liang, Y. Research on the thermo-physical properties of corncob residues as gasification feedstock and assessment for characterization of corncob ash from gasification. BioResources 2016, 11, 9823–9841. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, K.; Li, L.; Zhao, Z.; Jiang, L.; Huang, Z.; Wei, G.; He, F.; Li, H. Quantitative comparison of different chemical pretreatment methods on chemical structure and pyrolysis characteristics of corncobs. J. Energy Inst. 2018, 91, 676–682. [Google Scholar] [CrossRef]
- Kemp, W. Organic spectroscopy. J. Chem. Educ. 2002, 79, 26. [Google Scholar] [CrossRef]
- Ciolacu, D.; Kovac, J.; Kokol, V. The effect of the cellulose-binding domain from Clostridium cellulovorans on the supramolecular structure of cellulose fibers. Carbohydr. Res. 2010, 345, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lu, L.; Guo, W.; Liu, M.; Cao, Y. On preparation, structure and performance of high porosity bulk cellulose aerogel. Plast. Rubber Compos. 2015, 44, 26–32. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008. Available online: https://books.google.co.in/books?id=FkaNOdwk0FQC (accessed on 30 November 2022).
- Ling, Z.; Wang, T.; Makarem, M.; Cintrón, M.S.; Cheng, H.N.; Kang, X.; Bacher, M.; Potthast, A.; Rosenau, T.; King, H.; et al. Effects of ball milling on the structure of cotton cellulose. Cellulose 2019, 26, 305–328. [Google Scholar] [CrossRef]
- Stark, N.M.; Yelle, D.J.; Agarwal, U.P. Techniques for Characterizing Lignin. In Lignin in Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–66. [Google Scholar] [CrossRef]
- Virtanen, T.; Rudolph, G.; Lopatina, A.; Al-Rudainy, B.; Schagerlöf, H.; Puro, L.; Kallioinen, M.; Lipnizki, F. Analysis of membrane fouling by Brunauer-Emmet-Teller nitrogen adsorption/desorption technique. Sci. Rep. 2020, 10, 3427. [Google Scholar] [CrossRef]
- Sammons, R.J.; Harper, D.P.; Labbé, N.; Bozell, J.J.; Elder, T.; Rials, T.G. Characterization of organosolv lignins using thermal and FT-IR spectroscopic analysis. BioResources 2013, 8, 2752–2767. [Google Scholar] [CrossRef]
- Adapa, P.K.; Tabil, L.G.; Schoenau, G.J.; Canam, T.; Dumonceaux, T. Quantitative Analysis of Lignocellulosic Components of Non-Treated and Steam Exploded Barley, Canola, Oat and Wheat Straw Using Fourier Transform Infrared Spectroscopy. J. Agric. Sci. Technol. B 2011, 1, 177–188. [Google Scholar]
- Xu, F.; Wang, D. Analysis of Lignocellulosic Biomass Using Infrared Methodology. In Pretreatment of Biomass; Elsevier: Amsterdam, The Netherlands, 2015; pp. 7–25. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Characterization of lignocellulose of Opuntia (Cactaceae) species using FTIR spectroscopy: Possible candidates for renewable raw material. Biomass Convers. Biorefinery 2020, 12, 5165–5174. [Google Scholar] [CrossRef]
- Deng, Z.; Xia, A.; Liao, Q.; Zhu, X.; Huang, Y.; Fu, Q. Laccase pretreatment of wheat straw: Effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol. Biofuels 2019, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Jiang, Z.; Wu, P.; Yi, J.; Li, J.; Hu, C. Fractionation for further conversion: From raw corn stover to lactic acid. Sci. Rep. 2016, 6, 38623. [Google Scholar] [CrossRef] [PubMed]
- Salim, R.M.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Vandsburger, L.; Blanchet, P. Modification of hardwood samples in the flowing afterglow of N2–O2 dielectric barrier discharges open to ambient air Determination of active species in the modification of hardwood samples in the flowing afterglow of N2 dielectric barrier discharges open to ambient air. Cellulose 2014, 22, 811–827. [Google Scholar] [CrossRef]
- Casas, A.; Oliet, M.; Alonso, M.V.; Rodríguez, F. Dissolution of Pinus radiata and Eucalyptus globulus woods in ionic liquids under microwave radiation: Lignin regeneration and characterization. Sep. Purif. Technol. 2012, 97, 115–122. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and opportunities in the characterization of cellulose—An important regulator of cell wall growth and mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform—Materials Analysis; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Moosavinejad, S.M.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of degradation in chemical compounds of wood in historical buildings using Ft-Ir And Ft-Raman vibrational spectroscopy. Maderas Cienc. Tecnol. 2019, 21, 381–392. [Google Scholar] [CrossRef]
- Chi, X.; Liu, C.; Bi, Y.H.; Yu, G.; Zhang, Y.; Wang, Z.; Li, B.; Cui, Q. A clean and effective potassium hydroxide pretreatment of corncob residue for the enhancement of enzymatic hydrolysis at high solids loading. RSC Adv. 2019, 9, 11558–11566. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose—Structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Gong, X.; Li, Q.; Li, T.; Li, C.; Huang, J.; Zhou, N.; Jia, X. Chemical composition and monolignin in alkali and acid treated corncob affect sugar release. Ind. Crops Prod. 2022, 176, 114317. [Google Scholar] [CrossRef]
- Yu, Q.; Zhu, Y.; Bian, S.; Chen, L.; Zhuang, X.; Zhang, Z.; Wang, W.; Yuan, Z.; Hu, J.; Chen, J. Structural characteristics of corncob and eucalyptus contributed to sugar release during hydrothermal pretreatment and enzymatic hydrolysis. Cellulose 2017, 24, 4899–4909. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Kafle, K.; Shin, H.; Lee, C.M.; Park, S.; Kim, S.H. Progressive structural changes of Avicel, bleached softwood, and bacterial cellulose during enzymatic hydrolysis. Sci. Rep. 2015, 5, 15102. [Google Scholar] [CrossRef]
- Ioelovich, M. Characterization of Various Kinds of Nanocellulose. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017; pp. 51–100. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels. 2010, 3, 10. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Y.L.; Fu, N.; Li, D.; Adhikari, B.; Chen, X.D. Isolation and characterization of corncob cellulose fibers using microwave-assisted chemical treatments. Int. J. Food Eng. 2014, 10, 427–436. [Google Scholar] [CrossRef]
- Sahare, P.; Singh, R.; Laxman, R.S.; Rao, M. Effect of Alkali Pretreatment on the Structural Properties and Enzymatic Hydrolysis of Corn Cob. Appl. Biochem. Biotechnol. 2012, 168, 1806–1819. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Liu, W.; Wu, R.; Wang, B.; Hu, Y.; Hou, Q.; Zhang, P.; Wu, R. Comparative study on different pretreatment on enzymatic hydrolysis of corncob residues. Bioresour. Technol. 2020, 295, 122244. [Google Scholar] [CrossRef]
- Gupta, R.; Khasa, Y.P.; Kuhad, R.C. Evaluation of pretreatment methods in improving the enzymatic saccharification of cellulosic materials. Carbohydr. Polym. 2011, 84, 1103–1109. [Google Scholar] [CrossRef]
- Kawee-Ai, A.; Srisuwun, A.; Tantiwa, N.; Nontaman, W.; Boonchuay, P.; Kuntiya, A.; Chaiyaso, T.; Seesuriyachan, P. Eco-friendly processing in enzymatic xylooligosaccharides production from corncob: Influence of pretreatment with sonocatalytic-synergistic Fenton reaction and its antioxidant potentials. Ultrason. Sonochem. 2016, 31, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kim, T.H.; Lee, Y.Y.; Chen, R.; Elander, R.T. Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. Appl. Biochem. Biotechnol. 2006, 129–132, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Mardawati, E.; Pratiwi, S.M.; Andoyo, R.; Rialita, T.; Djali, M. Ozonation Pre-treatment Evaluation for Xylanase Crude Extract Production from Corncob under Solid-State Fermentation. J. Ind. Inf. Technol. Agric. 2017, 1, 27–34. [Google Scholar]


| Corn Variety/ Reference | Sample | %AIL | %ASL | %Glucan | %Xylan | %Galactan | %Arabinan | %Mannan | %Protein (Structural) | %Water Extractives | %Ethanol Extractives | %Sucrose | %Acetate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC1 | CO | 14.52 ± 0.23 | 1.85 ± 0.13 | 36.68 ± 0.13 | 25.42 ± 0.26 | 10.1 ± 0.04 | 5.29 ± 0.26 | 0 ± 0.38 | 0.62 ± 0.1 | 2.26 ± 0.15 | 1.17 ± 0.22 | 2.58 ± 0.2 | 5.24 ± 0.38 |
| CP | 11.11 ± 0.16 | 1.72 ± 0.12 | 39.13 ± 0.37 | 24.39 ± 0.34 | 11.14 ± 0.05 | 6.28 ± 0.28 | 0 ± 0.29 | 0.39 ± 0.13 | 3.49 ± 0.05 | 1.58 ± 0.04 | 3.84 ± 0.31 | 5.21 ± 0.07 | |
| CC2 | CO | 15.44 ± 0.33 | 2.04 ± 0.31 | 37.04 ± 0.36 | 25.77 ± 0.19 | 11.45 ± 0.24 | 5.77 ± 0.06 | 0 ± 0.31 | 0.79 ± 0.05 | 2.46 ± 0.37 | 1.55 ± 0.15 | 2.89 ± 0.27 | 5.84 ± 0.2 |
| CP | 11.18 ± 0.24 | 2.11 ± 0.35 | 39.66 ± 0.35 | 25.39 ± 0.1 | 11.52 ± 0.39 | 7.39 ± 0.12 | 0 ± 0.25 | 0.48 ± 0.13 | 3.59 ± 0.07 | 1.96 ± 0.25 | 4 ± 0.29 | 5.73 ± 0.19 | |
| CC3 | CO | 14.52 ± 0.15 | 2.51 ± 0.12 | 37.22 ± 0.26 | 25.86 ± 0.1 | 10.63 ± 0.16 | 6.55 ± 0.12 | 0 ± 0.34 | 0.69 ± 0.37 | 2.28 ± 0.36 | 1.77 ± 0.39 | 2.87 ± 0.08 | 5.57 ± 0.2 |
| CP | 11.42 ± 0.14 | 2.49 ± 0.37 | 40.44 ± 0.06 | 24.89 ± 0.17 | 11.26 ± 0.16 | 7.16 ± 0.05 | 0 ± 0.2 | 0.49 ± 0.32 | 3.35 ± 0.36 | 1.68 ± 0.28 | 4.19 ± 0.1 | 5.56 ± 0.13 | |
| CC4 | CO | 15.52 ± 0.14 | 2.1 ± 0.26 | 37.71 ± 0.21 | 26.66 ± 0.09 | 11.65 ± 0.17 | 5.93 ± 0.03 | 0 ± 0.04 | 0.7 ± 0.14 | 2.85 ± 0.19 | 1.64 ± 0.29 | 2.76 ± 0.16 | 5.87 ± 0.33 |
| CP | 12.04 ± 0.17 | 2.25 ± 0.11 | 39.64 ± 0.18 | 25.14 ± 0.34 | 12.15 ± 0.1 | 7.72 ± 0.33 | 0 ± 0.23 | 0.52 ± 0.07 | 3.37 ± 0.07 | 1.9 ± 0.27 | 4.21 ± 0.25 | 5.25 ± 0.3 | |
| Reference | CL | 0.33 ± 0.27 | 0.35 ± 0.07 | 66.66 ± 0.24 | 15.47 ± 0.26 | N.D | N.D | 10.8 ± 0.2 | 0 ± 0.26 | 0.34 ± 0.07 | 0.25 ± 0.14 | 0 ± 0.15 | 0 ± 0.3 |
| AC | 0 ± 0.04 | 0.32 ± 0.1 | 71.88 ± 0.11 | 15.83 ± 0.13 | N.D | N.D | 9.77 ± 0.36 | 0 ± 0.16 | 0.09 ± 0.34 | 0.07 ± 0.3 | 0 ± 0.25 | 0 ± 0.36 |
| Corn Variety/ Reference | Sample | % NDF | % ADF | % ADL | % Hemicellulose | % Cellulose | % Lignin |
|---|---|---|---|---|---|---|---|
| CC1 | CO | 87.17 ± 0.3 | 45.25 ± 0.14 | 6.75 ± 0.07 | 41.92 ± 0.07 | 38.5 ± 0.15 | 6.75 ± 0.1 |
| CP | 92.76 ± 0.1 | 49.35 ± 0.16 | 1.7 ± 0.32 | 43.41 ± 0.16 | 47.65 ± 0.32 | 1.7 ± 0.12 | |
| CC2 | CO | 85.56 ± 0.08 | 47.88 ± 0.1 | 9.47 ± 0.31 | 37.68 ± 0.3 | 38.41 ± 0.17 | 9.47 ± 0.15 |
| CP | 95.62 ± 0.25 | 51.77 ± 0.22 | 4.12 ± 0.31 | 43.85 ± 0.13 | 47.65 ± 0.18 | 4.12 ± 0.11 | |
| CC3 | CO | 88.02 ± 0.28 | 46.91 ± 0.3 | 9.34 ± 0.13 | 41.11 ± 0.24 | 37.57 ± 0.28 | 9.34 ± 0.09 |
| CP | 94.43 ± 0.15 | 49.64 ± 0.24 | 2.36 ± 0.32 | 44.79 ± 0.24 | 47.28 ± 0.1 | 2.36 ± 0.11 | |
| CC4 | CO | 86.21 ± 0.09 | 46.31 ± 0.19 | 8.3 ± 0.25 | 39.9 ± 0.27 | 38.01 ± 0.3 | 8.3 ± 0.1 |
| CP | 95.1 ± 0.24 | 50.62 ± 0.21 | 1.8 ± 0.16 | 44.48 ± 0.12 | 48.82 ± 0.17 | 1.8 ± 0.19 | |
| Reference | CL | 98.1 ± 0.31 | 95.51 ± 0.13 | 0 | 2.59 ± 0.28 | 95.51 ± 0.21 | 0 |
| AC | 98.62 ± 0.17 | 97.31 ± 0.22 | 0 | 1.31 ± 0.11 | 97.31 ± 0.11 | 0 |
| CO-i | CO-o | CO-dc | CP-i | CP-o | CP-dc | AC-i | AC-o | CL-i | CL-o | |
|---|---|---|---|---|---|---|---|---|---|---|
| HC | 24.23 | 24.97 | 25.31 | 29.93 | 32.83 | 45.09 | 0 | 0 | 0 | 0 |
| CE | 51.85 | 45.88 | 18.03 | 48.64 | 49.1 | 31.20 | 94.76 | 86.58 | 100 | 87.95 |
| LG | 12.15 | 24.99 | 16.58 | 10.09 | 13.91 | 13.16 | 5.24 | 12.01 | 0 | 9.09 |
| A and C | 11.35 | 4 | N.A | 10.9 | 4 | 0 | 1.37 | 0 | 2.9 | |
| TC | 76.09 | 70.86 | 43.34 | 78.57 | 81.93 | 76.29 | 94.76 | 86.58 | 100 | 87.95 |
| HC/TC | 0.32 | 0.35 | 0.58 | 0.38 | 0.40 | 0.59 | 0 | 0 | 0 | 0 |
| LG/TC | 0.16 | 0.35 | 0.38 | 0.13 | 0.17 | 0.17 | 0.06 | 0.14 | 0 | 0.1 |
| Wave Number Range (cm−1) | Samples and Their Obtained Peaks (cm−1) | Generic Functional Group Assignment, Reference | Lignocellulose Specific Assignment | ||||
|---|---|---|---|---|---|---|---|
| CO | CP | AC | CL | LG | |||
| 3650–3600 | Non-bonded free -OH stretching. [82] | ||||||
| 3400–3200 | Bonded -OH stretching. [82] | ||||||
| 3584 | 3559 | 3571 | 3586 | Intramolecular hydrogen bond O(2)H-O(6). [83] | Cellulose | ||
| 3475 | 3453 | 3448 | 3465 | Intramolecular hydrogen bond O(2)H-O(6). [83] | Cellulose | ||
| 3430 | -OH (bonded) stretching. [84] | Lignin * | |||||
| 3358 | 3351 | 3355 | Intramolecular hydrogen bond O(3)H-O(5), [83] | Cellulose | |||
| 3179 | 3124 | 3112 | 3123 | Intermolecular hydrogen bond O(6)H-O(3), [83] | Cellulose | ||
| 3000–2850 | C-H stretching: Alkanes/O-H stretching carboxylic acid/Aldehyde. [85] | ||||||
| 2970–2860 | CH—stretching region (saturated aliphatic group frequencies). [86] | ||||||
| 2937 | C-H stretch methyl and methylene groups (2942 HW lignin, 2938 SW lignin). [87] | SW.Lignin | |||||
| 2886 | 2898 | 2904 | 2902 | Symmetric C-H stretching. [84] | Cellulose * | ||
| 2842 | C-H stretch O-CH3 group. [87] | Lignin | |||||
| 1780–1640 | C=O stretching: Ester/Aldehyde/Ketone/Carboxylic acid; C=C stretching: Alkene [85] | ||||||
| 1731 | 1733 | Ketone/Aldehyde C=O stretching (unconjugated) [88] | Hemicellulose * | ||||
| 1711 | Non–conjugated carbonyl [89] | Lignin | |||||
| 1643 | 1635 | 1639 | 1641 | 1643 | Intramolecular hydrogen bond/absorbed water/Aromatic ketones stretching [84] | ||
| 1600–1475 | C=C stretching–skeletal vibration of phenolic compounds such as lignin, -CH2 bend. [85] | ||||||
| 1606 | 1604 | Aromatic skeleton vibration [87] | Lignin * (S > G; G-con. > G-eth.) | ||||
| 1598 | The aromatic ring (C=C), C=O stretching vibrations [64]. | Lignin * (S > G; G-con. > G-eth.) | |||||
| 1516 | 1516 | 1510 | Aromatic ring (C=C) stretching [64]. | Lignin * (G > S) | |||
| 1456 | 1462 | 1458 | 1464 | Asymmetric bending of CH3 in methoxy groups//CH2 bending vibration [88] | Lignin * (S > G), Cellulose, Hemicellulose | ||
| 1425 | 1427 | 1429 | 1431 | Scissoring motion of -CH2 [60] | Cellulose-I * Crystallinity peak | ||
| O-CH3 C-H deformation symmetric [87] | Lignin | ||||||
| 1372 | 1374 | 1372 | 1372 | 1376 | Symmetric and asymmetric C-H deformation [85] | Cellulose, Hemicellulose, Lignin | |
| 1335 | 1337 | 1337 | C-H, -OH in-plane bending/weak C-O stretching [90] | Cellulose amorphous | |||
| 1327 | Stretching of C-O in syringyl ring [91] | Lignin-S * | |||||
| 1318 | 1316 | 1314 | -CH2 wagging [92] | Cellulose I crystalline | |||
| 1300–1000 | C=O/C-O-C/C-O-H; Alcohols, ethers, esters, carboxylic acids, anhydrides [93] | ||||||
| 1281 | 1281 | C-H bending [91] | Cellulose crystalline * | ||||
| 1269 | Aromatic ring vibration [85] | Lignin-G | |||||
| 1248 | 1251 | C-O-C and C-O Stretching [94] | Hemicellulose * | ||||
| 1220 | C=O stretching of guaiacyl ring [95] | Lignin G | |||||
| 1205 | 1203 | 1201 | 1203 | O-H in-plane bending [89] | Carbohydrates * | ||
| 1158 | 1162 | 1164 | 1166 | C-O-C stretching, Asymmetric stretching of C-O, C-C, O-H stretching of C-OH group [94] | Crystalline cellulose, β-glycosidic bond | ||
| 1137 | C-H (aromatic) in-plane deformation, secondary alcohols, C-O stretch [59], | Lignin G | |||||
| 1111 | 1113 | 1113 | 1115 | Asymmetric stretching of C-O-C; Cellulose characteristic peak [84] | Cellulose * | ||
| 1082 | C-O deformation, secondary alcohol, an aliphatic ether [87] | Lignin | |||||
| 993 | 993 | 987 | 986 | C-O and C-C, C-H bending or CH2 (amorphous band) stretching [96] | Cellulose | ||
| 1000–650 | Out-of-plane bend Alkenes/Aromatics, aromatic C-H stretching [85] | ||||||
| 899 | 899 | 897 | 895 | C-O-C stretching at β-1,4 glycosidic link [84] | Amorphous band * | ||
| 862 | 858 | C-H out of the plane in positions 2, 5, and 6 of G-ring [97] | Lignin-G | ||||
| 814 | 817 | The vibration of mannan. CH out-of-plane bending in phenyl rings [98] | Glucomannan, Lignin G | ||||
| 714 | 714 | Alcohol, OH out-of-plane bend. [99] | Cellulose Iβ * | ||||
| 668 | 668 | 668 | 668 | -OH out-of-plane-bending [100] | Cellulose amorphous | ||
| 607 | 617 | 619 | 617 | 617 | Alkyne C–H bend, Alcohol, OH out-of-plane bend [95] | Carbohydrates/Lignin | |
| 524 | 527 | 520 | 518 | 520 | C-O-C bending, C-C-C ring deform [101] | Cellulose, β-glycosidic bond | |
| Ratio | Wave Number Range (cm−1) | CO | CP | LG |
|---|---|---|---|---|
| S/G | 1462/1510–1508 | 1.34 | 1.38 | 0.52 |
| S/G | 1595/1510–1508 | 1.28 | 1.34 | 2.54 |
| LG/TC | 1510–1508/1374 | 1.03 | 0.71 | 8.75 |
| LG/TC | 1510–1508/1162 | 0.45 | 0.34 | N.A1 |
| LG/TC | 1510–1508/898 | 2.89 | 1.93 | N.A1 |
| XY/TC | 1734/1374 | 1.16 | 1.88 | N.A2 |
| XY/TC | 1734/1162 | 0.50 | 0.90 | N.A2 |
| LG/XY | 1510–1508/1734 | 0.88 | 0.37 | N.A1 |
| XRD Analysis | FTIR Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | CrI% | Crd% | Cra1% | Cra2% | L | d | TCI | LOI | HBI |
| CO | 70.0 | 93.0 | 26.48 | 25.20 | 5.75 | 0.34 | 2.82 | 2.35 | 2.46 |
| CP | 31.0 | 73.0 | 20.06 | 23.84 | 2.94 | 0.41 | 1.47 | 0.87 | 2.03 |
| AC | 93.0 | 78.0 | 48.04 | 48.04 | 4.67 | 0.40 | 1.72 | 1.29 | 2.15 |
| CL | 91.0 | 77.0 | 44.28 | 36.01 | 4.73 | 0.39 | 1.8 | 0.96 | 1.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandam, P.K.; Chinta, M.L.; Gandham, A.P.; Pabbathi, N.P.P.; Konakanchi, S.; Bhavanam, A.; Atchuta, S.R.; Baadhe, R.R.; Bhatia, R.K. A New Insight into the Composition and Physical Characteristics of Corncob—Substantiating Its Potential for Tailored Biorefinery Objectives. Fermentation 2022, 8, 704. https://doi.org/10.3390/fermentation8120704
Gandam PK, Chinta ML, Gandham AP, Pabbathi NPP, Konakanchi S, Bhavanam A, Atchuta SR, Baadhe RR, Bhatia RK. A New Insight into the Composition and Physical Characteristics of Corncob—Substantiating Its Potential for Tailored Biorefinery Objectives. Fermentation. 2022; 8(12):704. https://doi.org/10.3390/fermentation8120704
Chicago/Turabian StyleGandam, Pradeep Kumar, Madhavi Latha Chinta, A. Priyadarshini Gandham, Ninian Prem Prashanth Pabbathi, Srilekha Konakanchi, Anjireddy Bhavanam, Srinivasa R. Atchuta, Rama Raju Baadhe, and Ravi Kant Bhatia. 2022. "A New Insight into the Composition and Physical Characteristics of Corncob—Substantiating Its Potential for Tailored Biorefinery Objectives" Fermentation 8, no. 12: 704. https://doi.org/10.3390/fermentation8120704
APA StyleGandam, P. K., Chinta, M. L., Gandham, A. P., Pabbathi, N. P. P., Konakanchi, S., Bhavanam, A., Atchuta, S. R., Baadhe, R. R., & Bhatia, R. K. (2022). A New Insight into the Composition and Physical Characteristics of Corncob—Substantiating Its Potential for Tailored Biorefinery Objectives. Fermentation, 8(12), 704. https://doi.org/10.3390/fermentation8120704