Anti-Influenza Virus Potential of Probiotic Strain Lactoplantibacillus plantarum YML015 Isolated from Korean Fermented Vegetable
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of L. plantarum YML015
2.1.1. Isolation and Stock Preparation Process of L. plantarum YML015
2.1.2. DNA Extraction, PCR, and 16S rRNA Gene Sequencing of L. plantarum YML015
2.2. Characterization of L. plantarum YML015
2.2.1. Biochemical Characterization
2.2.2. Heat Stability Test
2.2.3. Antibiotic Susceptibility Assay
2.2.4. Hemolytic Phenomenon Assay
2.3. Culture of MDCK Cell
2.4. MTT Cell Viability Assay
2.5. Virus Culture
2.6. Antiviral Potential of L. plantarum YML015
2.6.1. Cytopathogenic Reduction Assay
2.6.2. Hemagglutination Inhibition Assay
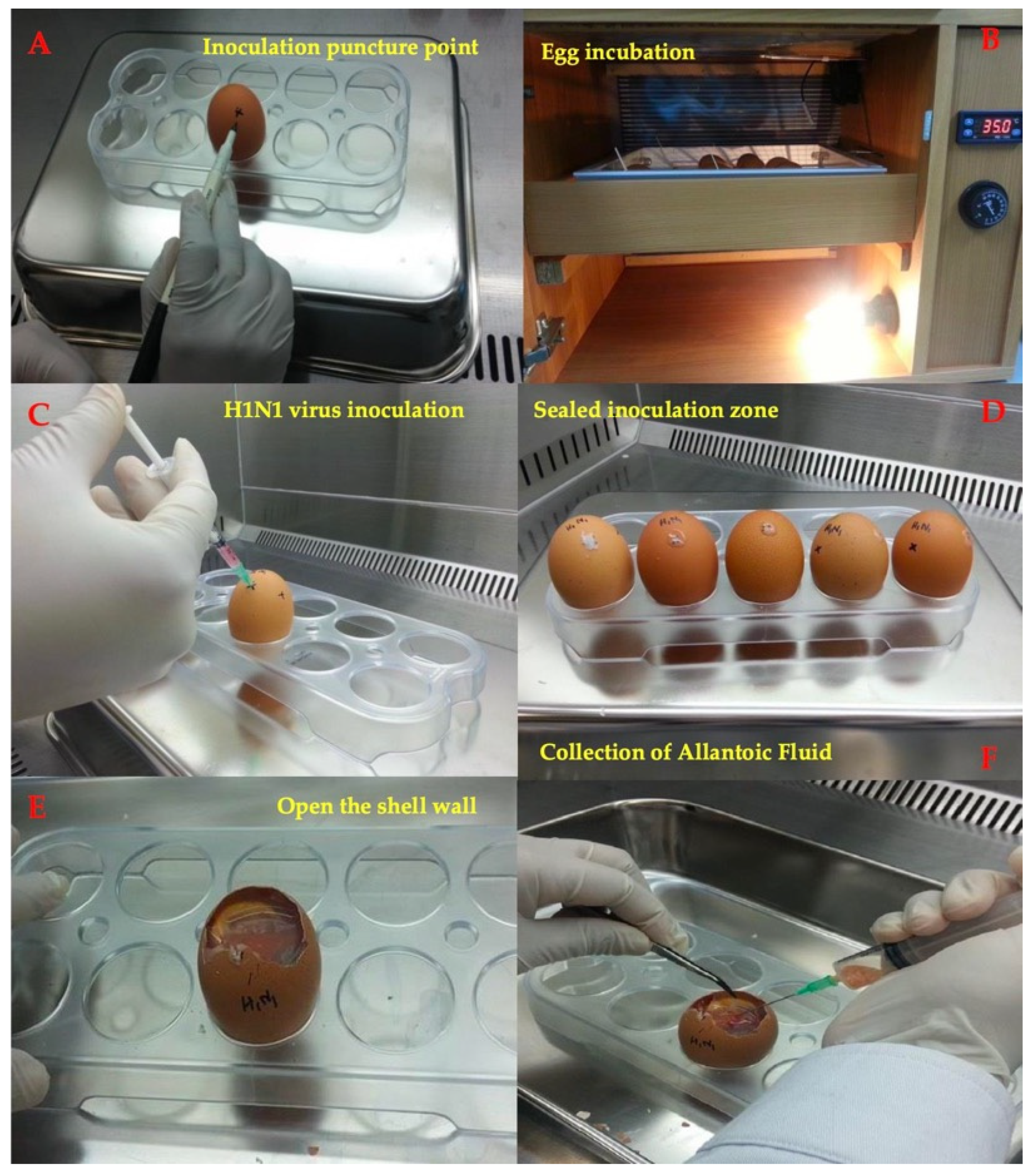
2.6.3. In Ovo Antiviral Assay
2.7. Statistical Analysis
3. Results
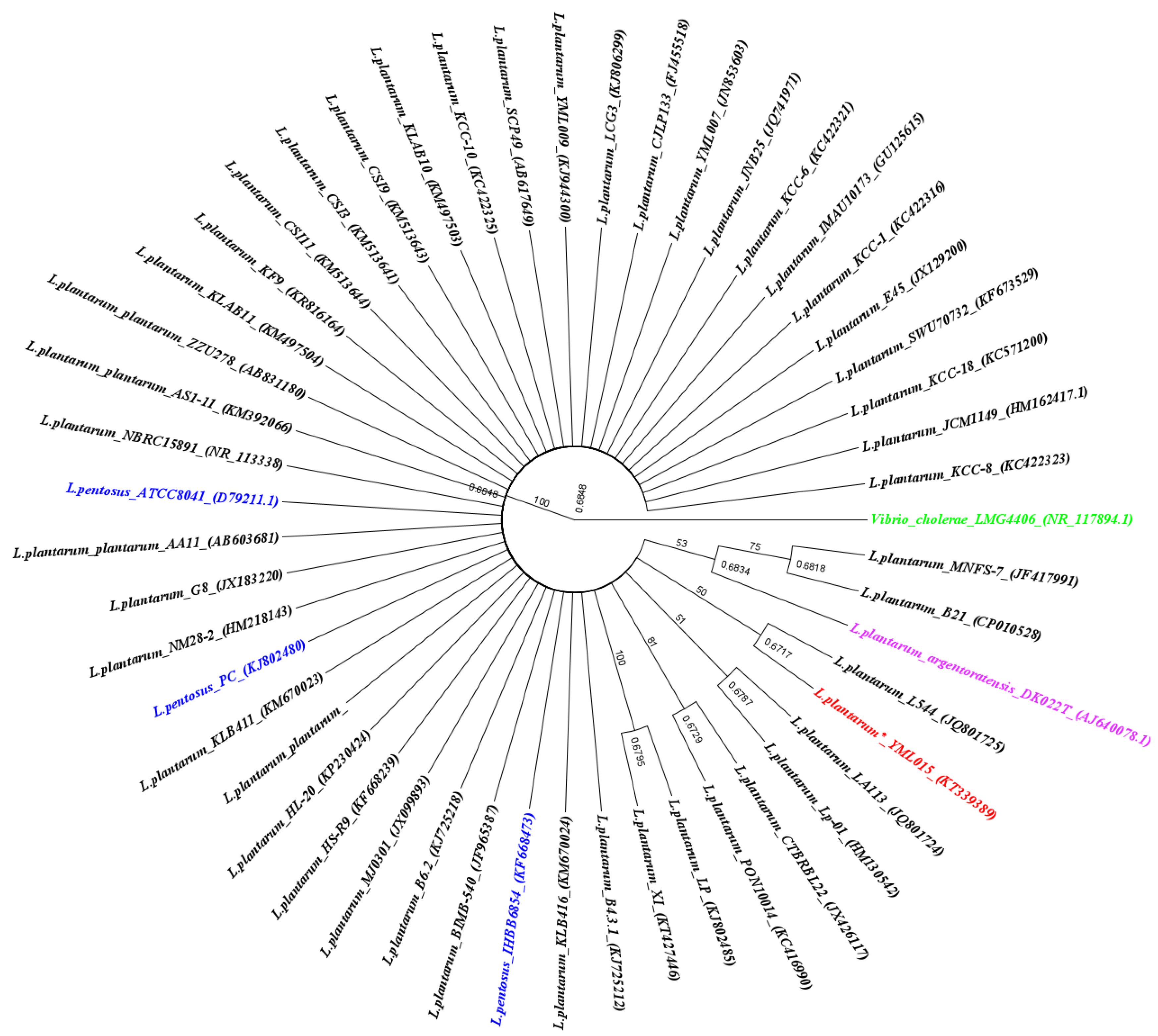
3.1. Isolation and Characterization of L. plantarum YMLO15
3.2. Biochemical Characterization of L. plantarum YML015
3.3. Antibiotic Susceptibility Assay
3.4. Heat Stability Test
3.5. Hemolytic Phenomenon Assay
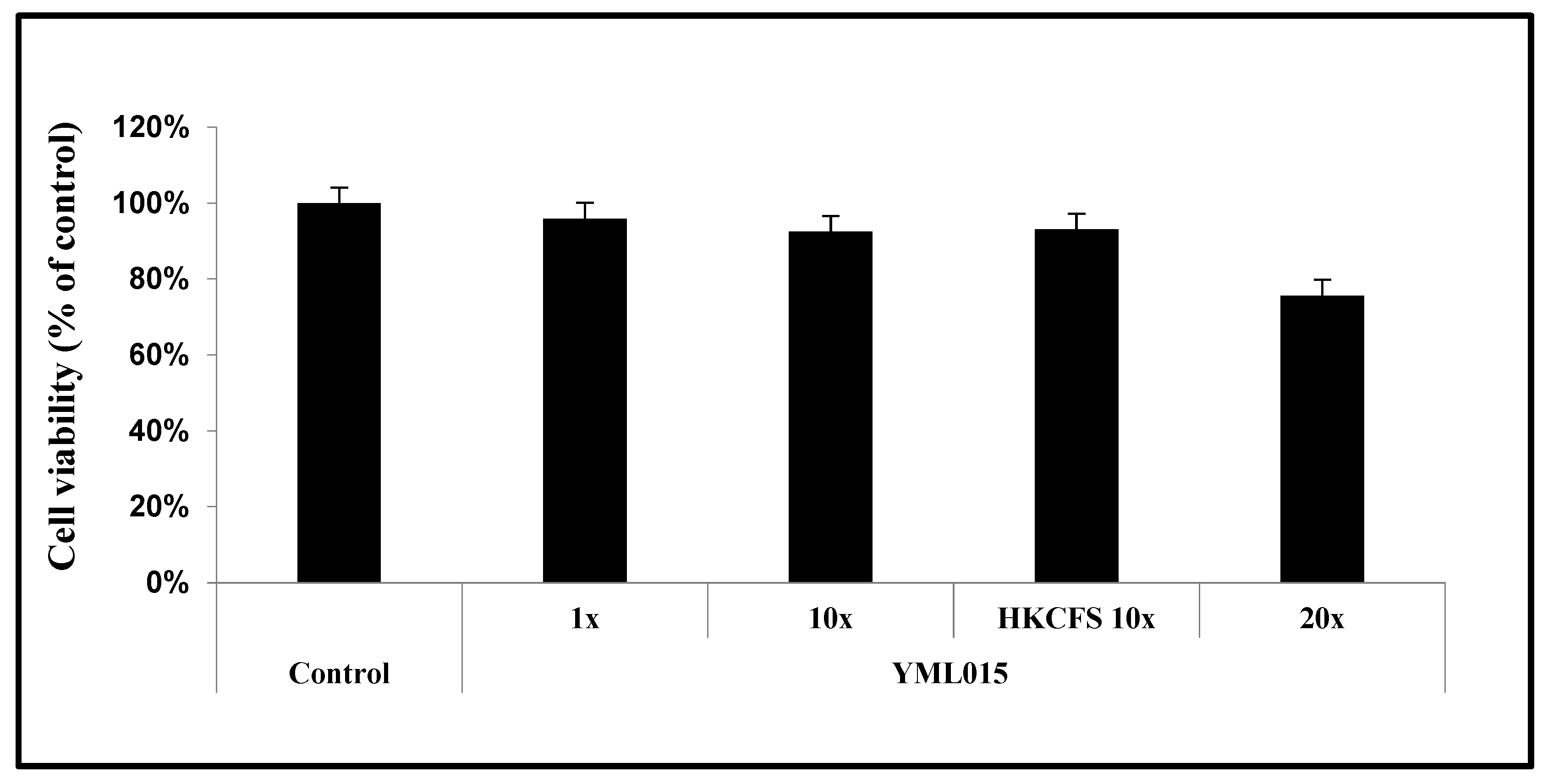
3.6. MTT Cell Viability Assay
3.7. Cytopathic Reduction Assay
3.8. Hemagglutination Inhibition Assay
3.9. In Ovo Antiviral Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foligne, B.; Nutten, S.; Grangette, C.; Dennin, V.; Goudercourt, D.; Poiret, S.; Dewulf, J.; Brassart, D.; Mercenier, A.; Pot, B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. WJG 2007, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Annuk, H.; Shchepetova, J.; Kullisaar, T.; Songisepp, E.; Zilmer, M.; Mikelsaar, M. Characterization of intestinal lactobacilli as putative probiotic candidates. J. Appl. Microbiol. 2003, 94, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zyrek, A.A.; Cichon, C.; Helms, S.; Enders, C.; Sonnenborn, U.; Schmidt, M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 2007, 9, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Pamer, E.G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016, 352, 535–538. [Google Scholar] [CrossRef]
- Draper, K.; Ley, C.; Parsonnet, J. Probiotic guidelines and physician practice: A cross-sectional survey and overview of the literature. Benef. Microbes 2017, 8, 507–519. [Google Scholar] [CrossRef]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Alexandre, Y.; Le Berre, R.; Barbier, G.; Le Blay, G. Screening of Lactobacillus spp. for the prevention of Pseudomonas aeruginosa pulmonary infections. BMC Microbiol. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Racedo, S.; Villena, J.; Medina, M.; Aguero, G.; Rodriguez, V.; Alvarez, S. Lactobacillus casei administration reduces lung injuries in a Streptococcus pneumoniae infection in mice. Microbes Infect 2006, 8, 2359–2366. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Drouault, S.; Corthier, G. Health effects of lactic acid bacteria ingested in fermented milk. Vet. Res. 2001, 32, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, T.; Hirose, R.; Saegusa, S.; Ametani, A.; Kiuchi, K.; Kaminogawa, S. Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int. J. Food Microbiol. 2003, 82, 255–264. [Google Scholar] [CrossRef]
- Maeda, N.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Kase, T.; Yoshikai, Y. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009, 9, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Yoda, K.; Miyazawa, K.; Hiramatsu, M. Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS Immunol. Med. Microbiol. 2012, 64, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-J.; Lee, Y.-T.; Ngo, V.L.; Cho, Y.-H.; Ko, E.-J.; Hong, S.-M.; Kim, K.-H.; Jang, J.-H.; Oh, J.-S.; Park, M.-K. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Harata, G.; He, F.; Hiruta, N.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Yausi, H. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett. Appl. Microbiol. 2010, 50, 597–602. [Google Scholar] [CrossRef]
- Hancock, K.; Veguilla, V.; Lu, X.; Zhong, W.; Butler, E.N.; Sun, H.; Liu, F.; Dong, L.; DeVos, J.R.; Gargiullo, P.M. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. New Engl. J. Med. 2009, 361, 1945–1952. [Google Scholar] [CrossRef]
- Beigel, J.; Bray, M. Current and future antiviral therapy of severe seasonal and avian influenza. Antivir. Res. 2008, 78, 91–102. [Google Scholar] [CrossRef]
- Youn, H.-N.; Lee, D.-H.; Lee, Y.-N.; Park, J.-K.; Yuk, S.-S.; Yang, S.-Y.; Lee, H.-J.; Woo, S.-H.; Kim, H.-M.; Lee, J.-B. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antivir. Res. 2012, 93, 138–143. [Google Scholar] [CrossRef]
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, D.; Inoue, S.; Wakabayashi, H.; Fujii, T. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int. Arch. Allergy Immunol. 2004, 135, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Yamaguchi, H.; Kurokawa, T.; Shirakami, T.; Tsuji, R.F.; Nishimura, I. Immunomodulatory effect of halophilic lactic acid bacterium Tetragenococcus halophilus Th221 from soy sauce moromi grown in high-salt medium. Int. J. Food Microbiol. 2008, 121, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nagao, F.; Nakayama, M.; Muto, T.; Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 2000, 64, 2706–2708. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Hirata, H.; Nishimura, A.; Kajimoto, O.; Fujiwara, S. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: A double-blind, placebo-controlled study. J. Dairy Sci. 2005, 88, 527–533. [Google Scholar] [CrossRef]
- Park, C.W.; Youn, M.; Jung, Y.-M.; Kim, H.; Jeong, Y.; Lee, H.-K.; Kim, H.O.; Lee, I.; Lee, S.W.; Kang, K.H. New functional probiotic Lactobacillus sakei probio 65 alleviates atopic symptoms in the mouse. J. Med. Food 2008, 11, 405–412. [Google Scholar] [CrossRef]
- Lin, W.-H.; Hwang, C.-F.; Chen, L.-W.; Tsen, H.-Y. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Food Microbiol. 2006, 23, 74–81. [Google Scholar] [CrossRef]
- Seo, B.; Rather, I.; Kumar, V.; Choi, U.; Moon, M.; Lim, J.; Park, Y. Evaluation of Leuconostoc mesenteroides YML003 as a probiotic against low-pathogenic avian influenza (H9N2) virus in chickens. J. Appl. Microbiol. 2012, 113, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rather Ahmad, I.; Seo, B.; Rejish Kumar, V.; Choi, U.H.; Choi, K.H.; Lim, J.; Park, Y.H. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML007 and its application as a food preservative. Lett. Appl. Microbiol. 2013, 57, 69–76. [Google Scholar] [CrossRef]
- Paju, S.; Bernstein, J.M.; Haase, E.M.; Scannapieco, F.A. Molecular analysis of bacterial flora associated with chronically inflamed maxillary sinuses. J. Med. Microbiol. 2003, 52, 591–597. [Google Scholar] [CrossRef]
- Rather, I.A.; Choi, K.-H.; Bajpai, V.K.; Park, Y.-H. Antiviral mode of action of Lactobacillus plantarum YML009 on Influenza virus H1N1. Bangladesh J. Pharmacol. 2015, 10, 475–482. [Google Scholar] [CrossRef]
- ISO 10932:2010; Milk and Milk Products: Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococal Lactic Acid Bacteria. ESA: Paris, France, 2012.
- Francis, T., Jr.; Pearson, H.E.; Salk, J.E.; Brown, P.N. Immunity in human subjects artificially infected with influenza virus, type B. Am. J. Public Health Nations Health 1944, 34, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Chesson, A.; Franklin, A.; Aumaître, A.; Sköld, O.; Leclercq, R.; von Wright, A.; Guillot, J. Opinion of the scientific committee on animal nutrition on the criteria for assessing the safety of microorganisms resistant to antibiotics of human and veterinary importance. In Directorate C—Scientific Opinions. European Commission Health and Consumer Protection Directorate-General, Brussels, Belgium; EFSA: Parma, Italy, 2002. [Google Scholar]
- Authority, E.F.S. The EFSA’s 2nd Scientific Colloquium Report-Qps. EFSA Support. Publ. 2005, 2, 109E. [Google Scholar]
- Maragkoudakis, P.A.; Mountzouris, K.C.; Psyrras, D.; Cremonese, S.; Fischer, J.; Cantor, M.D.; Tsakalidou, E. Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. Int. J. Food Microbiol. 2009, 130, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Aguirre, A.A.; Hamm, C.; Wang, Y.; Yu, Q.; Loh, P.C.; Yanagihara, R. Establishment, cryopreservation, and growth of 11 cell lines prepared from a juvenile Hawaiian monk seal, Monachus schauinslandi. Methods Cell Sci. 2000, 22, 115–124. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Moon, I.S.; Kim, J.-M.; Kong, I.-S. Anti-Alzheimers and anti-inflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. Influenza A virus isolation, culture and identification. Nat. Protoc. 2014, 9, 2663–2681. [Google Scholar] [CrossRef]
- Majumder, R.; Rather, I.A.; Bajpai, V.K.; Park, Y.-H. In vitro antiviral activity of Lactobacillus plantarum using SPF embryonated eggs and hemagglutination assay. Bangladesh J. Pharmacol. 2015, 10, 688–691. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Choi, H.-J.; Song, J.-H.; Ahn, Y.-J.; Baek, S.-H.; Kwon, D.-H. Antiviral activities of cell-free supernatants of yogurts metabolites against some RNA viruses. Eur. Food Res. Technol. 2009, 228, 945–950. [Google Scholar] [CrossRef]
- FAO; WHO. Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London; FAO: Québec, ON, Canada; WHO: Geneva, Switzerland, 2002.
- Sood, R.; Swarup, D.; Bhatia, S.; Kulkarni, D.; Dey, S.; Saini, M.; Dubey, S. Antiviral activity of crude extracts of Eugenia jambolana Lam. against highly pathogenic avian influenza (H5N1) virus. Indian J. Exp. Biol. 2012, 50, 179–186. [Google Scholar] [PubMed]
- Chang, J.H.; Shim, Y.; Cha, S.K.; Chee, K. Probiotic characteristics of lactic acid bacteria isolated from kimchi. J. Appl. Microbiol. 2010, 109, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-D.; Rhee, C.-H. Antimutagenic activity of Lactobacillus plantarum KLAB21 isolated from kimchi Korean fermented vegetables. Biotechnol. Lett. 2001, 23, 1583–1589. [Google Scholar] [CrossRef]
- EFSA. EFSA Scientific Colloquium. Summary Report. In QPS. Qualified Presumption of Safety of Microorganism in Food and Feed; European Food Safety Authority Brussels: Parma, Italy, 2004. [Google Scholar]
- Hummel, A.S.; Hertel, C.; Holzapfel, W.H.; Franz, C.M. Antibiotic resistances of starter and probiotic strains of lactic acid bacteria. Appl. Environ. Microbiol. 2007, 73, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Zoumpopoulou, G.; Foligne, B.; Christodoulou, K.; Grangette, C.; Pot, B.; Tsakalidou, E. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int. J. Food Microbiol. 2008, 121, 18–26. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Suchman, E.; Blair, C. Cytopathic Effects of Viruses Protocols. Microbe Library; American Society for Microbiology: Washington, DC, USA, 2014. [Google Scholar]
- Hirst, G.K. The agglutination of red cells by allantoic fluid of chick embryos infected with influenza virus. Science 1941, 94, 22–23. [Google Scholar] [CrossRef]


| Biochemical Characterization of YML015 by Carbohydrate Interpretation Assay | |||
|---|---|---|---|
| Carbohydrates List | Results | Carbohydrates List | Results |
| Control | − | Esculin | + |
| Glycerol | − | Salicin | + |
| Erythritol | − | d-cellobiose | + |
| d-arabinose | − | d-maltose | + |
| l-arabinose | + | d-lactose (bovine origin) | + |
| d-ribose | + | d-melibiose | + |
| d-xylose | − | d-saccharose | + |
| l-xylose | − | d-trehalose | − |
| d-adonitol | − | Lnulin | + |
| Methyl–β–d- xylopyranoside | + | d-melezitose | − |
| d-galactose | + | d-raffinose | − |
| d-glucose | + | Amidon (starch) | − |
| d-fructose | + | Glycogen | − |
| d-mannose | − | Xylitol | + |
| l-sorbose | − | Gentiobiose | − |
| l-rhamnose | − | d-turanose | − |
| Dulcitol | − | d-lyxose | + |
| Inositol | + | d-tagatose | - |
| d-mannitol | + | d-fuccose | − |
| d-sorbitol | − | l-fuccose | − |
| Methyl-a-d-mannopytanoside | + | d-arabitol | − |
| Methyl-a-d-glucopyranoside | + | l-arabitol | + |
| N-acetylglucosamine | + | Potassium Gluconate | − |
| Amygdalin | + | Potassium 2-Ketogluconate | − |
| Arbutin | − | Potassium 5-Ketogluconate | − |
| =MIC | <MIC | >MIC | mg/L | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | A 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | B 12 |
| Ampicillin | P | 0.032 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | N |
| Gentamycin | P | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | N |
| Kanamycin | P | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | N |
| Streptomycin | P | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | N |
| Erythromycin | P | 0.016 | 0.032 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | N |
| Clindamycin | P | 0.032 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | N |
| Tetracycline | P | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | N |
| Chloramphenicol | P | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | N |
| Temperature Stability Test CFS (10-Fold) | |||||
|---|---|---|---|---|---|
| Temperature | Time | 24 h | 48 h | 72 h | 96 h |
| 30 °C | 5 min | + | + | + | + |
| 10 min | + | + | + | + | |
| 15 min | + | + | + | + | |
| 45 °C | 5 min | + | + | + | + |
| 10 min | + | + | + | + | |
| 15 min | + | + | + | + | |
| 60 °C | 5 min | + | + | + | + |
| 10 min | + | + | + | + | |
| 15 min | + | + | + | + | |
| 90 °C | 5 min | + | + | + | + |
| 10 min | + | + | + | + | |
| 15 min | + | + | + | + | |
| 121 °C | 5 min | + | + | + | + |
| 10 min | + | + | + | + | |
| 15 min | + | + | + | + | |
| Cytopathogenic Reduction Effect of CSF of L. Plantarum YML015 | |||||
|---|---|---|---|---|---|
| Sample Name | Two-Fold Dilution | ||||
| 20 | 21 | 22 | 23 | 24 | |
| CSF (1-fold) | + | 0 | 0 | 0 | 0 |
| CSF (10-fold) | ++ | + | 0 | 0 | 0 |
| Hemagglutination Inhibition Effect of CSF of L. plantarum YML015 | |||||||
|---|---|---|---|---|---|---|---|
| Sample Name | Two-Fold Dilution | ||||||
| 20 | 21 | 22 | 23 | 24 | 25 | 26 | |
| a CSF (10-fold) | ++ | ++ | ++ | ++ | + | 0 | 0 |
| b HKCSF (10-fold) | ++ | ++ | ++ | ++ | + | 0 | 0 |
| Antiviral Activity of Lactobacillus plantarum YML015 Using Specific Pathogen-Free (SPF) Eggs | |||
|---|---|---|---|
| Group | Dilution | Survival/Total | % |
| IFVA (H1N1) + CFS a | 1:104 | 1/2 | 50% |
| IFVA (H1N1) + CFS (10 fold) b | 1:104 | 2/2 | 100% |
| IFVA (H1N1) + HKCFS (10 fold) c | 1:104 | 2/2 | 100% |
| IFVA (H1N1) + CM d | 1:104 (3 × 108 CFU/mL) | 1/2 | 50% |
| IFVA (H1N1) + T e | 5 mg/0.1 mL | 1/2 | 50% |
| IFVA (H1N1) + PBS f | 1:104 | 0/2 | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majumder, R.; Alam, M.B.; Paudel, K.R.; Ahmed, K.A.; Devkota, H.P.; Lee, S.-H.; Hansbro, P.M.; Park, Y.-H. Anti-Influenza Virus Potential of Probiotic Strain Lactoplantibacillus plantarum YML015 Isolated from Korean Fermented Vegetable. Fermentation 2022, 8, 572. https://doi.org/10.3390/fermentation8110572
Majumder R, Alam MB, Paudel KR, Ahmed KA, Devkota HP, Lee S-H, Hansbro PM, Park Y-H. Anti-Influenza Virus Potential of Probiotic Strain Lactoplantibacillus plantarum YML015 Isolated from Korean Fermented Vegetable. Fermentation. 2022; 8(11):572. https://doi.org/10.3390/fermentation8110572
Chicago/Turabian StyleMajumder, Rajib, Md Badrul Alam, Keshav Raj Paudel, Khandaker Asif Ahmed, Hari Prasad Devkota, Sang-Han Lee, Philip M. Hansbro, and Yong-Ha Park. 2022. "Anti-Influenza Virus Potential of Probiotic Strain Lactoplantibacillus plantarum YML015 Isolated from Korean Fermented Vegetable" Fermentation 8, no. 11: 572. https://doi.org/10.3390/fermentation8110572
APA StyleMajumder, R., Alam, M. B., Paudel, K. R., Ahmed, K. A., Devkota, H. P., Lee, S.-H., Hansbro, P. M., & Park, Y.-H. (2022). Anti-Influenza Virus Potential of Probiotic Strain Lactoplantibacillus plantarum YML015 Isolated from Korean Fermented Vegetable. Fermentation, 8(11), 572. https://doi.org/10.3390/fermentation8110572











