Effects of the Addition of Dendrobium officinale on Beer Yeast Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Brewing Process
2.3. Measurements of pH, Total Acidity, CO2 Loss, Foam Height, Yeast Number and Reducing Sugar and Alcohol Concentrations
2.4. Volatile Compounds
2.5. Consumer Test
2.6. Determination of Glucose Uptake by Yeast
2.7. Determination of the Cell Viability of Yeast
2.8. Determination of Glucose-6-Phosphate Dehydrogenase and Alcohol Dehydrogenase Activities
2.9. Determination of Total Phenolics and Antioxidant Activity
2.10. Statistical Analysis
3. Results
3.1. Effects of D. officinale on the Growth and Metabolism of S. cerevisiae
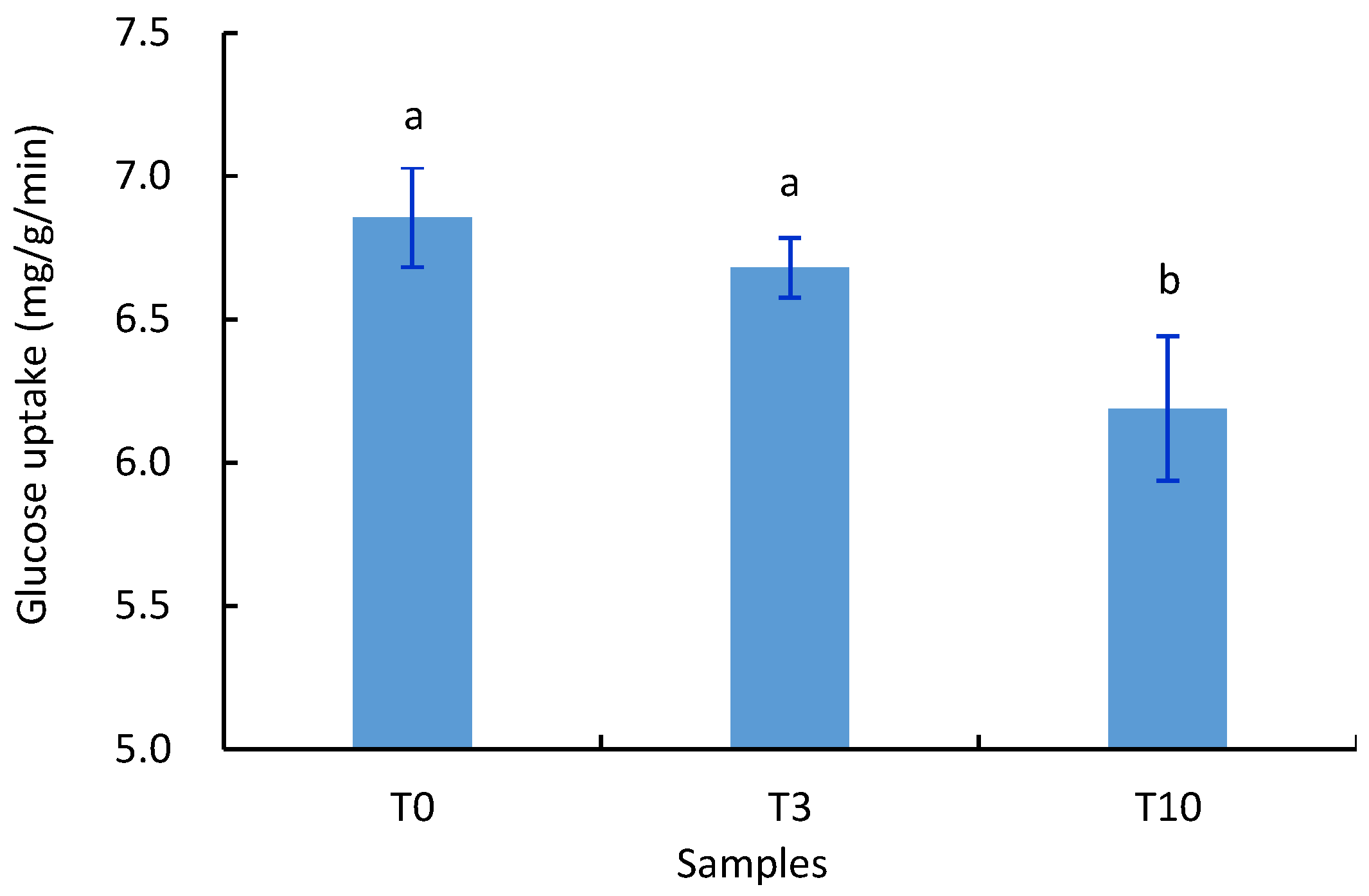
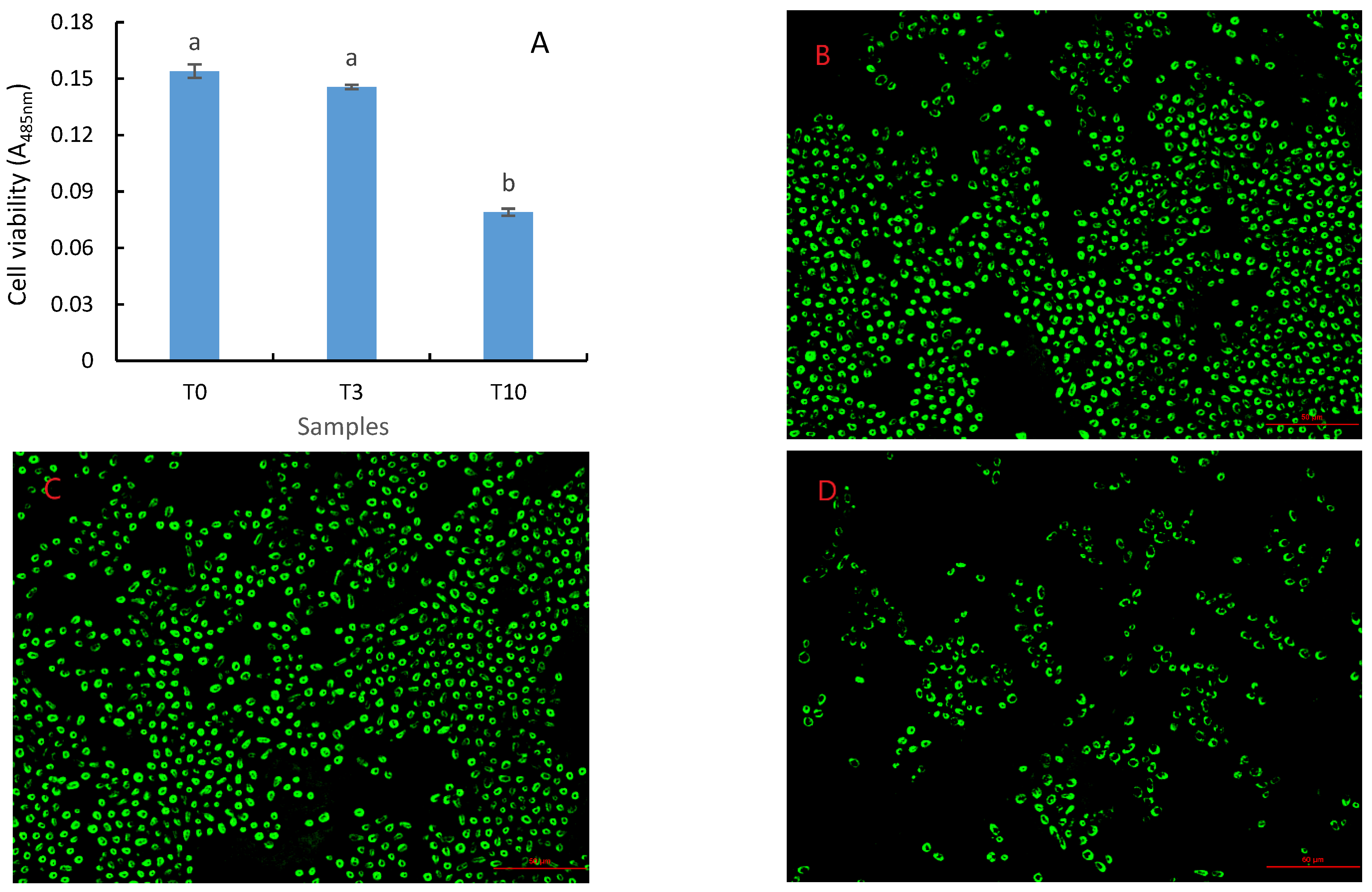
3.2. Effects of D. officinale on the Glucose Uptake and Cell Viability of S. cerevisiae
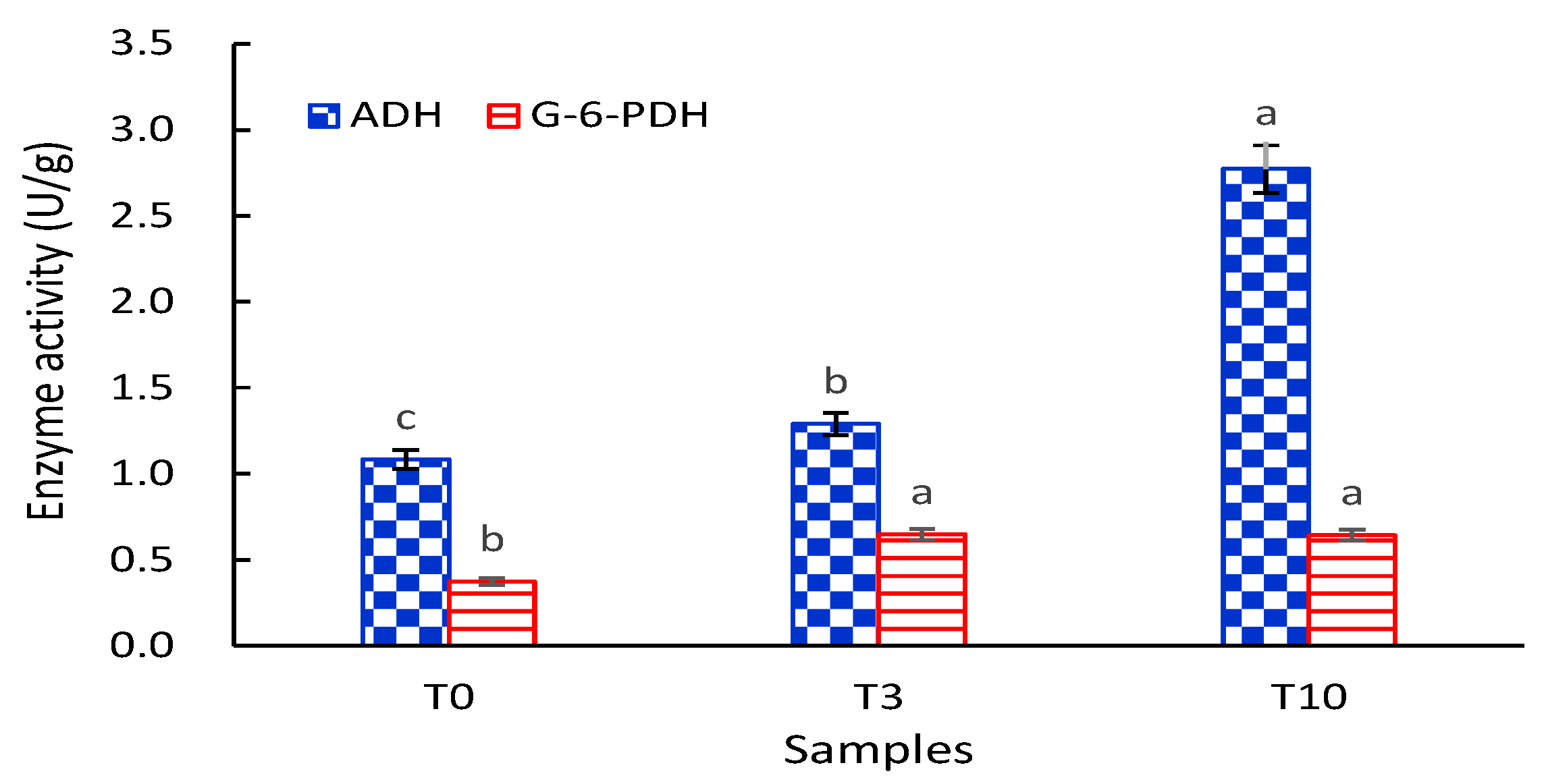
3.3. Effects of D. officinale on the ADH and G6PDH Activities of S. cerevisiae
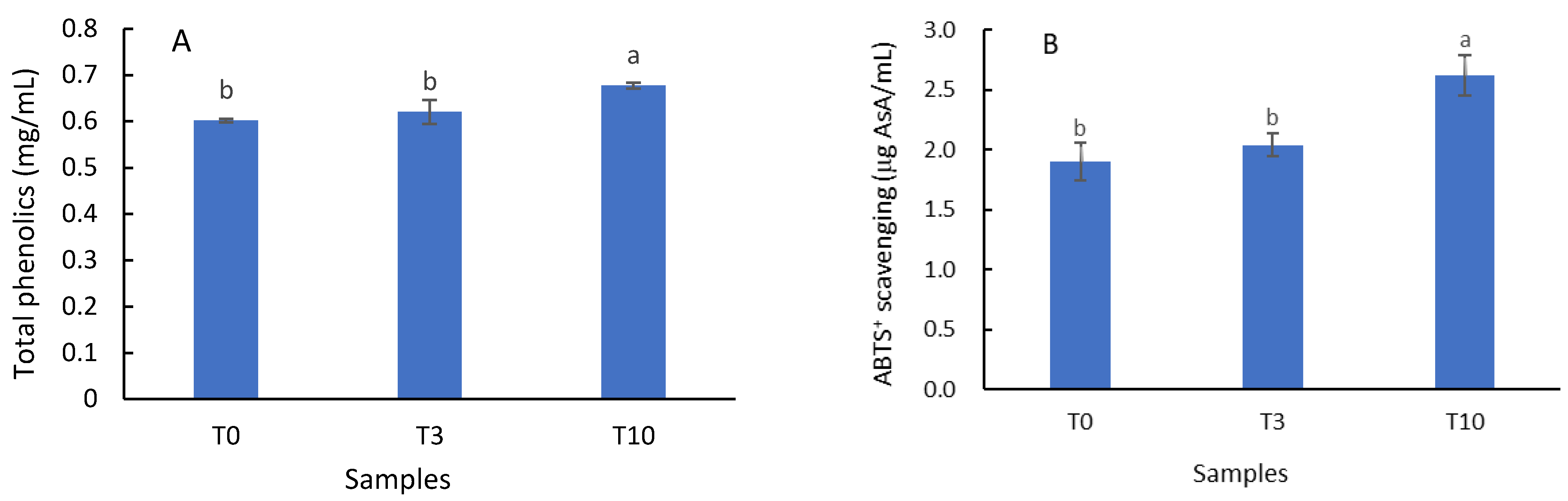
3.4. Total Phenolic Content and Antioxidant Activity
3.5. Consumer Acceptance
3.6. Volatile Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, R.C.N.; Galvan, D.; Effting, L.; Terhaag, M.M.; Yamashita, F.; Benassi, M.D.T.; Spinosa, W.A. Effects of adding spices with antioxidants compounds in red ale style craft beer: A simplex-centroid mixture design approach. Food Chem. 2021, 365, 130478. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Kim, J.; Bashir, K.M.I.; Cho, M.G. Antioxidant content of Aronia infused beer. Fermentation 2020, 6, 71. [Google Scholar] [CrossRef]
- Gómez-Corona, C.; Lelievre-Desmas, M.; Buendía, H.B.E.; Chollet, S.; Valentin, D. Craft beer representation amongst men in two different cultures. Food Qual. Prefer. 2016, 53, 19–28. [Google Scholar] [CrossRef]
- Fang, H.; Wu, H.L.; Wang, T.; Chen, Y.; Chang, Y.Y.; Ding, Y.J.; Yu, R.Q. Authentication of craft and industrial beers by excitation-emission matrix fluorescence spectroscopy and chemometrics. Microchem. J. 2022, 181, 107650. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Guglielmotti, M.; Passaghe, P.; Buiatti, S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J. Food Sci. 2020, 85, 2278–2285. [Google Scholar] [CrossRef]
- Nardini, M.; Foddai, M.S. Phenolics profile and antioxidant activity of special beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef]
- Paiva, R.A.M.; Mutz, Y.S.; Conte-Junior, C.A. A Review on the obtaining of functional beers by addition of non-cereal adjuncts rich in antioxidant compounds. Antioxidants 2021, 10, 1332. [Google Scholar] [CrossRef]
- Wan, J.; Gong, X.; Wang, F.; Wen, C.; Wei, Y.; Han, B.; Ouyang, Z. Comparative analysis of chemical constituents by HPLC–ESI–MSn and antioxidant activities of Dendrobium huoshanense and Dendrobium officinale. Biomed. Chromatogr. 2022, 36, e5250. [Google Scholar] [CrossRef]
- Guo, X.; Liu, S.; Wang, Z.; Zhang, G. Ultrasonic-assisted extraction of polysaccharide from Dendrobium officinale: Kinetics, thermodynamics and optimization. Biochem. Eng. J. 2022, 177, 108227. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liu, J.; Liang, J.; Si, J.; Wu, S. Dendrobium officinale leaves as a new antioxidant source. J. Funct. Foods 2017, 37, 400–415. [Google Scholar] [CrossRef]
- Luo, D.; Qu, C.; Zhang, Z.; Xie, J.; Xu, L.; Yang, H.; Li, C.; Lin, G.; Wang, H.; Su, Z. Granularity and laxative effect of ultrafine powder of Dendrobium officinale. J. Med. Food 2017, 20, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Duan, W.Y.; Zou, J.X.; Zhou, H.B.; Liu, C.W.; Yang, H.L. Flavor and antioxidant activity improvement of carrot juice by fermentation with Lactobacillus plantarum WZ-01. J. Food Meas. Charact. 2019, 13, 3366–3375. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Głowacki, A. Assessment of volatiles and polyphenol content, physicochemical parameters and antioxidant activity in beers with dotted hawthorn (Crataegus punctata). Foods 2020, 9, 775. [Google Scholar] [CrossRef]
- Lazzari, A.; Barbosa, H.D.; Machado, E.R.; da Silva, L.H.M.; Anjo, F.A.; Sato, F.; Rosa, C.I.L.F.; Pintro, P.T.M. Effect on bioactive compounds and antioxidant activity in the brewing process for beers using rubim and mastruz as hop replacements. J. Am. Soc. Brew. Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Somnath, D.B.; Mangesh, A.B.; Dheeraj, S.R.; Wadkar, G.H.; Hasabe, T.S. In vitro hypoglycemic effects of unripe and ripe fruits of Musa sapientum. Braz. J. Pharm. Sci. 2017, 53, e00159. [Google Scholar]
- Shi, C.; Wu, Y.; Fang, D.; Ma, N.; Mariga, A.M.; Hu, Q.; Yang, W. Nanocomposite packaging regulates extracellular ATP and programed cell death in edible mushroom (Flammulina velutipes). Food Chem. 2020, 309, 125702. [Google Scholar] [CrossRef]
- Yuan, Y.; Heinzle, E. Permeabilization of Corynebacterium glutamicum for NAD(P)H-dependent intracellular enzyme activity measurement. Comptes Rendus Chim. 2009, 12, 1154–1162. [Google Scholar] [CrossRef]
- van Rijswijck, I.M.H.; Kruis, A.J.; Wolkers-Rooijackers, J.C.M.; Abee, T.; Smid, E.J. Acetate-ester hydrolase activity for screening of the variation in acetate ester yield of Cyberlindnera fabianii, Pichia kudriavzevii and Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2019, 104, 8–15. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Cloudy wheat beer enriched with okra [Abelmoschus esculentus (L.) Moench]: Effects on volatile compound and sensorial attributes. Int. J. Food Prop. 2018, 21, 289–300. [Google Scholar] [CrossRef]
- Bustos, L.; Soto, E.; Parra, F.; Echiburu-Chau, C.; Parra, C. Brewing of a porter craft beer enriched with the plant Parastrephia lucida: A promising source of antioxidant compounds. J. Am. Soc. Brew. Chem. 2019, 77, 261–266. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci. Technol. 2021, 110, 754–764. [Google Scholar] [CrossRef]
- Đordević, S.; Popović, D.; Despotović, S.; Veljović, M.; Atanacković, M.; Cvejić, J.; Nedović, V.; Leskošek-Čukalović, I. Extracts of medicinal plants as functional beer additives. Chem. Ind. Chem. Eng. Q. 2016, 22, 301–308. [Google Scholar]
- Li, X.J.; Dong, L.; Guo, J.Q.; Zhao, S.J.; Zhao, C.X. Effects of beer barm of different generation on the activity of key enzymes of endocellular metabolism. Liquor Making Sci. Tech. 2009, 2, 40–42. (In Chinese) [Google Scholar]
- Rossi, F.G.; Silva, D.P.; Silva, J.B.A.E.; Taqueda, M.E.; Vitolo, M.; Pessoa, A., Jr. Effect of cultivation conditions on glucose-6-phosphate dehydrogenase production by genetically modified Saccharomyces cerevisiae. Braz. J. Chem. Eng. 2009, 26, 1–9. [Google Scholar] [CrossRef]
- Choi, J.T.; Joo, H.K.; Lee, S.K. The effect of schizandrae fructus extract on alcohol fermentation and enzyme activities of Saccharomyces cerevisiae. Agric. Chem. Biotechnol. 1995, 38, 278–282. [Google Scholar]
- Ji, X.Y. Comparative investigation of volatile components and bioactive compounds in beers by multivariate analysis. Flavour Frag. J. 2021, 36, 374–383. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Tuszyński, T. The effect of temperature on fermentation and beer volatiles at an industrial scale. J. Inst. Brew. 2018, 124, 230–235. [Google Scholar] [CrossRef]
- Yin, H.; He, Y.; Deng, Y.; Dong, J.J.; Lu, J.; Chen, L. Application of Plackett–Burman experimental design for investigating the effect of wort amino acids on flavour-active compounds production during lager yeast fermentation. J. Inst. Brew. 2017, 123, 300–311. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Zdaniewicz, M.; Pater, A.; Skoneczny, S. Impact of triticale malt application on physiochemical composition and profile of volatile compounds in beer. Eur. Food Res. Technol. 2019, 245, 1431–1437. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. LWT-Food Sci. Technol. 2019, 103, 139–146. [Google Scholar]
- Hu, J.; Huang, W.X.; Zhang, F.T.; Luo, X.D.; Chen, Y.L.; Xie, J.K. Variability of volatile compounds in the medicinal plant Dendrobium officinale from different regions. Molecules 2020, 25, 5046. [Google Scholar] [CrossRef]


| Parameters | Samples | ||||
|---|---|---|---|---|---|
| Control (T0) | T1 | T3 | T5 | T10 | |
| Reducing sugars (g/L) | 10.47 ± 3.63 a | 9.56 ± 0.70 b | 8.87 ± 0.21 b | 7.15 ± 0.20 bc | 9.14 ± 1.25 c |
| The number of yeasts (×107 yeast/mL) | 5.01 ± 0.83 c | 7.75 ± 0.88 ab | 9.83 ± 0.65 a | 9.12 ± 1.33 a | 5.49 ± 0.10 bc |
| Total acidity (g/100 mL) | 2.71 ± 0.07 a | 1.74 ± 0.12 c | 1.60 ± 0.04 cd | 1.50 ± 0.07 d | 2.01 ± 0.11 b |
| pH | 4.54 ± 0.02 b | 4.40 ± 0.01 d | 4.41 ± 0.02 d | 4.46 ± 0.02 c | 4.67 ± 0.02 a |
| Alcohol (%) | 4.70 ± 0.12 bc | 5.87 ± 0.31 ab | 6.27 ± 0.12 a | 6.30 ± 0.10 a | 4.10 ± 1.25 c |
| CO2 loss (g/100 mL) | 3.49 ± 0.25 cd | 4.22 ± 0.04 bc | 4.65 ± 0.05 ab | 5.41 ± 0.75 a | 2.95 ± 0.10 d |
| Foam height (mm) | 1.50 ± 0.50 d | 3.67 ± 0.42 c | 5.43 ± 0.49 b | 5.83 ± 0.35 b | 9.10 ± 0.10 a |
| Number | Compound | Molecular Weight | CAS Number | Samples | |
|---|---|---|---|---|---|
| T0 | T3 | ||||
| Alcohols | |||||
| 1 | 1-Propanol | 60.06 | 000071-23-8 | 0.051 | 0.010 |
| 2 | 1-Propanol, 2-methyl- | 74.07 | 000078-83-1 | 0.147 | 0.042 |
| 3 | 1-Butanol, 3-methyl- | 88.09 | 000123-51-3 | 4.403 | 1.188 |
| 4 | 1,6-Octadien-3-ol, 3,7-dimethyl- | 154.14 | 000078-70-6 | - | 0.006 |
| 5 | 1-Octanol | 130.14 | 000111-87-5 | - | 0.010 |
| 6 | 2-Furanmethanol | 98.04 | 000098-00-0 | 0.144 | 0.028 |
| 7 | 3-Methoxybenzyl alcohol | 138.07 | 006971-51-3 | - | 0.011 |
| 8 | Benzyl alcohol | 108.06 | 000100-51-6 | 0.005 | - |
| 9 | Phenylethyl alcohol | 122.07 | 000060-12-8 | 1.829 | 0.429 |
| Esters | |||||
| 10 | 1-Butanol, 3-methyl-, acetate | 130.1 | 000123-92-2 | 0.042 | 0.228 |
| 11 | Hexanoic acid, ethyl ester | 144.12 | 000123-66-0 | 0.016 | 0.036 |
| 12 | Octanoic acid, ethyl ester | 130.14 | 006169-06-8 | 0.090 | 0.333 |
| 13 | Undecanoic acid, ethyl ester | 172.15 | 000106-32-1 | - | 0.005 |
| 14 | 2-Furanmethanol, acetate | 140.05 | 000623-17-6 | 0.024 | 0.008 |
| 15 | Decanoic acid, ethyl ester | 200.18 | 000110-38-3 | - | 0.003 |
| 16 | Acetic acid, 2-phenylethyl ester | 164.08 | 000103-45-7 | - | 0.155 |
| 17 | Acetic acid, phenyl ester | 136.05 | 000122-79-2 | 0.007 | - |
| 18 | Tributyl phosphate | 266.17 | 000126-73-8 | - | 0.004 |
| 19 | 9,12-Octadecadienoic acid, methyl ester | 294.26 | 002566-97-4 | 0.009 | - |
| 20 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 278.15 | 000084-69-5 | - | 0.007 |
| 21 | Dibutyl phthalate | 278.15 | 000084-74-2 | 0.037 | 0.015 |
| 22 | Hexanedioic acid, bis(2-ethylhexyl) ester | 370.31 | 000103-23-1 | - | 0.007 |
| Acids | |||||
| 23 | Acetic acid | 60.02 | 000064-19-7 | - | 0.029 |
| 24 | Butanoic acid | 88.05 | 000107-92-6 | 0.012 | - |
| 25 | Hexanoic acid | 116.08 | 000142-62-1 | 0.319 | 0.078 |
| 26 | Octanoic acid | 144.12 | 000124-07-2 | 1.979 | 0.733 |
| 27 | Nonanoic acid | 158.13 | 000112-05-0 | 0.041 | 0.017 |
| 28 | n-Decanoic acid | 172.15 | 000334-48-5 | 0.258 | 0.020 |
| 29 | Benzoic acid | 122.04 | 000065-85-0 | 0.027 | 0.007 |
| 30 | 9-Decenoic acid | 170.13 | 014436-32-9 | 0.084 | 0.019 |
| 31 | Dodecanoic acid | 200.18 | 000143-07-7 | 0.037 | 0.003 |
| 32 | n-Hexadecanoic acid | 256.24 | 000057-10-3 | - | 0.015 |
| Aldehydes | |||||
| 33 | Furfural | 96.02 | 000098-01-1 | 0.024 | - |
| 34 | Benzaldehyde | 106.04 | 000100-52-7 | 0.035 | 0.002 |
| 35 | Benzeneacetaldehyde | 120.06 | 000122-78-1 | 0.051 | 0.014 |
| 36 | Benzaldehyde, 2,4-dimethyl- | 134.07 | 015764-16-6 | - | 0.014 |
| 37 | Benzaldehyde, 2,4,6-trimethyl- | 148.09 | 000487-68-3 | - | 0.016 |
| Others | |||||
| 38 | Isomaltol | 126.03 | 003420-59-5 | 0.069 | 0.010 |
| 39 | 1-Propanol, 3-(methylthio)- | 106.05 | 000505-10-2 | 0.005 | - |
| 40 | Ethanone, 1-(1H-pyrrol-2-yl)- | 109.05 | 001072-83-9 | - | 0.003 |
| 41 | 2(3H)-Furanone, dihydro-5-pentyl- | 156.12 | 000104-61-0 | - | 0.003 |
| 42 | 2-Methoxy-4-vinylphenol | 150.07 | 007786-61-0 | 0.178 | 0.056 |
| 43 | Phenol, 2,4-bis(1,1-dimethylethyl)- | 206.17 | 000096-76-4 | - | 0.06 |
| 44 | Benzofuran, 2,3-dihydro- | 120.06 | 000496-16-2 | - | 0.009 |
| 45 | Indole | 117.06 | 000120-72-9 | 0.016 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, L.; Yang, H.; Zhou, H. Effects of the Addition of Dendrobium officinale on Beer Yeast Fermentation. Fermentation 2022, 8, 595. https://doi.org/10.3390/fermentation8110595
Chen X, Li L, Yang H, Zhou H. Effects of the Addition of Dendrobium officinale on Beer Yeast Fermentation. Fermentation. 2022; 8(11):595. https://doi.org/10.3390/fermentation8110595
Chicago/Turabian StyleChen, Xiaolu, Linqiu Li, Hailong Yang, and Huabin Zhou. 2022. "Effects of the Addition of Dendrobium officinale on Beer Yeast Fermentation" Fermentation 8, no. 11: 595. https://doi.org/10.3390/fermentation8110595
APA StyleChen, X., Li, L., Yang, H., & Zhou, H. (2022). Effects of the Addition of Dendrobium officinale on Beer Yeast Fermentation. Fermentation, 8(11), 595. https://doi.org/10.3390/fermentation8110595






