Thermophilic Water Gas Shift Reaction at High Carbon Monoxide and Hydrogen Partial Pressures in Parageobacillus thermoglucosidasius KP1013
Abstract
1. Introduction
2. Materials and Methods
3. Results
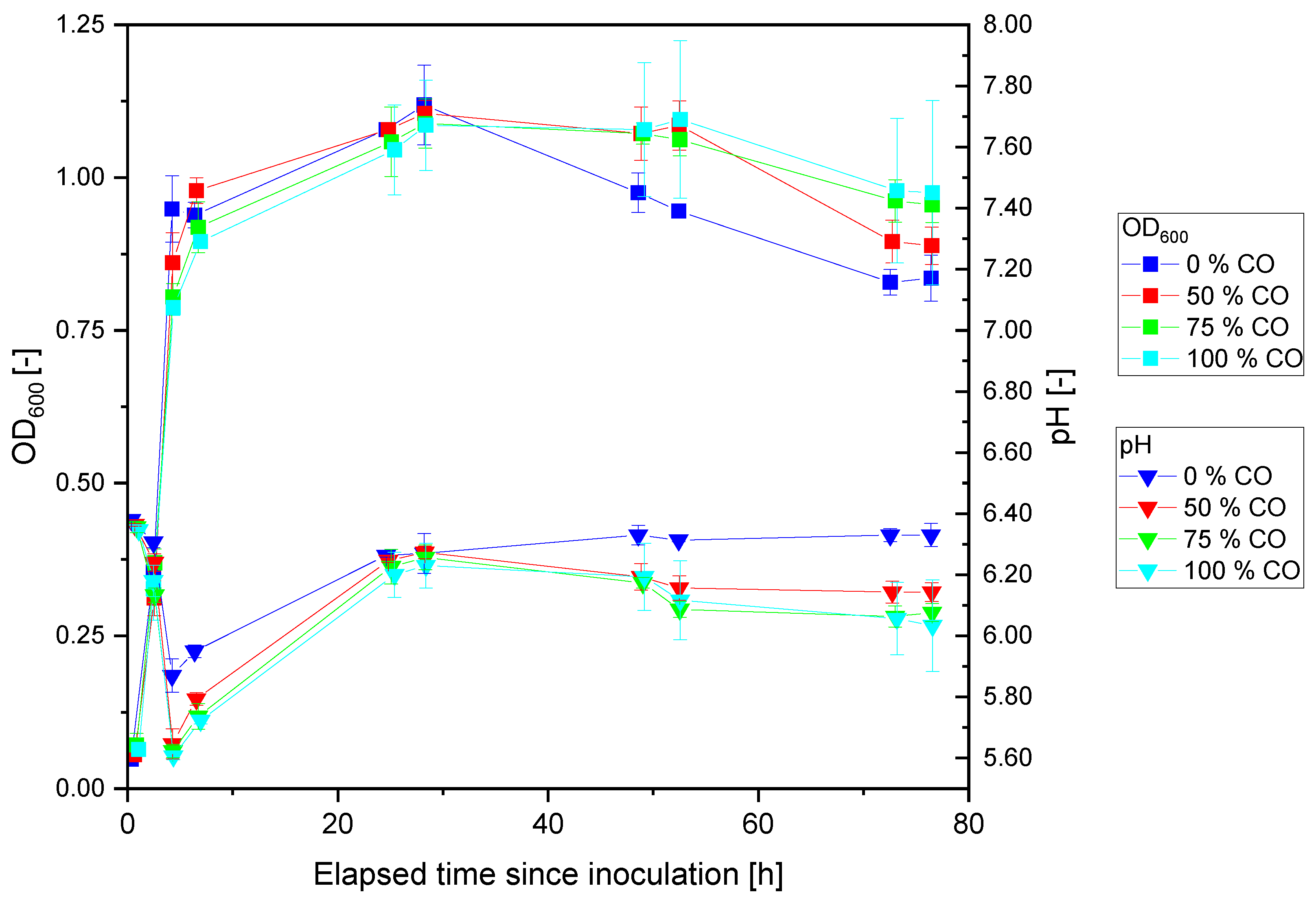
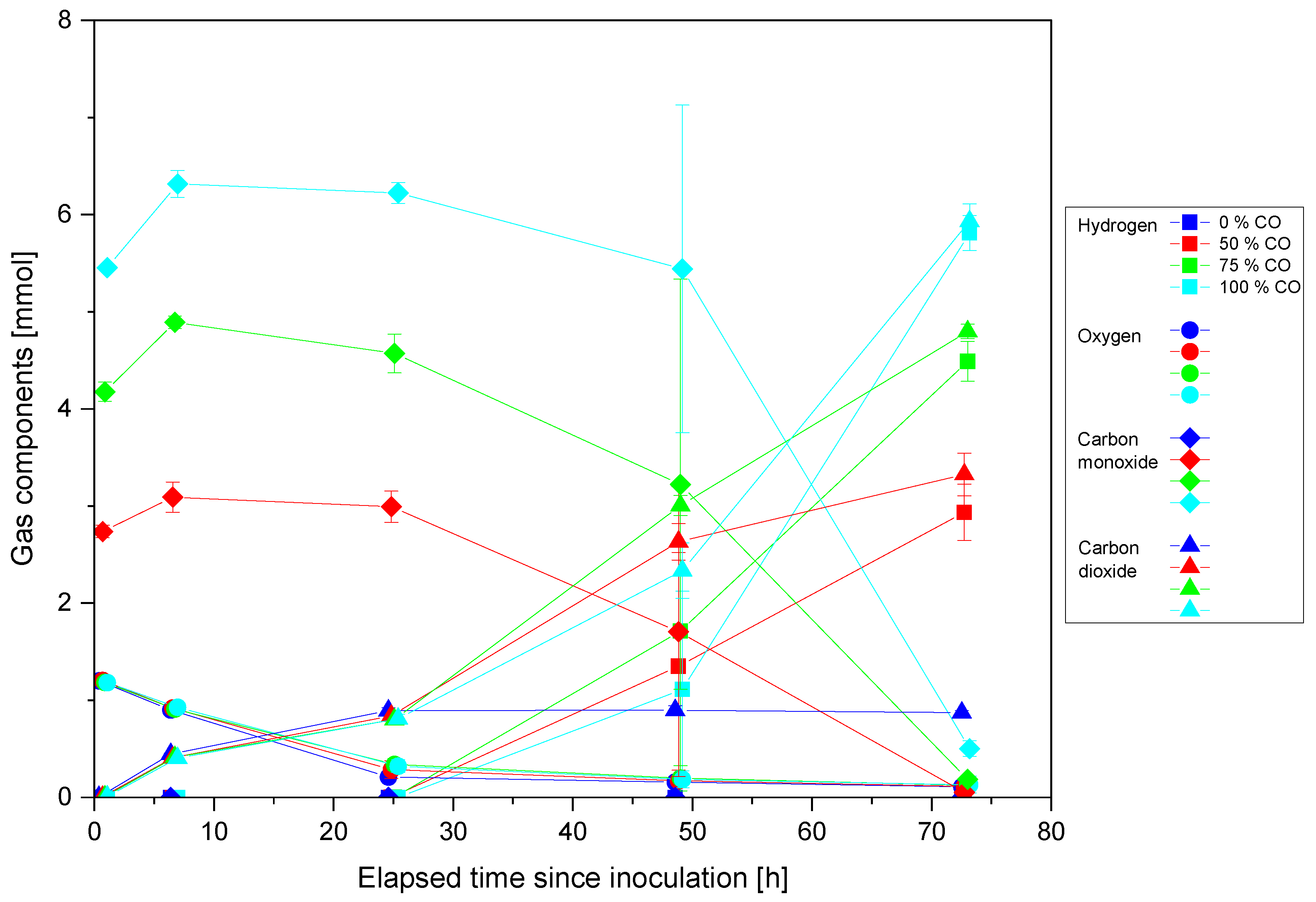
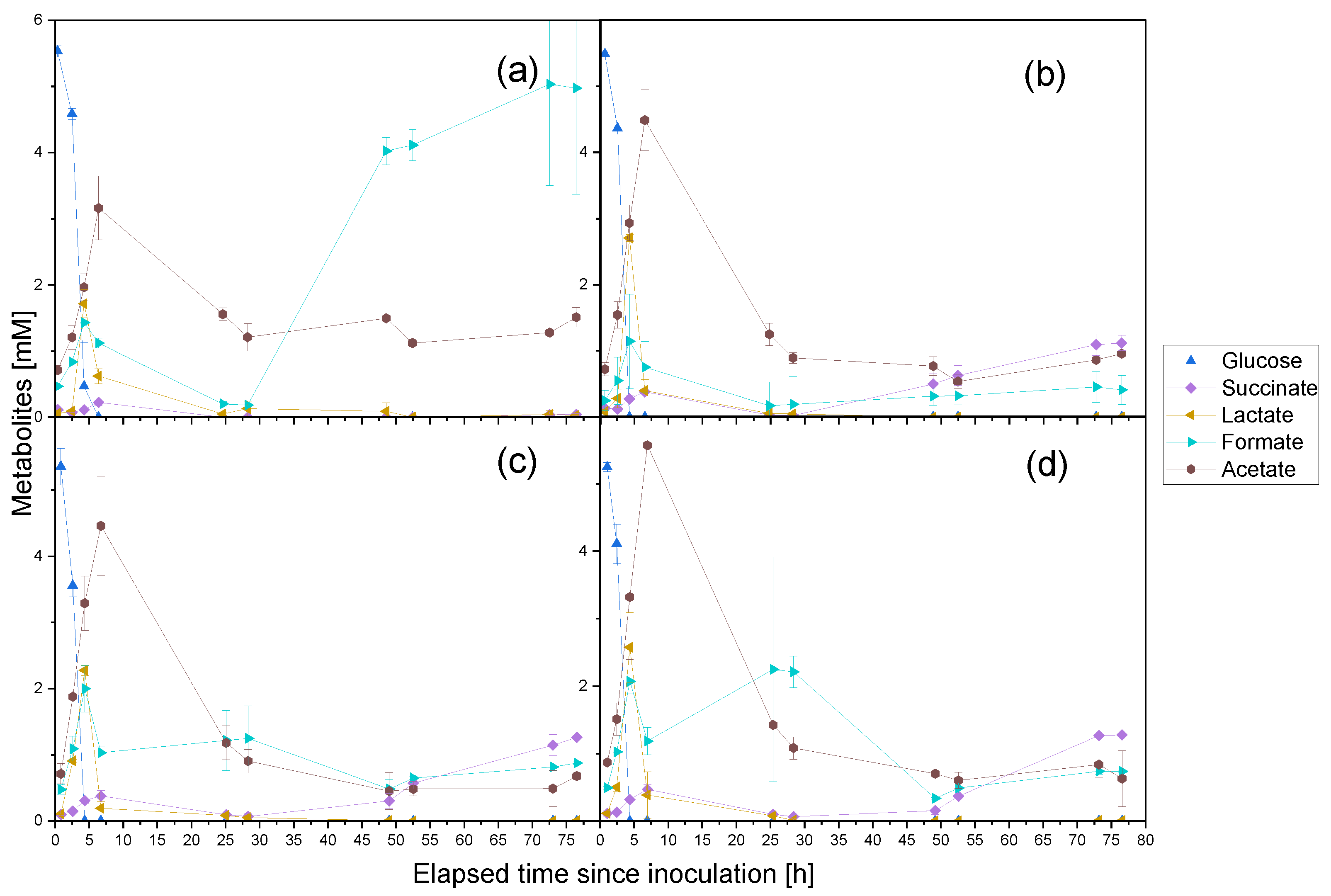
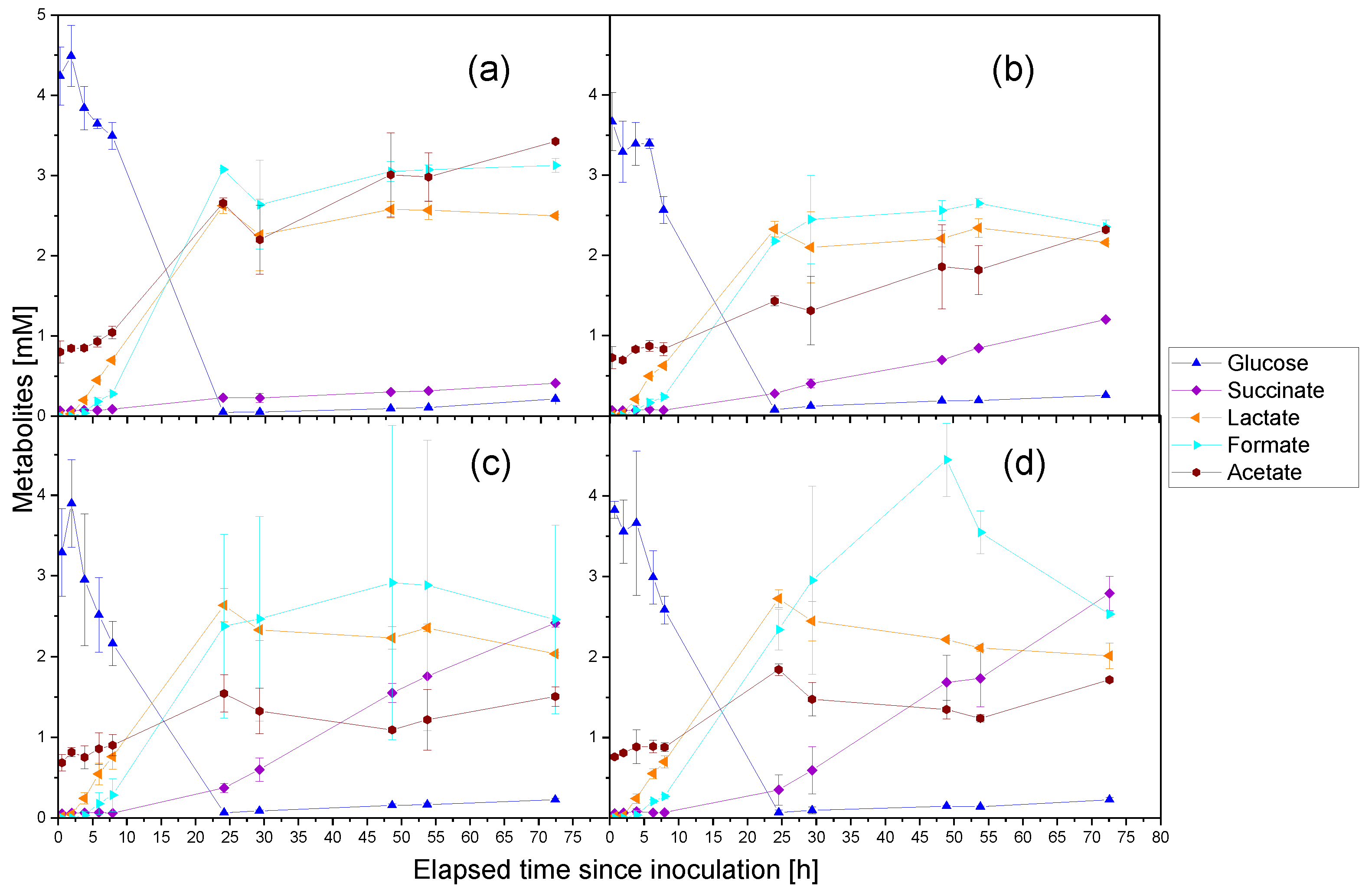
3.1. Cultivation of P. thermoglucosidasius KP1013 under Varying CO Concentrations at Atmospheric Pressure in the Presence of Oxygen
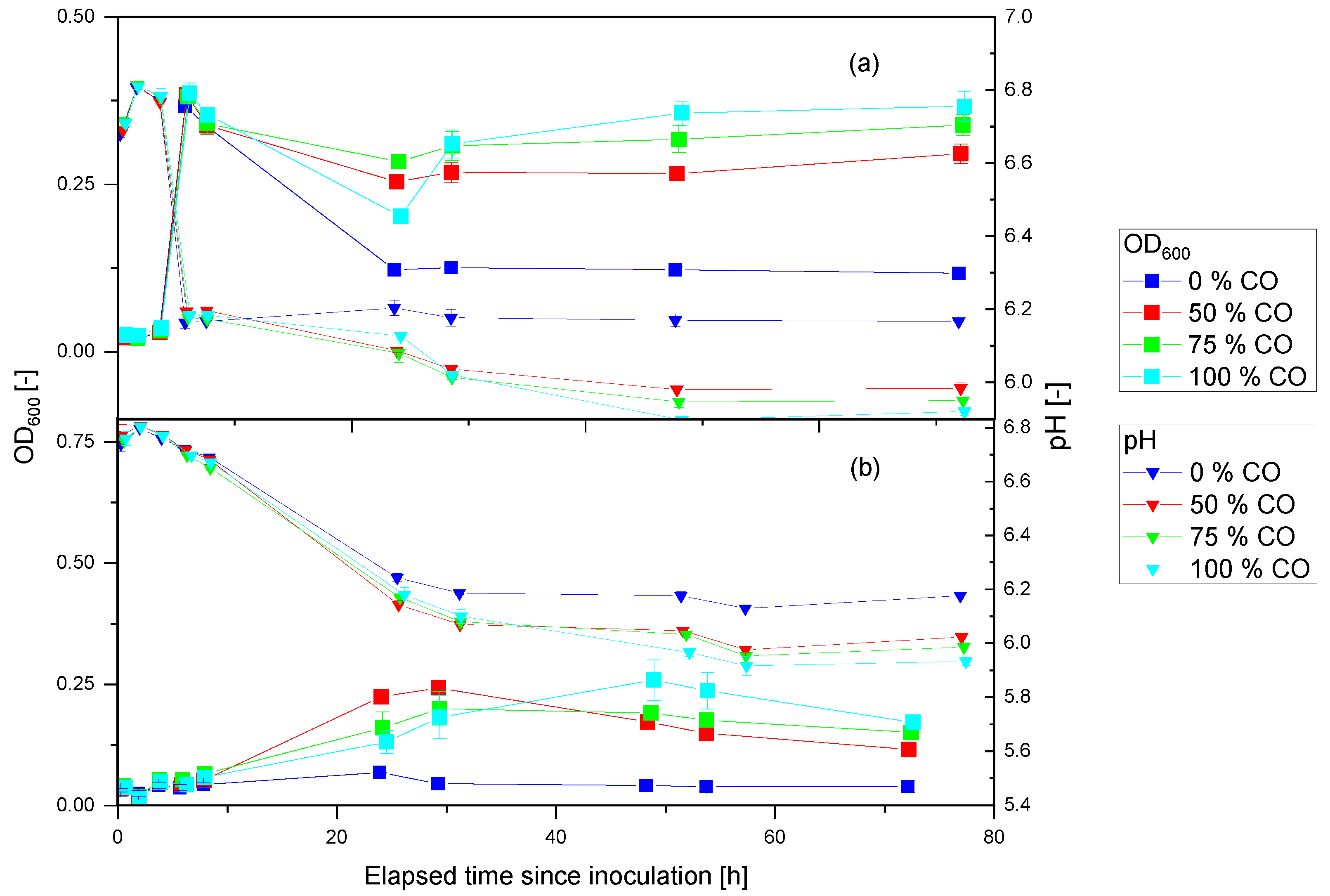
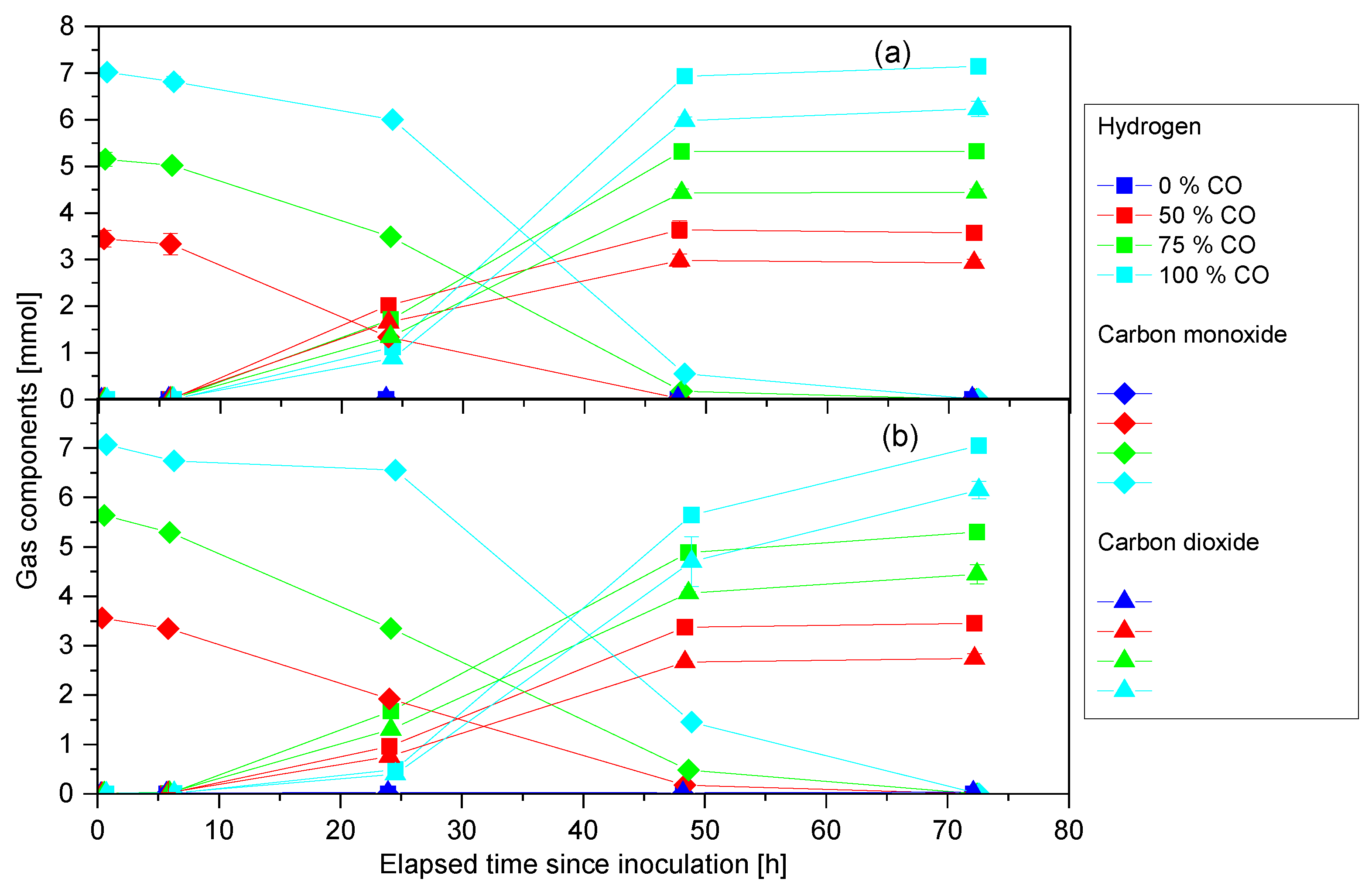
3.2. Anaerobic Cultivation of P. thermoglucosidasius DSM 6285 and KP1013 under Varying CO Concentrations at Atmospheric Pressure
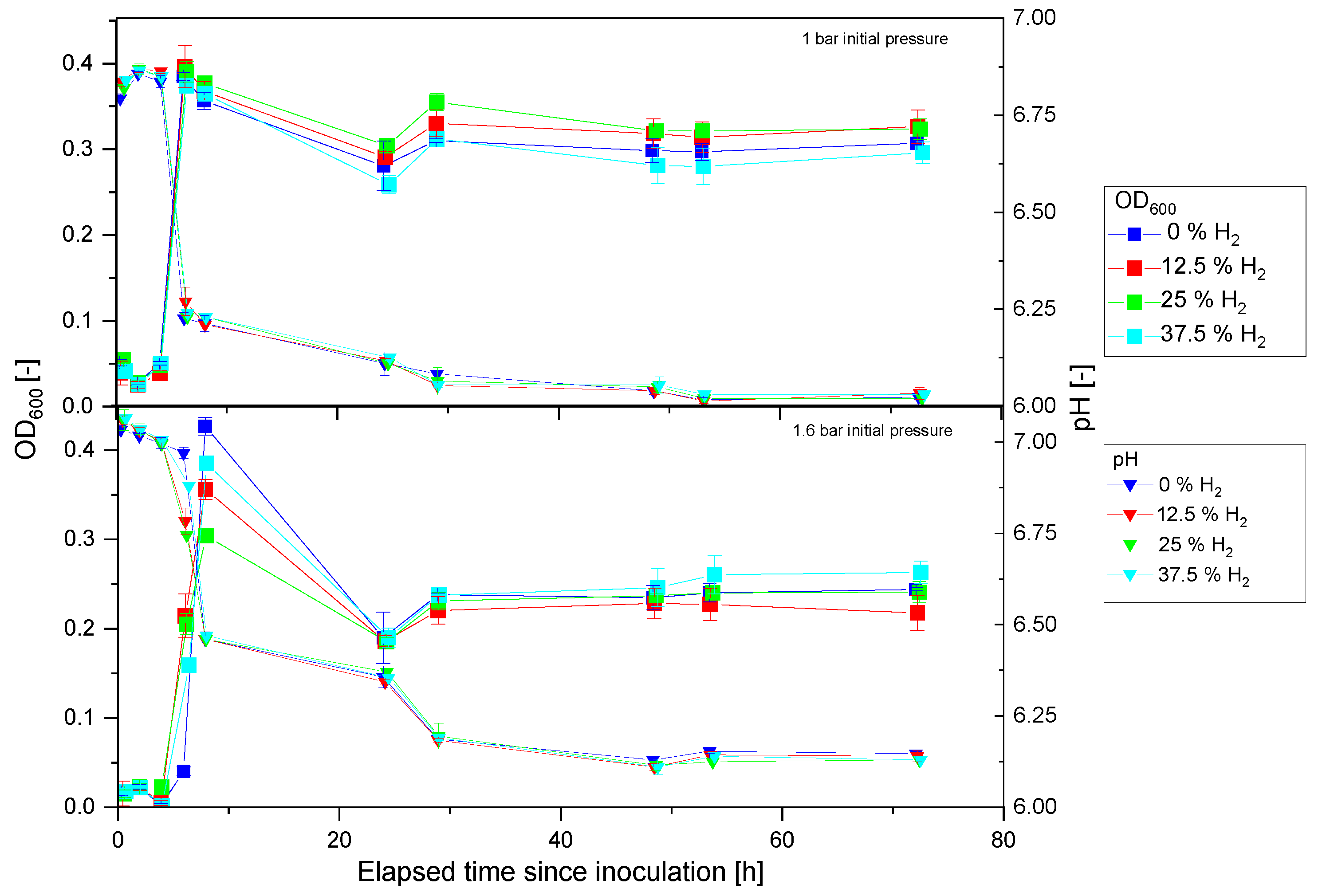
3.3. Anaerobic Cultivation of P. thermoglucosidasius KP1013 under Varying Hydrogen Concentrations
4. Discussion
4.1. Carbon Monoxide Induced Rerouting of Carbon Flux during Initial Aerobic Growth Phase
4.2. Hydrogen Enhanced P. thermoglucosidasius Growth and Does Not Inhibit the WGS Reaction at High Temperature
4.3. Initial Aerobic vs. Anaerobic Cultivation of P. thermoglucosidasius for WGS Reaction-Based Hydrogen Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winter, C.J. Hydrogen energy—Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Marchenko, O.; Solomin, S. The future energy: Hydrogen versus electricity. Int. J. Hydrogen Energy 2015, 40, 3801–3805. [Google Scholar] [CrossRef]
- Matar, S. Preface to First Edition. In Chemistry of Petrochemical Processes, 2nd ed.; Matar, S., Hatch, L.F., Eds.; Gulf Professional Publishing: Woburn, MA, USA, 2001; pp. xiii–xvi. [Google Scholar] [CrossRef]
- BP. BP Statistical Review of World Energy 2020; BP Plc: London, UK, 2020; pp. 1–6. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 20 September 2022).
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.W. Hydrogen-A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhao, W.; Xia, D. The diversity of hydrogen-producing bacteria and methanogens within an in situ coal seam. Biotechnol. Biofuels 2018, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, A.; Baskaran, D.; Pakshirajan, K. Sustainable biohydrogen production by dark fermentation using carbon monoxide as the sole carbon and energy source. Int. J. Hydrogen Energy 2019, 44, 13114–13125. [Google Scholar] [CrossRef]
- Ljunggren, M.; Willquist, K.; Zacchi, G.; van Niel, E.W. A kinetic model for quantitative evaluation of the effect of hydrogen and osmolarity on hydrogen production by Caldicellulosiruptor saccharolyticus. Biotechnol. Biofuels 2011, 4, 31. [Google Scholar] [CrossRef]
- van Niel, E.W.J.; Claassen, P.A.M.; Stams, A.J.M. Substrate and product inhibition of hydrogen production by the extreme thermophile, Caldicellulosiruptor saccharolyticus. Biotechnol. Bioeng. 2003, 81, 255–262. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Benemann, J.R. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Alfano, M.; Cavazza, C. The biologically mediated water–gas shift reaction: Structure, function and biosynthesis of monofunctional [NiFe]-carbon monoxide dehydrogenases. Sustain. Energy Fuels 2018, 2, 1653–1670. [Google Scholar] [CrossRef]
- Kung, Y.; Drennan, C.L. A role for nickel–iron cofactors in biological carbon monoxide and carbon dioxide utilization. Curr. Opin. Chem. Biol. 2011, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Soboh, B.; Linder, D.; Hedderich, R. Purification and catalytic properties of a CO-oxidizing: H2-evolving enzyme complex from Carboxydothermus hydrogenoformans. Eur. J. Biochem. 2002, 269, 5712–5721. [Google Scholar] [CrossRef]
- Maness, P.C.; Huang, J.; Smolinski, S.; Tek, V.; Vanzin, G. Energy Generation from the CO Oxidation-Hydrogen Production Pathway in Rubrivivax gelatinosus. Appl. Environ. Microbiol. 2005, 71, 2870–2874. [Google Scholar] [CrossRef]
- Welte, C.; Deppenmeier, U. Chapter thirteen—Proton Translocation in Methanogens. In Methods in Methane Metabolism, Part A; Rosenzweig, A.C., Ragsdale, S.W., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 494, pp. 257–280. [Google Scholar] [CrossRef]
- Schoelmerich, M.C.; Müller, V. Energy conservation by a hydrogenase-dependent chemiosmotic mechanism in an ancient metabolic pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 6329–6334. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Rittmann, S.K.M.; Lee, H.S.; Lim, J.K.; Kim, T.W.; Lee, J.H.; Kang, S.G. One-carbon substrate-based biohydrogen production: Microbes, mechanism, and productivity. Biotechnol. Adv. 2015, 33, 165–177. [Google Scholar] [CrossRef]
- Mohr, T.; Aliyu, H.; Küchlin, R.; Zwick, M.; Cowan, D.; Neumann, A.; de Maayer, P. Comparative genomic analysis of Parageobacillus thermoglucosidasius Strains Distinct hydrogenogenic capacities. BMC Genom. 2018, 19, 880. [Google Scholar] [CrossRef]
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Phylogenomic re-assessment of the thermophilic genus Geobacillus. Syst. Appl. Microbiol. 2016, 39, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kishigami, T.; Inoue, K.; Mizoguchi, Y.; Eto, N.; Takagi, M.; Abe, S. Bacillus thermoglucosidasius sp. Nov., A New Species Obligately Thermophilic Bacilli. Syst. Appl. Microbiol. 1983, 4, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Nazina, T.N.; Tourova, T.P.; Poltaraus, A.B.; Novikova, E.V.; Grigoryan, A.A.; Ivanova, A.E.; Lysenko, A.M.; Petrunyaka, V.V.; Osipov, G.A.; Belyaev, S.S.; et al. Taxonomic study of aerobic thermophilic bacilli: Descriptions of Geobacillus Subterraneus Gen. Nov., Sp. Nov. Geobacillus Uzenensis Sp. Nov. Pet. Reserv. Transf. Bacillus Stearothermophilus, Bacillus Thermocatenulatus, Bacillus Thermoleovorans, Bacillus Kaustophilus, Bacillus Thermodenitrificans Geobacillus New Comb. G. Stearothermophilus, G. Thermodenitrificans. Int. J. Syst. Evol. Microbiol. 2001, 51, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, D. The Geobacillus Paradox: Why Is A Thermophilic Bact. Genus So Prevalent A Mesophilic Planet? Microbiology 2013, 160 Pt 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.H.; Lisowska, B.K.; Leak, D.J. Chapter One—The Genus Geobacillus and Their Biotechnological Potential. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2015; Volume 92, pp. 1–48. [Google Scholar] [CrossRef]
- Takami, H.; Nishi, S.; Lu, J.; Shimamura, S.; Takaki, Y. Genomic characterization of thermophilic Geobacillus Species isolated from the deepest sea mud of the Mariana Trench. Extremophiles 2004, 8, 351–356. [Google Scholar]
- Marchant, R.; Franzetti, A.; Pavlostathis, S.G.; Tas, D.O.; Erdbrűgger, I.; Űnyayar, A.; Mazmanci, M.A.; Banat, I.M. Thermophilic bacteria in cool temperate soils: Are they metabolically active or continually added by global atmospheric transport? Appl. Microbiol. Biotechnol. 2008, 78, 841–852. [Google Scholar] [CrossRef]
- Zhou, J.; Lian, J.; Rao, C.V. Metabolic engineering of Parageobacillus thermoglucosidasius efficient production (2R, 3R)-Butanediol. Appl. Microbiol. Biotechnol. 2020, 104, 4303–4311. [Google Scholar] [CrossRef]
- Styles, M.Q.; Nesbitt, E.A.; Hoffmann, T.D.; Queen, J.; Ortenzi, M.V.; Leak, D.J. The heterologous production of terpenes by the thermophile Parageobacillus thermoglucosidasius A Consol. Bioprocess Using Waste Bread. Metab. Eng. 2021, 65, 146–155. [Google Scholar] [CrossRef]
- Fong, J.C.; Svenson, C.J.; Nakasugi, K.; Leong, C.T.; Bowman, J.P.; Chen, B.; Glenn, D.R.; Neilan, B.A.; Rogers, P.L. Isolation and characterization of two novel ethanol-tolerant facultative-anaerobic thermophilic bacteria strains from waste compost. Extremophiles 2006, 10, 363–372. [Google Scholar] [CrossRef]
- Tang, Y.J.; Sapra, R.; Joyner, D.; Hazen, T.C.; Myers, S.; Reichmuth, D.; Blanch, H.; Keasling, J.D. Analysis of metabolic pathways and fluxes in a newly discovered thermophilic and ethanol-tolerant Geobacillus Strain. Biotechnol. Bioeng. 2009, 102, 1377–1386. [Google Scholar] [CrossRef]
- Cripps, R.; Eley, K.; Leak, D.; Rudd, B.; Taylor, M.; Todd, M.; Boakes, S.; Martin, S.; Atkinson, T. Metabolic engineering of Geobacillus thermoglucosidasius High Yield Ethanol production. Metab. Eng. 2009, 11, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Eley, K.L.; Martin, S.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Thermophilic ethanologenesis: Future prospects for second-generation bioethanol production. Trends Biotechnol. 2009, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, H.; de Maayer, P.; Neumann, A. Not All That Glitters Is Gold: The Paradox of CO-dependent Hydrogenogenesis in Parageobacillus thermoglucosidasius. Front. Microbiol. 2021, 12, 3644. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, H.; Kastner, R.; Maayer, P.; Neumann, A. Carbon Monoxide Induced Metabolic Shift in the Carboxydotrophic Parageobacillus thermoglucosidasius DSM 6285. Microorganisms 2021, 9, 1090. [Google Scholar] [CrossRef]
- Meyer, O.; Schlegel, H.G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu. Rev. Microbiol. 1983, 37, 277–310. [Google Scholar] [CrossRef]
- Mohr, T.; Aliyu, H.; Küchlin, R.; Polliack, S.; Zwick, M.; Neumann, A.; Cowan, D.; de Maayer, P. CO-dependent hydrogen production by the facultative anaerobe Parageobacillus thermoglucosidasius. Microb. Cell Factories 2018, 17, 108. [Google Scholar] [CrossRef]
- Adachi, Y.; Inoue, M.; Yoshida, T.; Sako, Y. Genetic Engineering of Carbon Monoxide-dependent Hydrogen-producing Machinery in Parageobacillus thermoglucosidasius. Microbes Environ. 2020, 35, ME20101. [Google Scholar] [CrossRef]
- Mol, V.; Bennett, M.; Sánchez, B.J.; Lisowska, B.K.; Herrgård, M.J.; Nielsen, A.T.; Leak, D.J.; Sonnenschein, N. Genome-scale metabolic modeling of P. thermoglucosidasius NCIMB 11955 reveals metabolic bottlenecks in anaerobic metabolism. Metab. Eng. 2021, 65, 123–134. [Google Scholar] [CrossRef]
- Shimizu, K. 1-Main metabolism. In Bacterial Cellular Metabolic Systems; Shimizu, K., Ed.; Woodhead Publishing Series in Biomedicine; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–54. [Google Scholar] [CrossRef]
- Reitzer, L. Catabolism of Amino Acids and Related Compounds. EcoSal Plus 2005, 1, 1–26. [Google Scholar] [CrossRef]
- Metcalfe, G.D.; Sargent, F.; Hippler, M. Hydrogen production in the presence of oxygen by Escherichia coli K-12. Microbiology 2022, 168. [Google Scholar] [CrossRef]
- Soini, J.; Ukkonen, K.; Neubauer, P. High cell density media for Escherichia coli are generally designed for aerobic cultivations–consequences for Large-Scale bioprocesses and shake flask cultures. Microb. Cell Factories 2008, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Wang, G.Y.; Zeng, J.; Zhang, J.A. Improved Succinate Production by Metabolic Engineering. BioMed Res. Int. 2013, 2013, 538790. [Google Scholar] [CrossRef] [PubMed]
- Fontecilla-Camps, J.C.; Amara, P.; Cavazza, C.; Nicolet, Y.; Volbeda, A. Structure–function relationships of anaerobic gas-processing metalloenzymes. Nature 2009, 460, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Hirshberg, B.; Gerber, R.B. Formation of Carbonic Acid in Impact of CO2 on Ice and Water. J. Phys. Chem. Lett. 2016, 7, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Scharlin, P.; Battino, R.; Silla, E.; Tunon, I.; Pascual-Ahuir, J. Solubility of gases in water: Correlation between solubility and the number of water molecules in the first solvation shell. Pure Appl. Chem. 1998, 70, 1895–1904. [Google Scholar] [CrossRef]
- Brumm, P.; Land, M.L.; Hauser, L.J.; Jeffries, C.D.; Chang, Y.J.; Mead, D.A. Complete genome sequence of Geobacillus Strain Y4. 1MC1, A novel co-utilizing Geobacillus thermoglucosidasius Strain isolated from bath hot spring in Yellowstone National Park. BioEnergy Res. 2015, 8, 1039–1045. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Sander, R.; Acree, W.E.; De Visscher, A.; Schwartz, S.E.; Wallington, T.J. Henry’s law constants (IUPAC Recommendations 2021). Pure Appl. Chem. 2022, 94, 71–85. [Google Scholar] [CrossRef]
- Schenk, A.; Aragno, M. Bacillus schlegelii, a new species of thermophilic, facultatively chemolithoautotrophic bacterium oxidizing molecular hydrogen. Microbiology 1979, 115, 333–341. [Google Scholar] [CrossRef]
- Islam, Z.F.; Welsh, C.; Bayly, K.; Grinter, R.; Southam, G.; Gagen, E.J.; Greening, C. A widely distributed hydrogenase oxidises atmospheric H2 during bacterial growth. ISME J. 2020, 14, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Enjalbert, B.; Millard, P.; Dinclaux, M.; Portais, J.C.; Létisse, F. Acetate fluxes in Escherichia coli are determined by the thermodynamic control of the Pta-AckA pathway. Sci. Rep. 2017, 7, 42135. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Kolter, R.; Losick, R. A widely conserved gene cluster required for lactate utilization in Bacillus subtilis and its involvement in biofilm formation. J. Bacteriol. 2009, 191, 2423–2430. [Google Scholar] [CrossRef] [PubMed]



| Experiment | Amount (mmol) | ||
|---|---|---|---|
| H2 | N2 | CO | |
| O2-containing with KP1013 | |||
| 0% CO | 0 | 6.90 | 0 |
| 50% CO | 0 | 3.76 | 3.09 |
| 75% CO | 0 | 2.23 | 4.89 |
| 100% CO | 0 | 0.69 | 6.32 |
| Anoxic with KP1013 | |||
| 0% CO | 0 | 7.74 | 0 |
| 50% CO | 0 | 4.56 | 3.44 |
| 75% CO | 0 | 2.23 | 5.15 |
| 100% CO | 0 | 0.39 | 7.02 |
| Anoxic with DSM 6285 | |||
| 0% CO | 0 | 7.43 | 0 |
| 50% CO | 0 | 3.87 | 3.56 |
| 75% CO | 0 | 1.89 | 5.64 |
| 100% CO | 0 | 0.48 | 7.07 |
| Anoxic at 1 bar initial pressure with KP1013 | |||
| 0% H2 | 0 | 3.79 | 3.64 |
| 12.5% H2 | 0.93 | 2.72 | 3.77 |
| 25% H2 | 1.91 | 1.73 | 3.78 |
| 37.5% H2 | 2.83 | 0.79 | 3.80 |
| Anoxic at 1.6 bar initial pressure with KP1013 | |||
| 0% H2 | 0 | 5.49 | 4.79 |
| 12.5% H2 | 1.22 | 4.18 | 4.79 |
| 25% H2 | 2.64 | 2.71 | 4.84 |
| 37.5% H2 | 3.87 | 1.50 | 4.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, D.B.; Neumann, A.; Aliyu, H. Thermophilic Water Gas Shift Reaction at High Carbon Monoxide and Hydrogen Partial Pressures in Parageobacillus thermoglucosidasius KP1013. Fermentation 2022, 8, 596. https://doi.org/10.3390/fermentation8110596
Díaz DB, Neumann A, Aliyu H. Thermophilic Water Gas Shift Reaction at High Carbon Monoxide and Hydrogen Partial Pressures in Parageobacillus thermoglucosidasius KP1013. Fermentation. 2022; 8(11):596. https://doi.org/10.3390/fermentation8110596
Chicago/Turabian StyleDíaz, Daniel Barón, Anke Neumann, and Habibu Aliyu. 2022. "Thermophilic Water Gas Shift Reaction at High Carbon Monoxide and Hydrogen Partial Pressures in Parageobacillus thermoglucosidasius KP1013" Fermentation 8, no. 11: 596. https://doi.org/10.3390/fermentation8110596
APA StyleDíaz, D. B., Neumann, A., & Aliyu, H. (2022). Thermophilic Water Gas Shift Reaction at High Carbon Monoxide and Hydrogen Partial Pressures in Parageobacillus thermoglucosidasius KP1013. Fermentation, 8(11), 596. https://doi.org/10.3390/fermentation8110596







