Microbial Communities in Home-Made and Commercial Kefir and Their Hypoglycemic Properties
Abstract
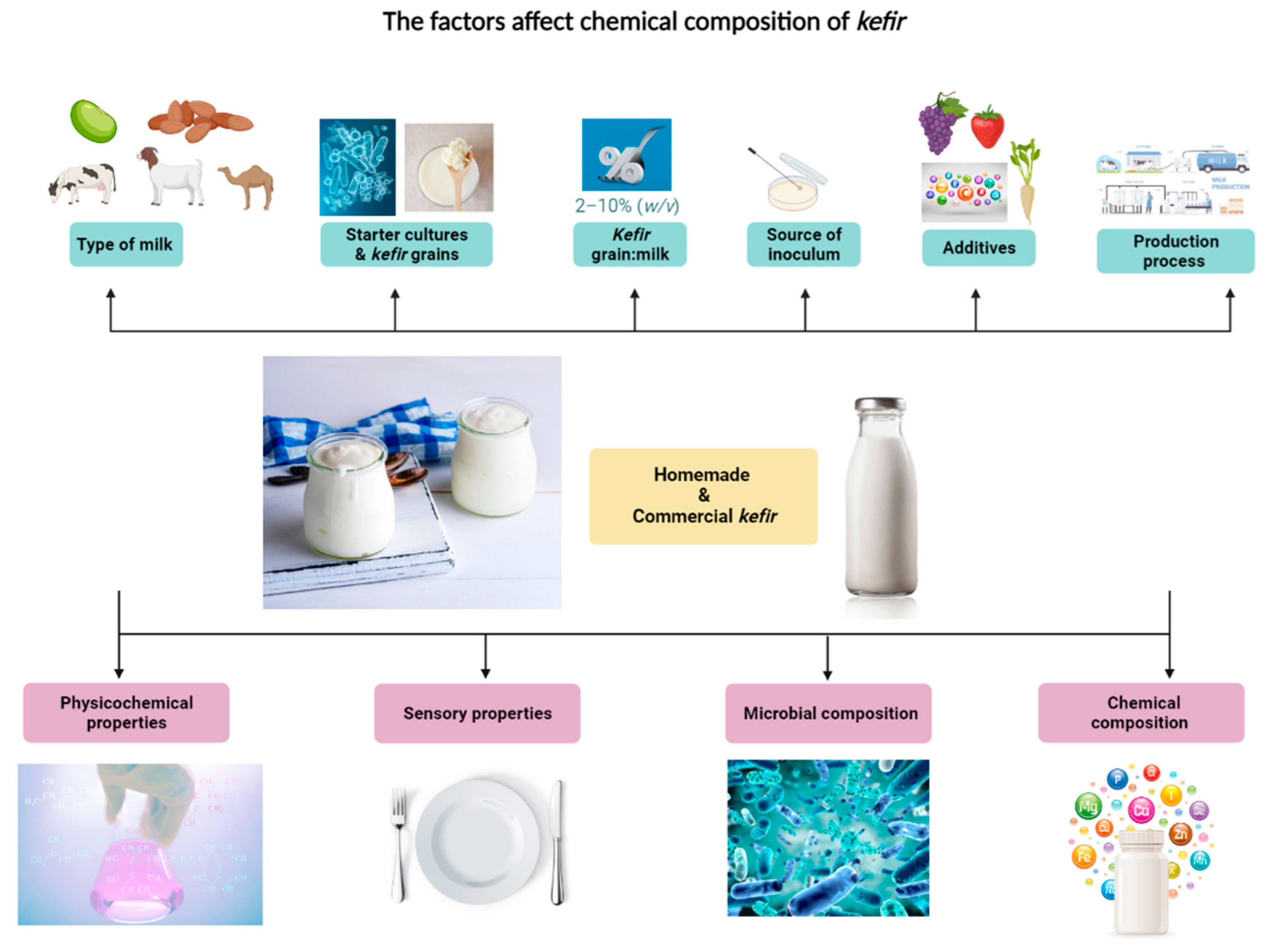
1. Introduction
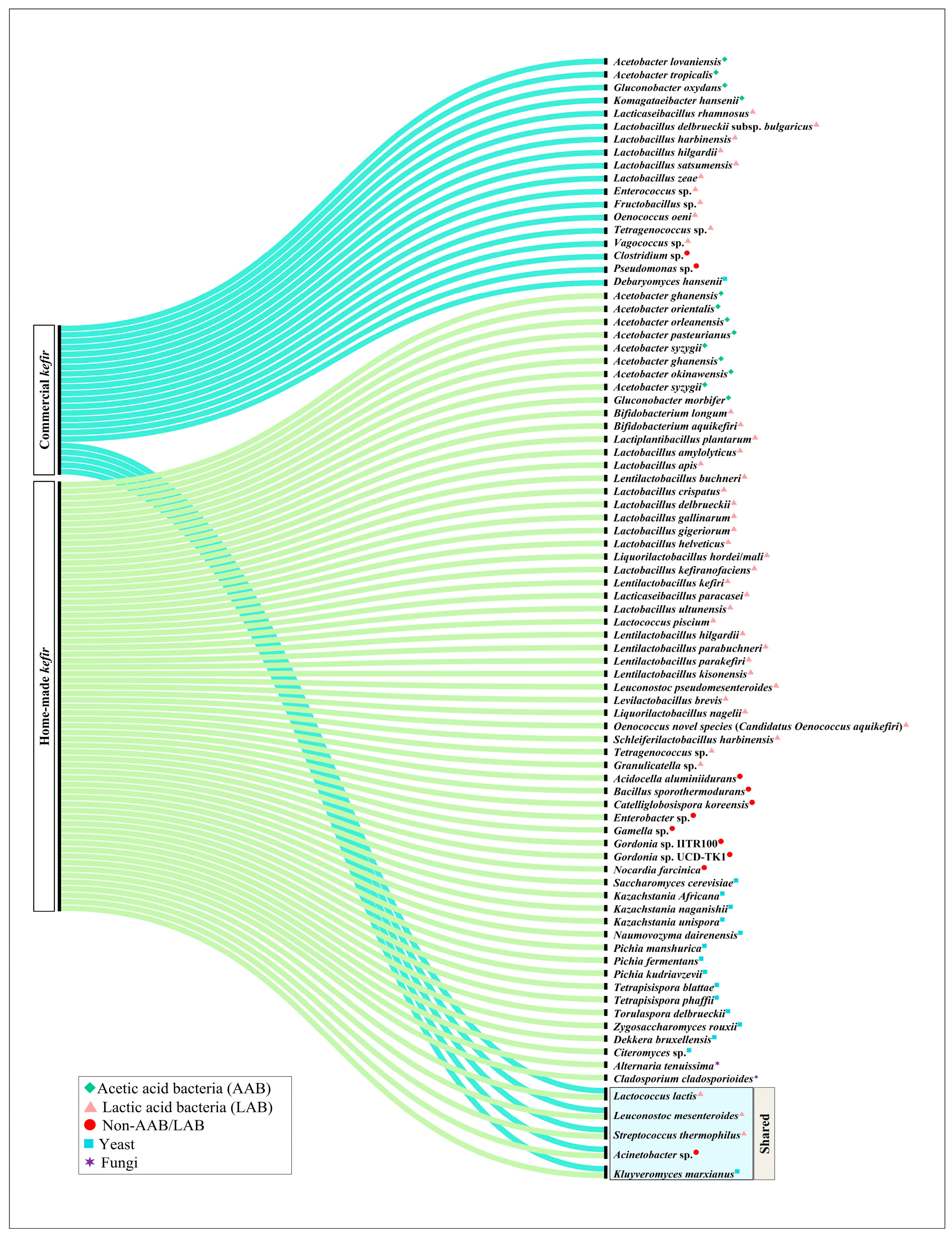
2. Microbial Composition of Home-Made and Commercial Kefir
2.1. Hypoglycemic Properties of Kefir
2.2. Animal Studies
2.3. Human Studies
3. Future Directions and Prospective
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef] [PubMed]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating fermented: Health benefits of LAB-fermented foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2019, 126, 686–700. [Google Scholar] [CrossRef]
- Glibowski, P.; Kowalska, A. Rheological, texture and sensory properties of kefir with high performance and native inulin. J. Food Eng. 2012, 111, 299–304. [Google Scholar] [CrossRef]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, D.; Kim, H.; Seo, K.-H. Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 1782–1793. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Du, L.; Chen, J.; Zheng, Q.; Li, P.; Du, B.; Fang, X.; Liao, Z. Kefir microbiota and metabolites stimulate intestinal mucosal immunity and its early development. Crit. Rev. Food Sci. Nutr. 2022, 1–14. [Google Scholar] [CrossRef]
- Tan, L.L.; Tan, C.H.; Ng, N.K.J.; Tan, Y.H.; Conway, P.L.; Loo, S.C.J. Potential Probiotic Strains From Milk and Water Kefir Grains in Singapore—Use for Defense Against Enteric Bacterial Pathogens. Front. Microbiol. 2022, 13, 857720. [Google Scholar] [CrossRef]
- International Diabetic Federation. 2021. Available online: https://idf.org/our-network/regions-members/europe/members/125-belgium.html (accessed on 4 October 2022).
- Farag, M.A.; Jomaa, S.A.; Abd El-Wahed, A.R.; El-Seedi, H. The many faces of kefir fermented dairy products: Quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Gheshlaghi, Z.B.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228. [Google Scholar] [PubMed]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Maske, B.L.; De Dea Lindner, J.; Vale, A.S.; Favero, G.R.; Viesser, J.; de Carvalho, J.C.; Góes-Neto, A.; Soccol, C.R. An updated review on bacterial community composition of traditional fermented milk products: What next-generation sequencing has revealed so far? Crit. Rev. Food Sci. Nutr. 2022, 62, 1870–1889. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Kalamaki, M.S.; Angelidis, A.S. High-throughput, sequence-based analysis of the microbiota of Greek kefir grains from two geographic regions. Food Technol. Biotechnol. 2020, 58, 138. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Salgado, S.; Castelán-Sánchez, H.G.; Dávila-Ramos, S.; Huerta-Saquero, A.; Rodríguez-Morales, S.; Merino-Pérez, E.; Roa de la Fuente, L.F.; Solis-Pereira, S.E.; Pérez-Rueda, E.; Lizama-Uc, G. Metagenomic analysis and antimicrobial activity of two fermented milk kefir samples. MicrobiologyOpen 2021, 10, e1183. [Google Scholar] [CrossRef]
- Biçer, Y.; Telli, A.E.; Sönmez, G.; Turkal, G.; Telli, N.; Uçar, G. Comparison of commercial and traditional kefir microbiota using metagenomic analysis. Int. J. Dairy Technol. 2021, 74, 528–534. [Google Scholar] [CrossRef]
- Kazou, M.; Grafakou, A.; Tsakalidou, E.; Georgalaki, M. Zooming into the microbiota of home-made and industrial kefir produced in Greece using classical microbiological and amplicon-based metagenomics analyses. Front. Microbiol. 2021, 12, 621069. [Google Scholar] [CrossRef]
- Kumar, M.R.; Yeap, S.K.; Mohamad, N.E.; Abdullah, J.O.; Masarudin, M.J.; Khalid, M.; Leow, A.T.C.; Alitheen, N.B. Metagenomic and phytochemical analyses of kefir water and its subchronic toxicity study in BALB/c mice. BMC Complement. Med. Ther. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Yusuf, B.; Gürkan, U. Analysis of the kefir and koumiss microbiota with the focus on certain functional properties of selected lactic-acid bacteria. Mljekarstvo: Časopis za unaprjeđenje proizvodnje i prerade mlijeka 2021, 71, 112–123. [Google Scholar] [CrossRef]
- Yegin, Z.; Yurt, M.N.Z.; Tasbasi, B.B.; Acar, E.E.; Altunbas, O.; Ucak, S.; Ozalp, V.C.; Sudagidan, M. Determination of bacterial community structure of Turkish kefir beverages via metagenomic approach. Int. Dairy J. 2022, 129, 105337. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Y.; Jia, H.; Wang, Z.; Gao, Z.; Luo, Y.; Sheng, Q.; Yuan, Y.; Yue, T. Metagenomic analysis of microflora structure and functional capacity in probiotic Tibetan kefir grains. Food Res. Int. 2022, 151, 110849. [Google Scholar] [CrossRef] [PubMed]
- Ilıkkan, Ö.K.; Bağdat, E.Ş. Comparison of bacterial and fungal biodiversity of Turkish kefir grains with high-throughput metagenomic analysis. LWT—Food Sci. Technol. 2021, 152, 112375. [Google Scholar] [CrossRef]
- Tu, C.; Azi, F.; Huang, J.; Xu, X.; Xing, G.; Dong, M. Quality and metagenomic evaluation of a novel functional beverage produced from soy whey using water kefir grains. LWT—Food Sci. Technol. 2019, 113, 108258. [Google Scholar] [CrossRef]
- Verce, M.; De Vuyst, L.; Weckx, S. Shotgun metagenomics of a water kefir fermentation ecosystem reveals a novel Oenococcus species. Front. Microbiol. 2019, 10, 479. [Google Scholar] [CrossRef]
- Sindi, A.; Badsha, M.B.; Ünlü, G. Bacterial populations in international artisanal kefirs. Microorganisms 2020, 8, 1318. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Maróti, G.; Jerzsele, Á.; Dubecz, A.; Patai, Á.V.; Judge, M.F.; Nagy, S.Á.; Makrai, L.; Bányai, K. A glimpse of antimicrobial resistance gene diversity in kefir and yoghurt. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- de Almeida Brasiel, P.G.; Dutra Medeiros, J.; Barbosa Ferreira Machado, A.; Schuchter Ferreira, M.; Gouveia Peluzio, M.d.C.; Potente Dutra Luquetti, S.C. Microbial community dynamics of fermented kefir beverages changes over time. Int. J. Dairy Technol. 2021, 74, 324–331. [Google Scholar] [CrossRef]
- Guangsen, T.; Xiang, L.; Jiahu, G. Microbial diversity and volatile metabolites of kefir prepared by different milk types. CYTA—J. Food 2021, 19, 399–407. [Google Scholar] [CrossRef]
- Ibacache-Quiroga, C.; González-Pizarro, K.; Charifeh, M.; Canales, C.; Díaz-Viciedo, R.; Schmachtenberg, O.; Dinamarca, M.A. Metagenomic and Functional Characterization of Two Chilean Kefir Beverages Reveals a Dairy Beverage Containing Active Enzymes, Short-Chain Fatty Acids, Microbial β-Amyloids, and Bio-Film Inhibitors. Foods 2022, 11, 900. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.E.; Moreira, N.C.; Sakamoto-Hojo, E.T. Mechanisms underlying the pathophysiology of type 2 diabetes: From risk factors to oxidative stress, metabolic dysfunction, and hyperglycemia. Mutat. Res. Genet. Toxicol. Environ. 2022, 874, 503437. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A mini review on antidiabetic properties of fermented foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; Cabral, C.C.; da Costa Lima, B.R.; Paschoalin, V.M.F.; Leandro, K.C.; Conte-Junior, C.A. Lactococcus lactis ssp. cremoris MRS47, a potential probiotic strain isolated from kefir grains, increases cis-9, trans-11-CLA and PUFA contents in fermented milk. J. Funct. Foods 2017, 31, 172–178. [Google Scholar] [CrossRef]
- Tiss, M.; Souiy, Z.; ben Abdeljelil, N.; Njima, M.; Achour, L.; Hamden, K. Fermented soy milk prepared using kefir grains prevents and ameliorates obesity, type 2 diabetes, hyperlipidemia and Liver-Kidney toxicities in HFFD-rats. J. Funct. Foods 2020, 67, 103869. [Google Scholar] [CrossRef]
- Peluzio, M.d.C.G.; Dias, M.d.M.e.; Martinez, J.A.; Milagro, F.I. Kefir and intestinal microbiota modulation: Implications in human health. Front. Nutr. 2021, 8, 638740. [Google Scholar] [CrossRef]
- Alihosseini, N.; Moahboob, S.; Farrin, N.; Mobasseri, M.; Taghizadeh, A.; Ostadrahimi, A. Effect of probiotic fermented milk (kefir) on serum level of insulin and homocysteine in type 2 diabetes patients. Acta Endocrinol. 2017, 13, 431. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Calatayud, M.; Börner, R.A.; Ghyselinck, J.; Verstrepen, L.; Medts, J.D.; Abbeele, P.V.d.; Boulangé, C.L.; Priour, S.; Marzorati, M.; Damak, S. Water kefir and derived pasteurized beverages modulate gut microbiota, intestinal permeability and cytokine production in vitro. Nutrients 2021, 13, 3897. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.; Fabritius, M.; Yang, B. Determination of vitamin K composition of fermented food. Food Chem. 2019, 275, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.R.; Al Bataineh, M.T. Metagenomic analysis of the gut microbiome reveals enrichment of menaquinones (vitamin K2) pathway in diabetes mellitus. Diabetes Metab. J. 2021, 45, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, M.; Ertekin, Y.H.; Satman, İ. The Effects of Kefir on Kidney Tissues and Functions in Diabetic Rats. Probiotics Antimicrob. Proteins 2021, 13, 375–382. [Google Scholar] [CrossRef]
- Akar, F.; Sumlu, E.; Alçığır, M.E.; Bostancı, A.; Sadi, G. Potential mechanistic pathways underlying intestinal and hepatic effects of kefir in high-fructose-fed rats. Food Res. Int. 2021, 143, 110287. [Google Scholar] [CrossRef]
- Rosa, D.D.; Grześkowiak, Ł.M.; Ferreira, C.L.; Fonseca, A.C.M.; Reis, S.A.; Dias, M.M.; Siqueira, N.P.; Silva, L.L.; Neves, C.A.; Oliveira, L.L. Kefir reduces insulin resistance and inflammatory cytokine expression in an animal model of metabolic syndrome. Food Funct. 2016, 7, 3390–3401. [Google Scholar] [CrossRef]
- Punaro, G.R.; Maciel, F.R.; Rodrigues, A.M.; Rogero, M.M.; Bogsan, C.S.; Oliveira, M.N.; Ihara, S.S.; Araujo, S.R.; Sanches, T.R.; Andrade, L.C. Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide 2014, 37, 53–60. [Google Scholar] [CrossRef]
- Nurliyani, A.; Harmayani, E.; Sunarti, S. Antidiabetic potential of kefir combination from goat milk and soy milk in rats induced with streptozotocin-nicotinamide. Korean J. Food Sci. Anim. Resour. 2015, 35, 847–858. [Google Scholar] [CrossRef]
- Kwon, J.H.; Lee, H.G.; Seo, K.H.; Kim, H. Combination of whole grapeseed flour and newly isolated kefir lactic-acid bacteria reduces high-fat-induced hepatic steatosis. Mol. Nutr. Food Res. 2019, 63, 1801040. [Google Scholar] [CrossRef]
- Nurliyani; Harmayani, E.; Sunarti. Synbiotic goat milk kefir improves health status in rats fed a high-fat and high-fructose diet. Vet. World 2022, 15, 173–181. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.-H. The effect of dietary glycaemic index on glycaemia in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.L.; Hendrich, S. Glycemic index, insulinemic index, and satiety index of kefir. J. Am. Coll. Nutr. 2012, 31, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Judiono, J.; Hadisaputro, S.; Indranila, K.; Cahyono, B.; Suzery, M.; Widiastuti, Y.; Purnawan, A.I. Effects of clear kefir on biomolecular aspects of glycemic status of type 2 diabetes mellitus (T2DM) patients in Bandung, West Java [study on human blood glucose, c peptide and insulin]. Funct. Foods Health Dis. 2014, 4, 340–348. [Google Scholar] [CrossRef]
- El-Bashiti, T.A.; Zabut, B.M.; Abu Safia, F.F. Effect of probiotic fermented milk (Kefir) on some blood biochemical parameters among newly diagnosed type 2 diabetic adult males in Gaza governorate. Curr. Res. Nutr. Food Sci. 2019, 7, 568–575. [Google Scholar] [CrossRef]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Akyon, Y.; Aydin-Kose, F.; Karagozlu, C.; Ozgen, A.G.; Brinkmann, A.; Nitsche, A.; Ergunay, K.; Yilmaz, E.; et al. Effects of Regular Kefir Consumption on Gut Microbiota in Patients with Metabolic Syndrome: A Parallel-Group, Randomized, Controlled Study. Nutrients 2019, 11, 2089. [Google Scholar] [CrossRef]
- da Silva Ghizi, A.C.; de Almeida Silva, M.; de Andrade Moraes, F.S.; da Silva, C.L.; Endringer, D.C.; Scherer, R.; Lenz, D.; de Lima, E.M.; Brasil, G.A.; Maia, J.F. Kefir improves blood parameters and reduces cardiovascular risks in patients with metabolic syndrome. PharmaNutrition 2021, 16, 100266. [Google Scholar] [CrossRef]
- Hosainzadegn, H.; Hosainzadegan, M. Traditional probiotic (Kefir) effects on glycated hemoglobin a level and weight of an indexed diabetic patient. Int. J. Prev. Med. 2021, 12, 139. [Google Scholar] [PubMed]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Karagozlu, C.; Aydin-Kose, F.; Ozgen, A.G.; Buyuktuncer, Z. Probiotic kefir consumption improves serum apolipoprotein A1 levels in metabolic syndrome patients: A randomized controlled clinical trial. Nutr. Res. 2022, 102, 59–70. [Google Scholar] [CrossRef]
- Javid, A.Z.; Aminzadeh, M.; Haghighi-Zadeh, M.H.; Jamalvandi, M. The effects of synbiotic supplementation on glycemic status, lipid profile, and biomarkers of oxidative stress in type 1 diabetic patients. A placebo-controlled, double-blind, randomized clinical trial. Diabetes. Metab. Syndr. Obes. 2020, 13, 607. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, B.; Elibol, E.; Shangpliang, H.N.J.; Ozogul, F.; Tamang, J.P. Microbial Communities in Home-Made and Commercial Kefir and Their Hypoglycemic Properties. Fermentation 2022, 8, 590. https://doi.org/10.3390/fermentation8110590
Yilmaz B, Elibol E, Shangpliang HNJ, Ozogul F, Tamang JP. Microbial Communities in Home-Made and Commercial Kefir and Their Hypoglycemic Properties. Fermentation. 2022; 8(11):590. https://doi.org/10.3390/fermentation8110590
Chicago/Turabian StyleYilmaz, Birsen, Emine Elibol, H. Nakibapher Jones Shangpliang, Fatih Ozogul, and Jyoti Prakash Tamang. 2022. "Microbial Communities in Home-Made and Commercial Kefir and Their Hypoglycemic Properties" Fermentation 8, no. 11: 590. https://doi.org/10.3390/fermentation8110590
APA StyleYilmaz, B., Elibol, E., Shangpliang, H. N. J., Ozogul, F., & Tamang, J. P. (2022). Microbial Communities in Home-Made and Commercial Kefir and Their Hypoglycemic Properties. Fermentation, 8(11), 590. https://doi.org/10.3390/fermentation8110590






