Effect of Starters on Quality Characteristics of Hongsuantang, a Chinese Traditional Sour Soup
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Strains and Starter Culture
2.3. HST Fermented by Different Single-Strain
2.4. Sample Quality Analysis
2.4.1. Determination of pH, Total Titration Acid, and Viable Count
2.4.2. Determination of Organic Acid, Total Phenols, Total Carotenoids, and Lycopene
2.4.3. Antioxidant Capacity Assay
- DPPH Method
- ABTS Method
2.4.4. Determination of Color
2.4.5. Analysis of Volatile Flavor Substances
2.4.6. Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
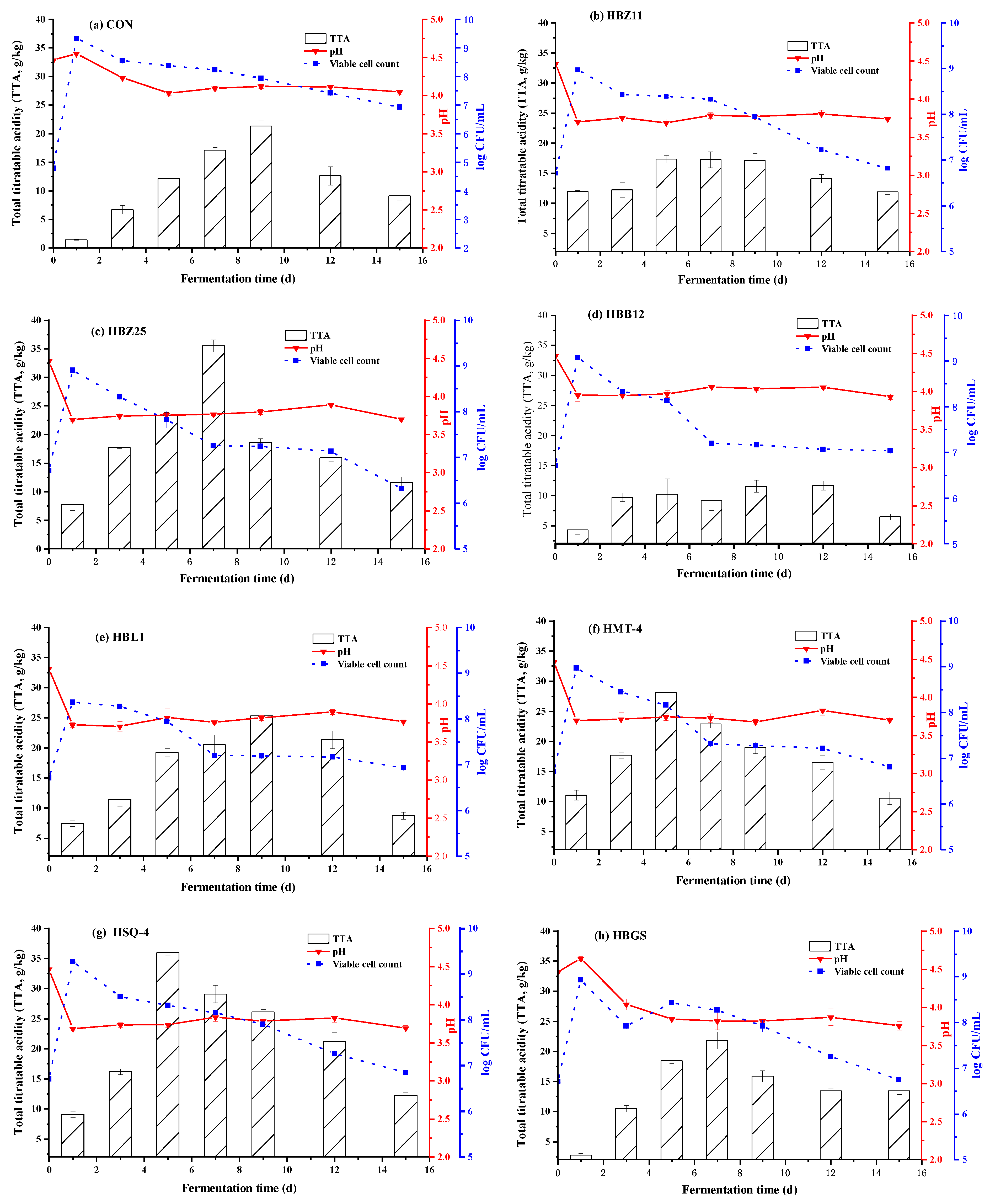
3.1. Changes in pH, TTA, and Microorganisms
3.2. Effect of Fermentation on Organic Acid, Total Phenols, Total Carotenoids, Lycopene, and Antioxidant Activity of HST
3.3. Effect of Fermentation on the Color of HST
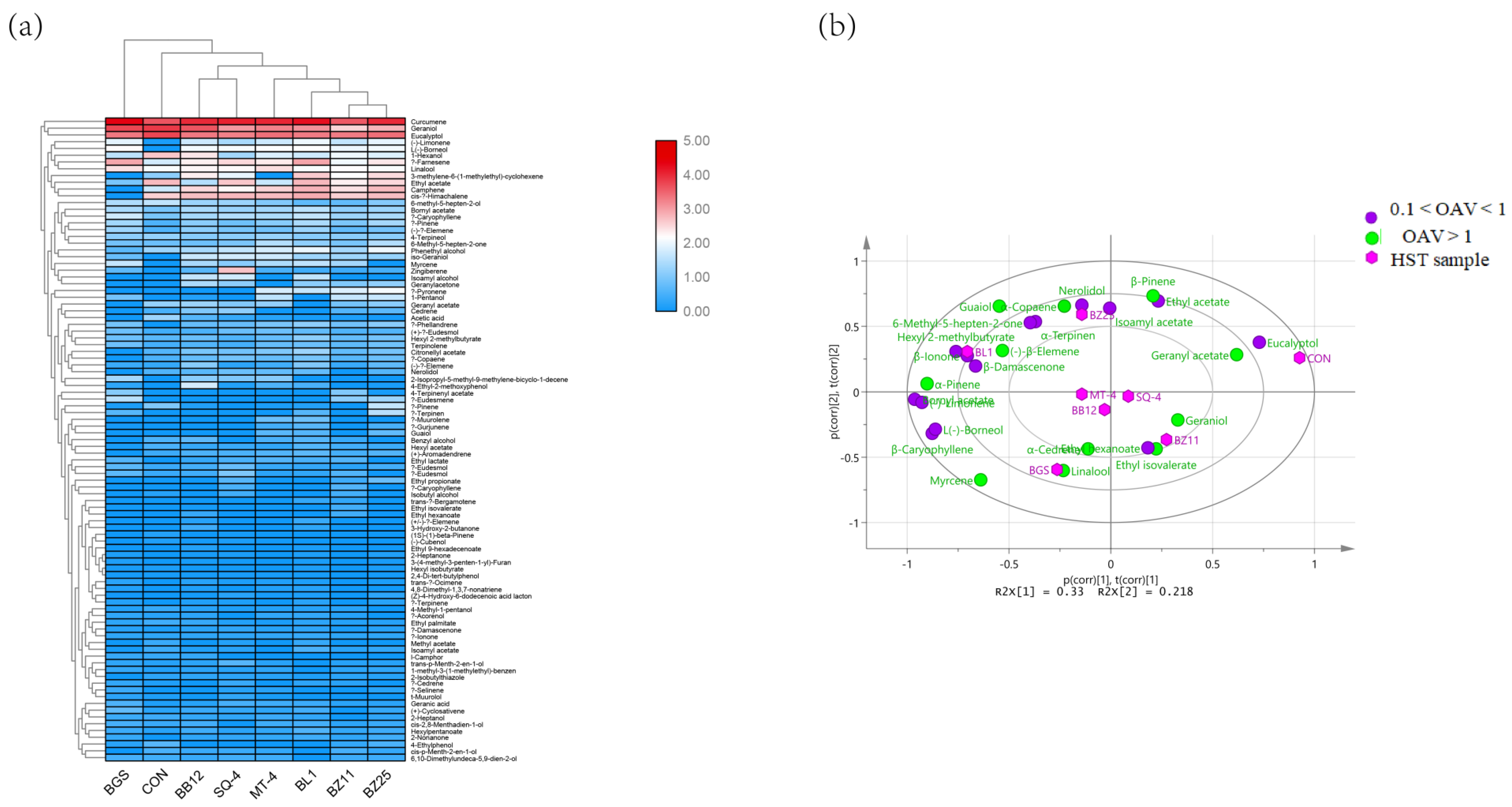
3.4. PCA of the Volatile Substances of HST
| Compounds (μg/100 mL) | RT (min) | Odor Description | Odor Threshold(μg/L) | OAVs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HBZ11 | HBZ25 | HBB12 | HBL1 | HMT-4 | HSQ-4 | HBGS | ||||
| Ethyl acetate | 3.447 | Pineapple, fruity, balsamic | 97.8 a | 0.59 | 0.40 | 0.48 | 0.19 | 0.60 | 0.23 | 0.54 | 0.10 |
| Ethyl isovalerate | 7.191 | Banana, sweet fruity | 3 a | ND | 1.27 | ND | 0.12 | ND | ND | ND | 0.06 |
| Isoamyl acetate | 8.653 | Banana, fruity, sweet | 30 a | 0.11 | 0.06 | 0.08 | 0.04 | 0.15 | ND | 0.06 | 0.02 |
| Ethyl hexanoate | 11.832 | Flowery, fruity | 5 a | ND | 0.74 | ND | 0.57 | ND | ND | ND | ND |
| Hexyl 2-methylbutyrate | 15.512 | Sweet fruit | 18 a | 0.33 | 0.40 | 0.50 | 0.43 | 0.67 | 0.40 | 0.28 | 0.45 |
| Bornyl acetate | 18.811 | Woody, herbal | 75 b | 0.13 | 0.17 | 0.19 | 0.22 | 0.26 | 0.20 | 0.18 | 0.22 |
| Geranyl acetate | 21.002 | Sweet, floral | 9 c | 1.48 | 1.00 | 1.30 | 1.59 | ND | 1.42 | 1.18 | ND |
| α-Pinene | 5.991 | Woody, fir needle, cooling, minty | 6 b | 0.81 | 1.64 | 2.61 | 1.97 | 2.79 | 1.88 | 1.37 | 2.58 |
| Myrcene | 9.732 | Spicy, mint | 4.9 b | ND | 3.17 | ND | 3.30 | 4.14 | 3.50 | 2.35 | 4.37 |
| β-Pinene | 9.805 | Woody, pine, minty, camphor | 6 b | 1.71 | ND | 3.53 | 0.20 | 0.23 | 0.12 | ND | 0.15 |
| α-Terpinene | 10.038 | Woody, lemon, citrus | 80 c | ND | ND | 0.24 | ND | 0.07 | ND | ND | 0.07 |
| (–)-Limonene | 10.853 | Citrus | 34 b | 0.02 | 0.62 | 0.85 | 0.68 | 0.91 | 0.68 | 0.48 | 0.84 |
| α-Copaene | 17.528 | Woody, spicy, honey | 6 b | 0.77 | 0.96 | 1.31 | 1.19 | 1.54 | 1.20 | 1.46 | ND |
| α-Cedrene | 18.706 | Floral, herbal | 2.13 d | 0.47 | 0.83 | ND | ND | 0.95 | ND | 1.37 | 1.20 |
| β-Caryophyllene | 19.918 | Fried, wood | 64 d | 0.15 | 0.23 | 0.26 | 0.25 | 0.33 | 0.27 | 0.28 | 0.36 |
| (–)-β-Elemene | 18.959 | Spicy, fennel | 0.12 b | 22.53 | 24.84 | 54.57 | 94.70 | 85.93 | 30.83 | 17.04 | 25.56 |
| Linalool | 18.249 | Spicy, citrus, woody | 30 b | 1.14 | 1.19 | 1.15 | 1.25 | 1.12 | 1.50 | 1.22 | 1.47 |
| Geraniol | 22.068 | Sweet, floral, fruity | 130 e | 1.05 | 0.37 | 0.47 | 0.88 | 0.59 | 0.65 | 0.56 | 0.97 |
| Nerolidol | 24.021 | Fir, linoleum | 10 d | 0.51 | ND | 0.55 | 0.59 | 0.69 | 0.69 | 0.78 | ND |
| Guaiol | 25.173 | Smoky, bitter, woody | 0.1 f | ND | ND | 67.18 | ND | 54.74 | 59.51 | ND | ND |
| L(–)-Borneol | 20.428 | Camphoraceous, pine | 180 b | ND | 0.17 | 0.18 | 0.18 | 0.20 | 0.19 | 0.18 | 0.20 |
| 6-Methyl-5-hepten-2-one | 14.501 | Sweet, fruity | 68 g | 0.12 | 0.14 | 0.27 | 0.12 | 0.18 | 0.27 | 0.14 | 0.13 |
| β-Damascenone | 21.828 | Sweet, woody, fruity | 2.5 b | 0.39 | 0.29 | 0.64 | 0.72 | 0.85 | 0.66 | 0.90 | 0.63 |
| β-Ionone | 23.117 | Balsamic, rose, violet | 5 b | 0.43 | 0.43 | 0.44 | 0.46 | 0.57 | 0.49 | 0.46 | 0.45 |
| Eucalyptol | 11.104 | Eucalyptus, camphor | 550 b | 0.23 | 0.16 | 0.18 | 0.16 | 0.16 | 0.17 | 0.16 | 0.17 |
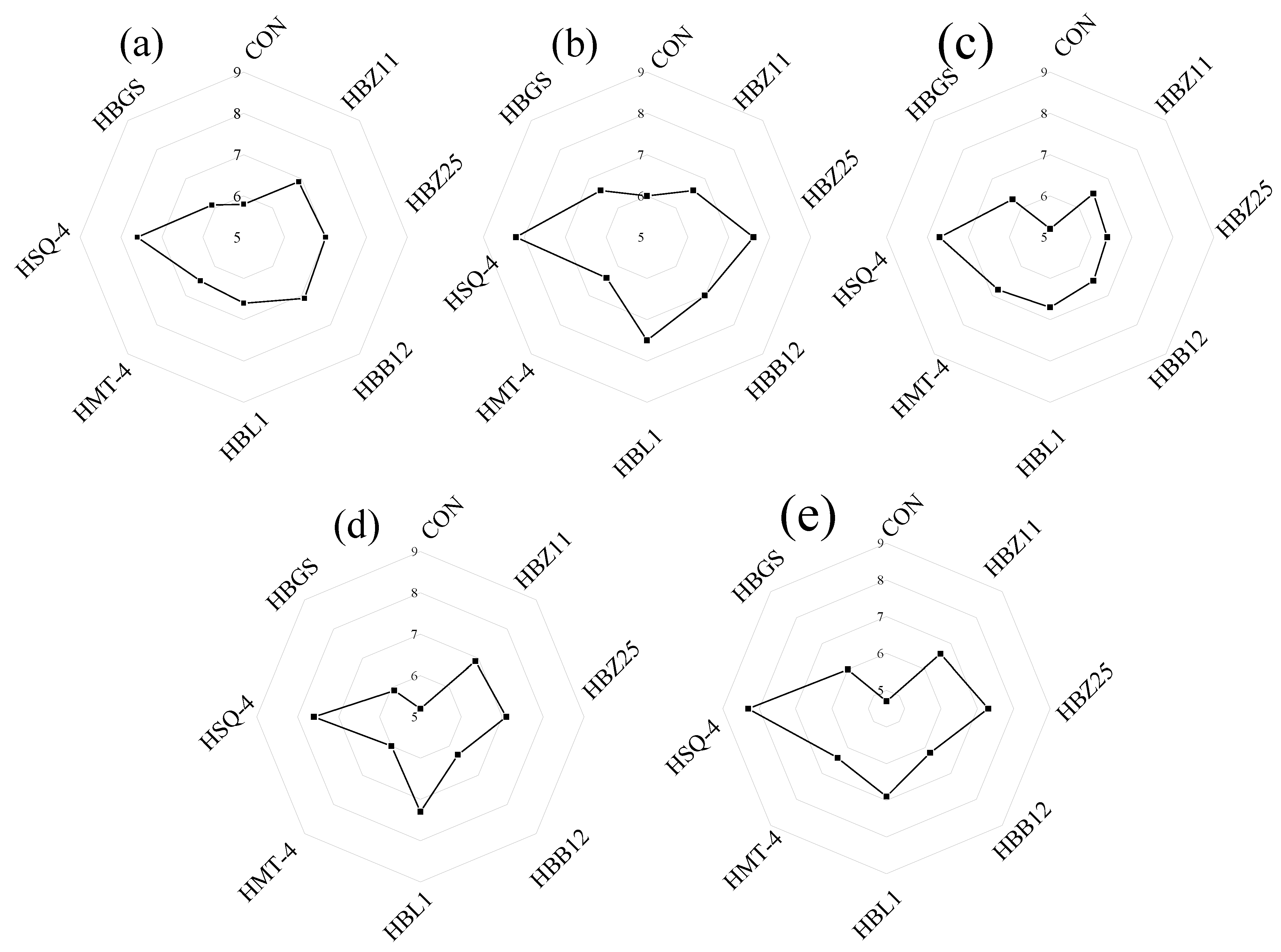
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2019, 128, 108773. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, H.; Wang, X.; Lin, X.; Ji, C.; Li, S.; Liang, H. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. LWT-Food Sci. Technol. 2020, 118, 108773. [Google Scholar] [CrossRef]
- Li, D.; Duan, F.; Tian, Q.; Zhong, D.; Wang, X.; Jia, L. Physiochemical, microbiological and flavor characteristics of traditional Chinese fermented food Kaili Red Sour Soup. LWT-Food Sci. Technol. 2021, 142, 110933. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; He, L.; Li, C. Determination of the microbial communities of Guizhou Suantang, a traditional Chinese fermented sour soup, and correlation between the identified microorganisms and volatile compounds. Food Res. Int. 2020, 138, 109820. [Google Scholar] [CrossRef]
- Xiong, K.; Han, F.; Wang, Z.; Ming, D.; Chen, Y.; Tang, Y.; Wang, Z. Screening of dominant strains in red sour soup from Miao nationality and the optimization of inoculating fermentation conditions. Food Sci. Nutr. 2020, 9, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Du Fang, M.; Zeng, J.; Liang, Z.J.; Zhang, X.Y.; Gao, X.Y. Deep insights into fungal diversity in traditional Chinese sour soup by Illumina MiSeq sequencing. Food Res. Int. 2020, 137, 109439. [Google Scholar] [CrossRef]
- Evivie, S.E.; Huo, G.-C.; Igene, J.O.; Bian, X. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. Food Nutr. Res. 2017, 61, 1318034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Z.; Xie, L.; Li, L.; Zhou, W.; Zhao, L. The correlation mechanism between dominant bacteria and primary metabolites during fermentation of Red Sour Soup. Foods 2022, 11, 341. [Google Scholar] [CrossRef]
- García-Hernández, J.; Hernández-Pérez, M.; Peinado, I.; Andrés, A.; Heredia, A. Tomato-antioxidants enhance viability of L. reuteri under gastrointestinal conditions while the probiotic negatively affects bioaccessibility of lycopene and phenols. J. Funct. Foods 2018, 43, 1–7. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef]
- Azabou, S.; Abid, Y.; Sebii, H.; Felfoul, I.; Gargouri, A.; Attia, H. Potential of the solid-state fermentation of tomato by products by Fusarium solani pisi for enzymatic extraction of lycopene. LWT-Food Sci. Technol. 2016, 68, 280–287. [Google Scholar] [CrossRef]
- Jabbari, S.-S.; Jafari, S.M.; Dehnad, D.; Shahidi, S.-A. Changes in lycopene content and quality of tomato juice during thermal processing by a nanofluid heating medium. J. Food Eng. 2018, 230, 1–7. [Google Scholar] [CrossRef]
- Watts, E.G.; Janes, M.E.; Prinyawiwatkul, W.; Shen, Y.; Xu, Z.; Johnson, D. Microbiological changes and their impact on quality characteristics of red hot chilli pepper mash during natural fermentation. Int. J. Food Sci. Technol. 2018, 53, 1816–1823. [Google Scholar] [CrossRef]
- Kizhakkayil, J.; Sasikumar, B. Diversity, characterization and utilization of ginger: A review. Plant Genet. Resour. 2011, 9, 464–477. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wu, W.; Jiang, J.; Feng, D.; Shi, Y.; Hu, P. Comparison of fermentation behaviors and characteristics of tomato sour soup between natural fermentation and dominant bacteria-enhanced fermentation. Microorganisms 2022, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Yangbo, H.; Yongfu, L.; Xingbang, L.; Guolin, L.; Zhaoyan, D.; Chaojun, C. Effects of thermal and nonthermal processing technology on the quality of red sour soup after storage. Food Sci. Nutr. 2021, 9, 3863–3872. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Li, Z.; Yu, L.; Liu, Y.; Hu, Y.; Bi, Y.; Cheng, M. Integrative proteomic and lipidomic analysis of Kaili Sour Soup-mediated attenuation of high-fat diet-induced nonalcoholic fatty liver disease in a rat model. Nutr. Metab. 2021, 18, 26. [Google Scholar] [CrossRef]
- Yang, H.; Xie, J.; Wang, N.; Zhou, Q.; Lu, Y.; Qu, Z.; Wang, H. Effects of Miao sour soup on hyperlipidemia in high-fat diet-induced obese rats via the AMPK signaling pathway. Food Sci. Nutr. 2021, 9, 4266–4277. [Google Scholar] [CrossRef]
- Lin, L.-J.; Zeng, J.; Tian, Q.-M.; Ding, X.-Q.; Zhang, X.-Y.; Gao, X.-Y. Effect of the bacterial community on the volatile flavour profile of a Chinese fermented condiment–Red sour soup–During fermentation. Food Res. Int. 2022, 155, 111059. [Google Scholar] [CrossRef]
- Song, X. Screening and Performance Evaluation of Cholesterol-Reducing Lactic Acid Bacteria Suitable for Meat Fermentation. Master’s Thesis, Guizhou Univesrsity, Guiyang, China, 2016. (In Chinese). [Google Scholar]
- Zhang, R. Screening and Evalutation of Cholesterol-Lowing Bifidobacteium from Guizhou Xiang Pig. Master’s Thesis, Guizhou Univesrsity, Guiyang, China, 2014. (In Chinese). [Google Scholar]
- Zhang, R.; He, L.; Zhang, L.; Li, C.; Zhu, Q. Screening of cholesterol-lowering Bifidobacterium from Guizhou Xiang Pigs, and evaluation of its tolerance to oxygen, acid, and bile. Korean J. Food Sci. Anim. Resour. 2016, 36, 37–43. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.-R.; Lee, M.-A. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Fasogbon, M.B. Chemical and antioxidant properties of snake tomato (Trichosanthes cucumerina) juice and Pineapple (Ananas comosus) juice blends and their changes during storage. Food Chem. 2017, 220, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Peinado, I.; Rosa, E.; Heredia, A.; Andrés, A. Use of isomaltulose to formulate healthy spreadable strawberry products. Application of response surface methodology. Food Biosci. 2015, 9, 47–59. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W.; Górecki, A.; Błaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jeliński, T. A preliminary study about the influence of high hydrostatic pressure processing in parallel with oak chip maceration on the physicochemical and sensory properties of a young red wine. Food Chem. 2016, 194, 545–554. [Google Scholar] [CrossRef]
- Xu, Y.X.; Zhang, M.; Fang, Z.X.; Sun, J.C.; Wang, Y.Q. How to improve bayberry (Myrica rubra Sieb. et Zucc.) juice flavour quality: Effect of juice processing and storage on volatile compounds. Food Chem. 2014, 151, 40–46. [Google Scholar] [CrossRef]
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Liu, L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE–GC–MS, e-nose and sensory evaluation. Food Chem. 2016, 207, 205–213. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Probiotication of tomato juice by lactic acid bacteria. J. Microbiol. 2004, 42, 315–318. [Google Scholar]
- Markovic, M.; Markov, S.; Pejin, D.; Mojovic, L.; Vukasinovic, M.; Pejin, J.; Joković, N. The possibility of lactic acid fermentation in the triticale stillage. Chem. Ind. Chem. Eng. Q. 2011, 17, 153–162. [Google Scholar] [CrossRef]
- Koh, J.-H.; Kim, Y.; Oh, J.-H. Chemical characterization of tomato juice fermented with Bifidobacteria. J. Food Sci. 2010, 75, C428–C432. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Surico, R.F.; Paradiso, A.; De Angelis, M.; Salmon, J.-C.; Buchin, S.; De Gara, L.; Gobbetti, M. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food Microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jun, Z.; Shuaishuai, W.; Lihua, Z.; Qilong, M.; Xi, L.; Mengyang, N.; Tong, Z.; Hongli, Z. Culture-dependent and -independent analysis of bacterial community structure in Jiangshui, a traditional Chinese fermented vegetable food. LWT 2018, 96, 244–250. [Google Scholar] [CrossRef]
- Zhu, Y.; Sims, C.A.; Klee, H.J.; Sarnoski, P.J. Sensory and flavor characteristics of tomato juice from garden gem and Roma tomatoes with comparison to commercial tomato juice. J. Food Sci. 2017, 83, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Shen, Y.; Yu, H.; He, Y.; Chen, C. Effects of 4 Probiotic strains in coculture with traditional starters on the flavor profile of yogurt. J. Food Sci. 2017, 82, 1693–1701. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Mousavi, M. The effect of fermentation by Lactobacillus plantarum on the physicochemical and functional properties of liquorice root extract. LWT-Food Sci. Technol. 2019, 105, 164–168. [Google Scholar] [CrossRef]
- García-Alonso, F.J.; González-Barrio, R.; Martín-Pozuelo, G.; Hidalgo, N.; Navarro-González, I.; Masuero, D.; Soini, E.; Vrhovsek, U.; Periago, M.J. A study of the prebiotic-like effects of tomato juice consumption in rats with diet-induced non-alcoholic fatty liver disease (NAFLD). Food Funct. 2017, 8, 3542–3552. [Google Scholar] [CrossRef]
- Singhvi, M.; Zendo, T.; Sonomoto, K. Free lactic acid production under acidic conditions by lactic acid bacteria strains: Challenges and future prospects. Appl. Microbiol. Biotechnol. 2018, 102, 5911–5924. [Google Scholar] [CrossRef]
- Ye, J.-H.; Huang, L.-Y.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Saa, D.L.T.; Gianotti, A. Changes in carotenoids, phenolic acids and antioxidant capacity in bread wheat doughs fermented with different lactic acid bacteria strains. Food Chem. 2019, 292, 211–216. [Google Scholar] [CrossRef]
- Managa, M.G.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Physicochemical parameters and bioaccessibility of lactic acid bacteria fermented chayote leaf (Sechium edule) and pineapple (Ananas comosus) smoothies. Front. Nutr. 2021, 8, 649189. [Google Scholar] [CrossRef] [PubMed]
- Hornero-Méndez, D.; Cerrillo, I.; Ortega, Á.; Rodríguez-Griñolo, M.-R.; Escudero-López, B.; Martín, F.; Fernández-Pachón, M.-S. β-Cryptoxanthin is more bioavailable in humans from fermented orange juice than from orange juice. Food Chem. 2018, 262, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Dagostin, J.L.A.; Carpiné, D.; Masson, M.L. Influence of acidification method on composition, texture, psychrotrophs, and lactic acid bacteria in Minas frescal cheese. Food Bioprocess Technol. 2012, 6, 3017–3028. [Google Scholar] [CrossRef]
- Markkinen, N.; Laaksonen, O.; Nahku, R.; Kuldjärv, R.; Yang, B. Impact of lactic acid fermentation on acids, sugars, and phenolic compounds in black chokeberry and sea buckthorn juices. Food Chem. 2019, 286, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Ferns, G.A.; Banach, M. A high consumption of tomato and lycopene is associated with a lower risk of cancer mortality: Results from a multiethnic cohort. Public Health Nutr. 2020, 23, 1569–1575. [Google Scholar] [CrossRef]
- Lu, Y.; Mu, K.; McClements, D.J.; Liang, X.; Liu, X.; Liu, F. Fermentation of tomato juice improves in vitro bioaccessibility of lycopene. J. Funct. Foods 2020, 71, 104020. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xie, B.; Sun, Z. Influence of lactic acid bacteria fermentation on physicochemical properties and antioxidant activity of chickpea yam milk. J. Food Qual. 2021, 2021, 5523356. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef]
- Oh, B.-T.; Jeong, S.-Y.; Velmurugan, P.; Park, J.-H.; Jeong, D.-Y. Probiotic-mediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. J. Biosci. Bioeng. 2017, 124, 542–550. [Google Scholar] [CrossRef]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2021, 43, 189–198. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Gallego, J.; Medina, E.; Sánchez, B.; Benítez-Cabello, A.; Arroyo-López, F.N. Role of lactic acid bacteria in fermented vegetables. Grasas Y Aceites 2020, 71, 358. [Google Scholar] [CrossRef]
- Tatol, W.M.M. Color difference delta E—A surve. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.; Chenchaiah, M.; Cullen, P. Effect of ozonation on the rheological and colour characteristics of hydrocolloid dispersions. Food Res. Int. 2008, 41, 1035–1043. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: Process optimisation and product stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef]
- Wang, H.; Yin, L.-J.; Cheng, Y.-Q.; Li, L.-T. Effect of sodium chloride on the color, texture, and sensory attributes of douchi during post-fermentation. Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2018, 27, 30–36. [Google Scholar] [CrossRef]
- Huang, Z.-R.; Guo, W.-L.; Zhou, W.-B.; Li, L.; Xu, J.-X.; Hong, J.-L.; Liu, H.-P.; Zeng, F.; Bai, W.-D.; Liu, B.; et al. Microbial communities and volatile metabolites in different traditional fermentation starters used for Hong Qu glutinous rice wine. Food Res. Int. 2018, 121, 593–603. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Deng, F.; Zhou, H. SPME/GC-MS characterization of volatile compounds of Chinese traditional-chopped pepper during fermentation. Int. J. Food Prop. 2019, 22, 1863–1872. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016, 60, 29630. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Li, A.-H.; Dizy, M.; Ullah, N.; Sun, W.-X.; Tao, Y.-S. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef]
- Sun, X.; Du, J.; Xiong, Y.; Cao, Q.; Wang, Z.; Li, H.; Zhang, F.; Chen, Y.; Liu, Y. Characterization of the key aroma compounds in Chinese JingJiu by quantitative measurements, aroma recombination, and omission experiment. Food Chem. 2021, 352, 129450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Yang, W.; Sun, B.; Zhou, Y.; Zheng, Y.; Huang, M.; Yang, W. Characterization of the potent odorants in Zanthoxylum armatum DC Prodr. pericarp oil by application of gas chromatography–mass spectrometry–olfactometry and odor activity value. Food Chem. 2020, 319, 126564. [Google Scholar] [CrossRef]
- Hou, Z.-W.; Wang, Y.-J.; Xu, S.-S.; Wei, Y.-M.; Bao, G.-H.; Dai, Q.-Y.; Deng, W.-W.; Ning, J.-M. Effects of dynamic and static withering technology on volatile and nonvolatile components of Keemun black tea using GC-MS and HPLC combined with chemometrics. LWT 2020, 130, 109547. [Google Scholar] [CrossRef]
- Zhao, L.; Ruan, S.; Yang, X.; Chen, Q.; Shi, K.; Lu, K.; He, L.; Liu, S.; Song, Y. Characterization of volatile aroma compounds in litchi (Heiye) wine and distilled spirit. Food Sci. Nutr. 2021, 9, 5914–5927. [Google Scholar] [CrossRef]
- Yang, C.; Luo, L.; Zhang, H.; Yang, X.; Lv, Y.; Song, H. Common aroma-active components of propolis from 23 regions of China. J. Sci. Food Agric. 2010, 90, 1268–1282. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Zini, C.A. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial effects of tomato juice fermented by Lactobacillus plantarum and Lactobacillus casei: Antioxidation, antimicrobial effect, and volatile profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Zhao, C.; Zhu, Y. Metagenomic insights into the changes in microbial community and antimicrobial resistance genes associated with different salt content of red pepper (Capsicum annuum L.) sauce. Food Microbiol. 2020, 85, 103295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Che, Z.; Xu, W.; Yue, P.; Li, R.; Li, Y.; Pei, X.; Zeng, P. Dynamics of physicochemical factors and microbial communities during ripening fermentation of Pixian Doubanjiang, a typical condiment in Chinese cuisine. Food Microbiol. 2019, 86, 103342. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Sample | Oxalic Acid (mg/100 mL) | Ascorbic Acid (mg/100 mL) | Lactic Acid (mg/100 mL) | Citric Acid (mg/100 mL) | Succinic Acid (mg/100 mL) | Total Phenols (mg/mL) | Total Carotenoids (mg/mL) | Lycopene (µg/mL) | ABTS% | DPPH% |
|---|---|---|---|---|---|---|---|---|---|---|
| UNF | 24.11 ± 0.47 c | ND | ND | 26.54 ± 0.73 b | 59.95 ± 0.34 g | 6.61 ± 0.11 c | 0.91 ± 0.01 h | 18.31 ± 1.01 c | 18.83 ± 0.71 f | 5.11 ± 0.57 c |
| CON | 11.43 ± 1.01 e | 6.99 ± 0.08 b | 317.26 ± 8.5 b | ND | 104.69 ± 3.54 b | 12.89 ± 0.03 a | 1.17 ± 0.09 g | 14.70 ± 0.48 e | 73.94 ± 0.51 e | 62.88 ± 1.74 b |
| HBZ11 | 27.07 ± 1.3 b | 6.90 ± 0.33 b | 260.74 ± 3.53 d | 21.37 ± 1.26 c | 77.90 ± 1.41 f | 12.87 ± 0.04 a | 3.52 ± 0.05 a | 22.19 ± 0.73 b | 81.55 ± 1.23 cd | 67.23 ± 2.37 a |
| HBZ25 | 31.84 ± 0.54 a | 5.25 ± 0.16 c | 234.72 ± 6.31 e | 26.27 ± 1.88 b | 90.53 ± 0.34 d | 12.91 ± 0.03 a | 3.15 ± 0.03 c | 24.39 ± 0.46 a | 84.27 ± 0.94 ab | 69.13 ± 1.74 a |
| HBB12 | 30.60 ± 0.79 a | 7.60 ± 0.29 a | 143.46 ± 3.59 f | 31.98 ± 0.91 a | 75.72 ± 0.71 f | 12.89 ± 0.03 a | 2.98 ± 0.02 d | 14.59 ± 0.86 e | 80.38 ± 1.55 d | 68.75 ± 1.5 a |
| HBL1 | 31.50 ± 0.62 a | 5.42 ± 0.07 c | 235.24 ± 4.74 e | 25.65 ± 0.67 b | 87.01 ± 1.00 e | 12.92 ± 0.05 a | 2.08 ± 0.04 f | 16.49 ± 0.33 d | 81.92 ± 1.87 bcd | 69.89 ± 1.14 a |
| HMT-4 | 31.54 ± 0.20 a | ND | 380.95 ± 2.15 a | 21.39 ± 0.88 c | 107.45 ± 1.32 a | 12.89 ± 0.10 a | 3.42 ± 0.05 b | 14.73 ± 0.10 e | 83.80 ± 0.88 abc | 69.70 ± 1.83 a |
| HSQ-4 | 21.45 ± 0.25 d | ND | 269.76 ± 0.93 c | 22.20 ± 1.23 c | 86.37 ± 1.67 e | 12.90 ± 0.04 a | 2.83 ± 0.04 e | 23.41 ± 0.15 ab | 86.15 ± 0.29 a | 70.27 ± 1.83 a |
| HBGS | 26.86 ± 0.43 b | 5.48 ± 0.12 c | 324.07 ± 8.13 b | ND | 97.90 ± 1.29 c | 8.74 ± 0.04 b | 3.49 ± 0.02 ab | 22.60 ± 1.43 b | 83.90 ± 2.19 abc | 69.32 ± 1.97 a |
| 0 Day | 1 Day | 3 Days | 5 Days | 7 Days | 9 Days | 12 Days | 15 Days | ||
|---|---|---|---|---|---|---|---|---|---|
| CON | L* | 23.80 ± 0.70 b | 25.72 ± 0.80 a | 26.49 ± 0.36 a | 25.74 ± 0.22 a | 26.84 ± 0.85 a | 25.68 ± 0.63 a | 26.16 ± 0.53 a | 26.52 ± 0.50 a |
| a* | 11.53 ± 0.61 e | 14.61 ± 0.70 bcd | 15.22 ± 0.44 abc | 14.02 ± 0.46 cd | 14.22 ± 0.77 bcd | 15.42 ± 0.44 ab | 16.02 ± 0.82 a | 13.40 ± 0.87 d | |
| b* | 7.67 ± 0.40 bc | 4.60 ± 0.35 d | 7.49 ± 0.57 bc | 7.09 ± 0.74 c | 8.37 ± 0.22 ab | 7.93 ± 0.69 abc | 8.75 ± 0.57 a | 8.04 ± 0.76 abc | |
| ΔE* | 0 | 4.75 ± 0.45 a | 4.75 ± 0.59 a | 4.75 ± 0.59 a | 4.20 ± 0.99 b | 4.36 ± 0.74 b | 5.24 ± 0.74 a | 3.48 ± 0.41 b | |
| HBZ11 | L* | 23.80 ± 0.70 c | 26.93 ± 0.55 b | 27.19 ± 0.39 b | 27.42 ± 0.52 b | 29.36 ± 0.84 a | 29.68 ± 0.73 a | 29.92 ± 0.93 a | 29.77 ± 0.91 a |
| a* | 11.53 ± 0.61 d | 13.67 ± 0.44 c | 13.85 ± 0.28 c | 14.86 ± 0.42b | 16.26 ± 0.76 a | 15.72 ± 0.69 ab | 16.35 ± 0.10 a | 13.70 ± 0.61 c | |
| b* | 7.67 ± 0.40 cd | 6.20 ± 0.61 e | 7.03 ± 0.39 de | 9.29 ± 0.88 ab | 9.49 ± 0.89 ab | 8.84 ± 0.22 bc | 10.20 ± 0.62 a | 8.82 ± 0.96 bc | |
| ΔE* | 0 | 4.18 ± 0.42 c | 4.22 ± 0.29 c | 5.33 ± 0.84 bc | 7.54 ± 1.22 a | 7.37 ± 1.10 a | 8.23 ± 0.95 a | 6.51 ± 1.74 ab | |
| HBZ25 | L* | 23.80 ± 0.70 d | 28.26 ± 0.05 b | 27.47 ± 0.32 bc | 27.29 ± 0.56 bc | 27.18 ± 0.35 c | 26.59 ± 0.20 c | 29.86 ± 0.78 b | 30.07 ± 0.99 a |
| a* | 11.53 ± 0.61 c | 14.96 ± 0.60 b | 14.66 ± 0.47 b | 15.37 ± 0.62 b | 15.52 ± 0.66 b | 14.96 ± 0.49 b | 18.92 ± 0.28 a | 15.58 ± 0.92 b | |
| b* | 7.67 ± 0.40 bcd | 7.39 ± 0.51 cd | 7.52 ± 0.59 cd | 8.34 ± 0.61 bc | 8.70 ± 0.86 b | 6.88 ± 0.35 d | 11.39 ± 0.52 a | 12.39 ± 0.77 a | |
| ΔE* | 0 | 5.72 ± 0.10 c | 4.91 ± 0.24 cd | 5.28 ± 0.16 cd | 5.54 ± 0.52 c | 4.55 ± 0.75 d | 10.28 ± 0.74 a | 8.40 ± 0.63 b | |
| HBB12 | L* | 23.80 ± 0.70 d | 26.80 ± 0.19 c | 27.38 ± 0.33 bc | 28.01 ± 0.84 b | 27.52 ± 0.69 bc | 27.90 ± 0.29 b | 29.26 ± 0.56 a | 28.23 ± 0.52 b |
| a* | 11.53 ± 0.61 e | 16.83 ± 0.27 a | 15.69 ± 0.54 b | 14.63 ± 0.67 c | 15.82 ± 0.37 b | 14.64 ± 0.71 c | 14.41 ± 0.41 cd | 13.60 ± 0.59 d | |
| b* | 7.67 ± 0.40 cd | 9.23 ± 0.55 ab | 6.91 ± 0.90 d | 7.80 ± 0.93 cd | 9.99 ± 0.56 a | 8.02 ± 0.10 bcd | 8.22 ± 0.79 bc | 8.74 ± 0.54 bc | |
| ΔE* | 0 | 6.32 ± 0.28 a | 5.71 ± 0.14 a | 5.46 ± 0.56 a | 6.19 ± 0.81 a | 5.18 ± 0.81 a | 6.28 ± 0.87 a | 5.13 ± 0.63 a | |
| HBL1 | L* | 23.80 ± 0.70 e | 26.57 ± 0.81 cd | 27.11 ± 0.66 cd | 27.86 ± 0.70 bc | 27.41 ± 0.17 bcd | 26.28 ± 0.96 d | 30.19 ± 0.63 a | 28.51 ± 0.78 b |
| a* | 11.53 ± 0.61 e | 17.01 ± 0.42 b | 16.24 ± 0.66 bc | 14.52 ± 0.80 d | 14.53 ± 0.75 d | 15.42 ± 0.44 cd | 18.17 ± 0.68 a | 15.53 ± 0.82 cd | |
| b* | 7.67 ± 0.40 c | 8.98 ± 0.51 ab | 8.80 ± 0.23 abc | 8.16 ± 0.77 bc | 8.62 ± 0.57 abc | 8.20 ± 0.78 bc | 9.41 ± 0.78 a | 9.51 ± 0.60 a | |
| ΔE* | 0 | 6.38 ± 0.90 b | 6.01 ± 0.14 b | 5.15 ± 0.47 c | 4.84 ± 0.47 c | 4.68 ± 0.46 c | 9.50 ± 0.36 a | 6.50 ± 0.18 b | |
| HMT-4 | L* | 23.80 ± 0.70 f | 29.32 ± 0.30 bc | 26.67 ± 0.21 cd | 27.53 ± 0.21 de | 28.11 ± 0.64 d | 26.94 ± 0.56 e | 30.61 ± 0.80 a | 30.08 ± 0.72 ab |
| a* | 11.53 ± 0.61 e | 14.72 ± 0.61 c | 15.33 ± 0.26 c | 14.24 ± 0.89 cd | 15.06 ± 0.66 c | 13.38 ± 0.37 d | 17.78 ± 0.62 a | 16.54 ± 0.25 b | |
| b* | 7.67 ± 0.40 de | 9.02 ± 0.44 b | 7.43 ± 0.83 bcd | 7.93 ± 0.50 cde | 8.51 ± 0.54 bcd | 6.94 ± 0.35 e | 8.92 ± 0.92 bc | 13.33 ± 0.64 a | |
| ΔE* | 0 | 6.60 ± 0.42 b | 5.74 ± 0.72 bc | 4.72 ± 0.98 cd | 5.69 ± 0.86 bc | 3.88 ± 0.84 d | 9.46 ± 0.52 a | 9.86 ± 0.37 a | |
| HSQ-4 | L* | 23.80 ± 0.70 e | 27.88 ± 0.33 c | 28.39 ± 0.85 d | 28.01 ± 0.18 c | 30.83 ± 0.64 a | 30.18 ± 0.25 ab | 30.73 ± 0.60 a | 29.69 ± 0.99 b |
| a* | 11.53 ± 0.61 d | 15.50 ± 0.44 b | 14.87 ± 0.17 b | 14.01 ± 0.54 c | 14.97 ± 0.70 bc | 17.28 ± 1.02 a | 17.16 ± 0.58 a | 14.04 ± 0.43 c | |
| b* | 7.67 ± 0.40 de | 8.32 ± 0.75 cd | 8.17 ± 0.32 d | 7.01 ± 0.94 d | 12.73 ± 0.77 a | 9.54 ± 0.94 bc | 10.34 ± 0.89 b | 9.88 ± 0.79 b | |
| ΔE* | 0 | 5.77 ± 0.10 bc | 4.88 ± 0.20 c | 5.09 ± 0.63 c | 9.36 ± 1.35 a | 8.87 ± 1.13 a | 9.36 ± 0.72 a | 6.87 ± 1.05 b | |
| HBGS | L* | 23.80 ± 0.70 d | 23.49 ± 0.18 d | 27.14 ± 0.23 bc | 28.04 ± 0.39 b | 27.10 ± 0.69 bc | 26.6 ± 0.43 c | 29.11 ± 0.80 a | 27.63 ± 0.95 bc |
| a* | 11.53 ± 0.61 c | 14.36 ± 0.51 ab | 14.93 ± 0.79 ab | 13.87 ± 0.15 b | 14.03 ± 0.59 b | 14.63 ± 0.89 ab | 15.47 ± 0.06 a | 14.00 ± 0.92 b | |
| b* | 7.67 ± 0.40 b | 5.78 ± 0.34 c | 5.51 ± 0.64 c | 7.41 ± 0.41 b | 9.07 ± 0.64 a | 8.90 ± 0.61 a | 8.49 ± 0.74 ab | 9.47 ± 0.89 a | |
| ΔE* | 0 | 3.47 ± 0.33 c | 5.36 ± 0.34 b | 4.88 ± 0.13 b | 4.61 ± 0.51 bc | 4.40 ± 0.86 bc | 6.74 ± 0.57 a | 4.95 ± 1.20 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhang, Q.; Wang, C.; He, L.; Tao, H.; Zeng, X.; Dai, Y. Effect of Starters on Quality Characteristics of Hongsuantang, a Chinese Traditional Sour Soup. Fermentation 2022, 8, 589. https://doi.org/10.3390/fermentation8110589
Li C, Zhang Q, Wang C, He L, Tao H, Zeng X, Dai Y. Effect of Starters on Quality Characteristics of Hongsuantang, a Chinese Traditional Sour Soup. Fermentation. 2022; 8(11):589. https://doi.org/10.3390/fermentation8110589
Chicago/Turabian StyleLi, Cuiqin, Qing Zhang, Chan Wang, Laping He, Han Tao, Xuefeng Zeng, and Yifeng Dai. 2022. "Effect of Starters on Quality Characteristics of Hongsuantang, a Chinese Traditional Sour Soup" Fermentation 8, no. 11: 589. https://doi.org/10.3390/fermentation8110589
APA StyleLi, C., Zhang, Q., Wang, C., He, L., Tao, H., Zeng, X., & Dai, Y. (2022). Effect of Starters on Quality Characteristics of Hongsuantang, a Chinese Traditional Sour Soup. Fermentation, 8(11), 589. https://doi.org/10.3390/fermentation8110589





