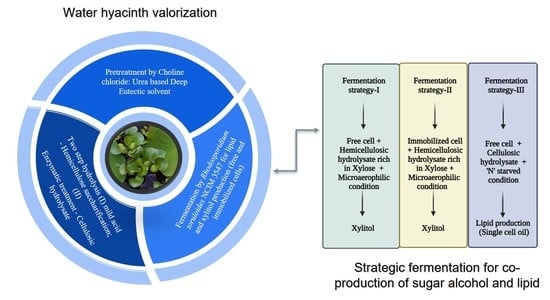
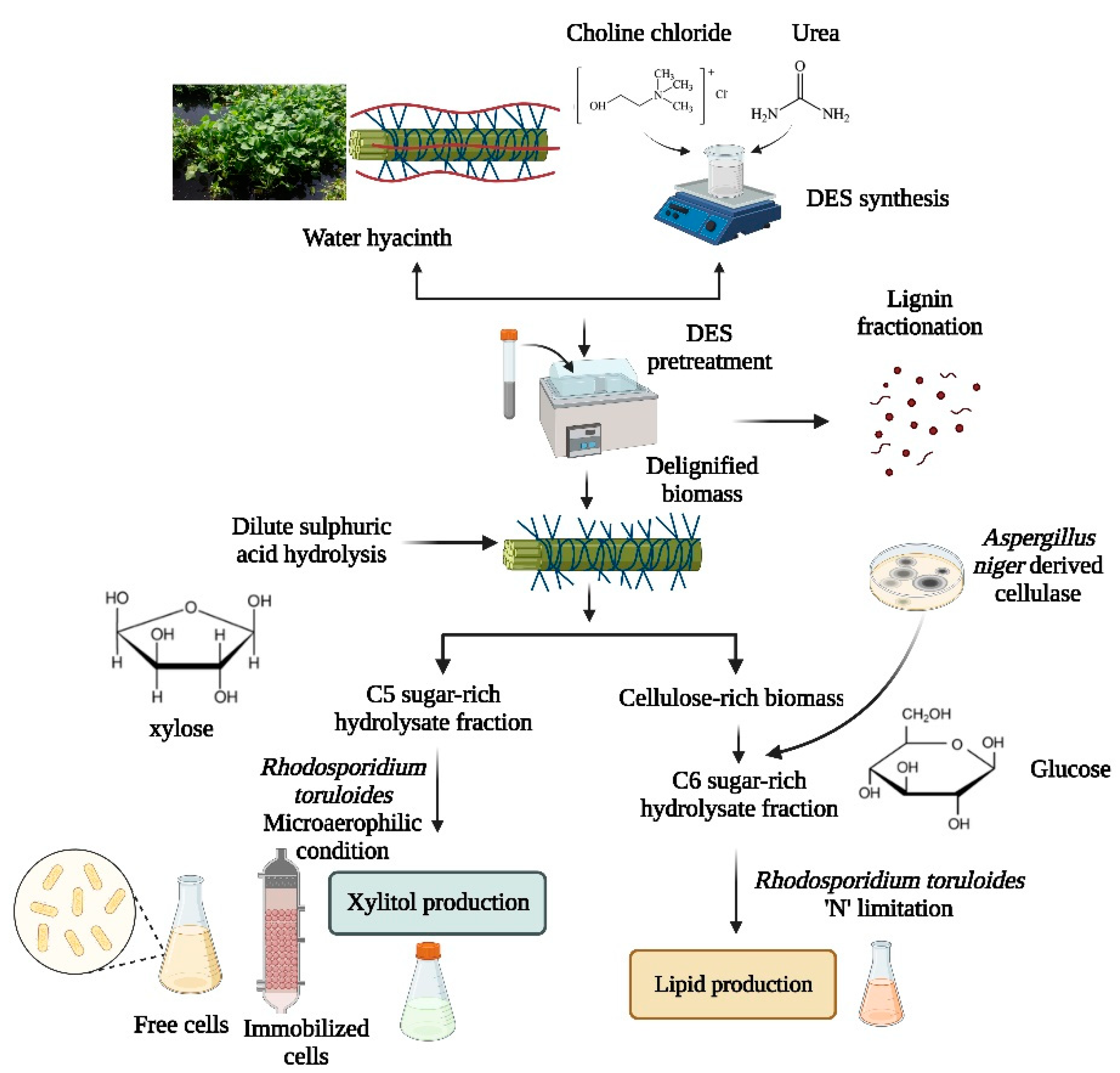
Deep Eutectic Solvent Pretreatment of Water Hyacinth for Improved Holocellulosic Saccharification and Fermentative Co-Production of Xylitol and Lipids Using Rhodosporidium toruloides NCIM 3547
Abstract
1. Introduction
- (i)
- A mild sulphuric acid (2%, v/v) for xylose (C5 sugar) recovery from hemicellulose;
- (ii)
- Enzymes (a combination of cellulase and xylanase) derived from Aspergillus niger for hydrolysis of holocellulose to yield glucose and xylose as reported earlier by our research group [13].
2. Materials and Methods
2.1. Substrate: Water Hyacinth (WH)
2.2. Microorganism and Inoculum Preparation
2.3. Adaptive Laboratory Evolution (ALE) Strategy for Rhodosporidium toruloides NCIM 3547 to Produce Xylitol
2.4. Synthesis of DES and Pretreatment
2.5. Pretreatment of WH by DES and Recovery of Lignin
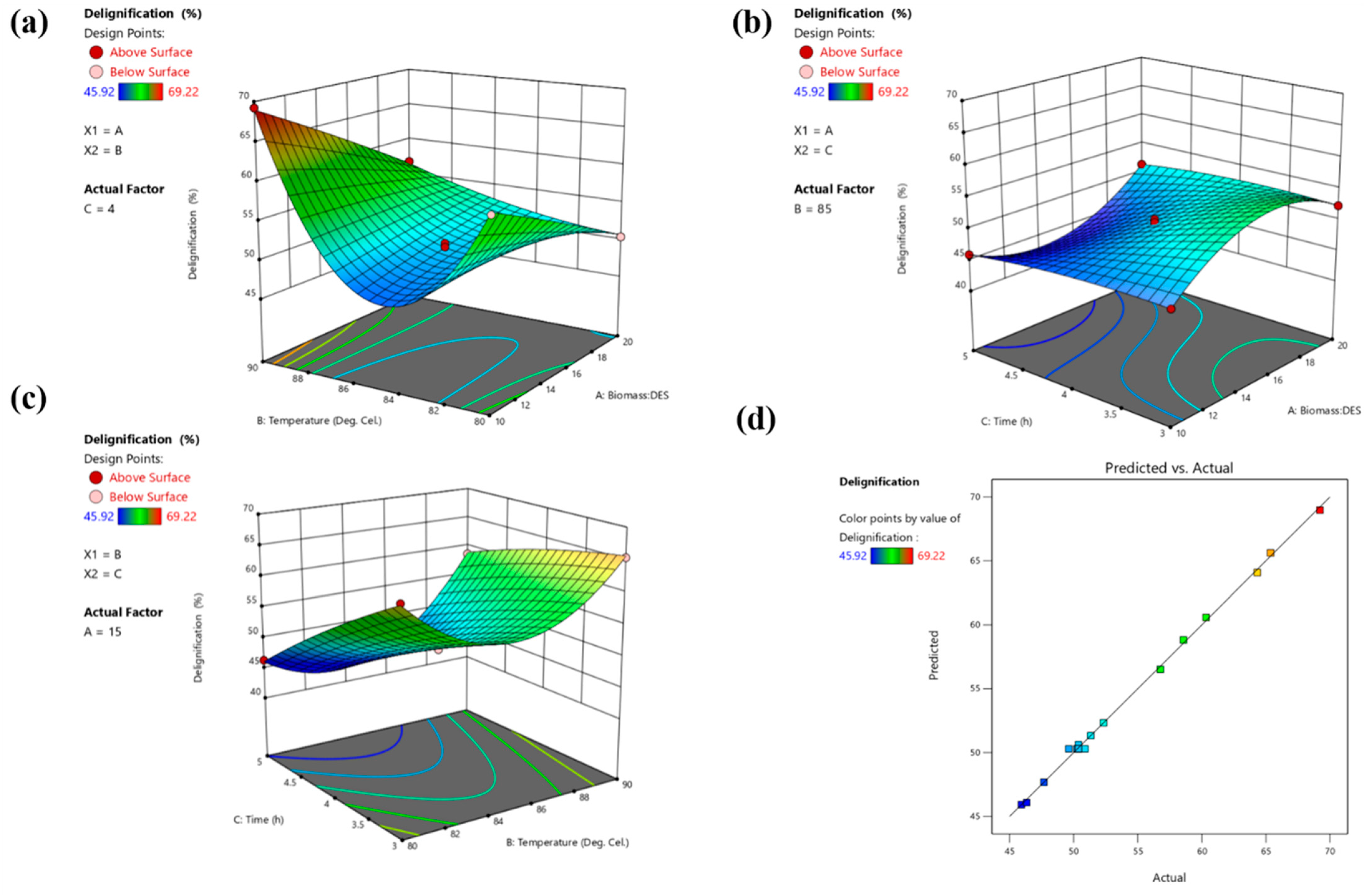
2.6. Statistical Optimization of DES Pretreatment by BBD-RSM
2.7. Recovery of Delignified WH and Lignin after DES Pretreatment
2.8. Characterization of Raw and Treated WH
2.9. Hemicellulosic Hydrolysate Preparation
2.10. Enzymatic Cellulose Hydrolysis of DES Delignified WH
2.11. Fermentation of Hemicellulosic and Cellulosic Hydrolysate for Co-Production of Xylitol and Lipids
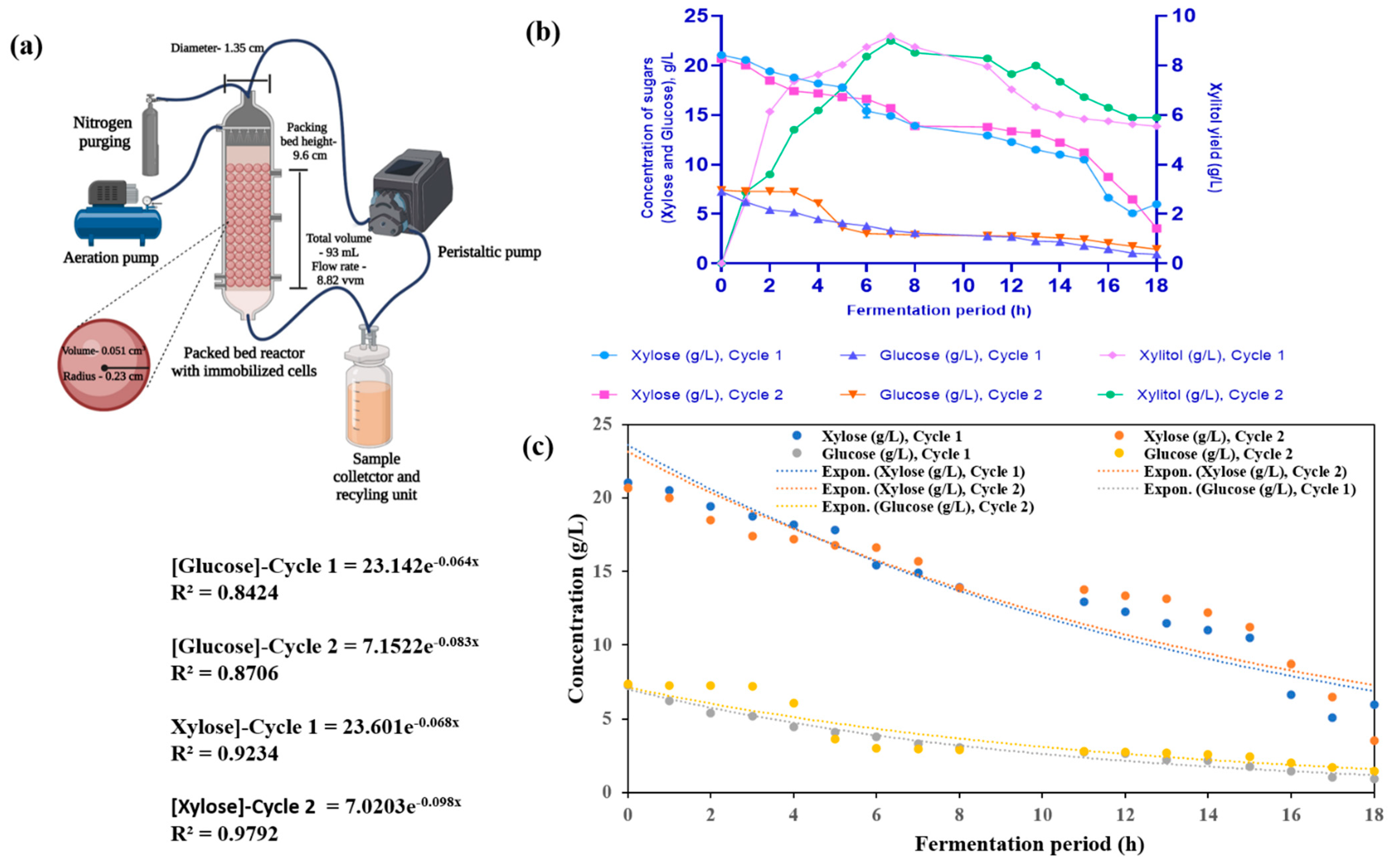
2.12. PBR Studies with Immobilized Rhodosporidium toruloides for Xylitol Production
2.13. Product Analytical Methods
2.13.1. Xylitol Analysis: Colorimetric Method and HPLC
2.13.2. Lipid and Fatty Acid Methyl Ester (FAME) Analysis by GC-MS
2.13.3. Kinetic Studies on Different Fermentative Strategies
3. Results
3.1. Proximate and Biochemical Analysis of the WH
3.2. Properties of Synthesized DES Using ChCl:Urea
3.3. Effect of Process Parameters Ascertained by OVAT Analysis
3.4. Optimization of DES Pretreatment by BBD-Based RSM
3.5. Effect of DES Pretreatment on WH Based on SEM, FTIR and XRD
3.6. Stepwise Saccharification of DES-Pretreated WH
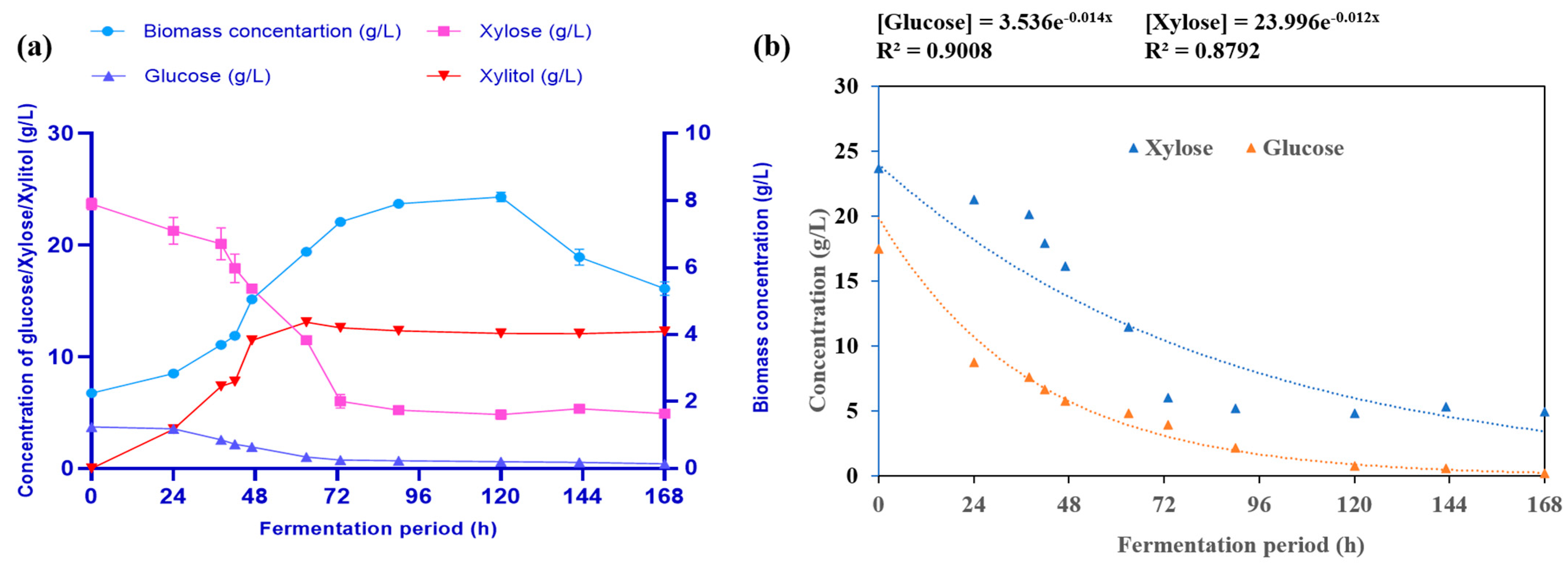
3.7. Co-Production of Xylitol and Lipids from Xylose by Free Cells of R. toruloides NCIM 3547
3.8. Production of Xylitol from Xylose by Immobilized R. toruloides NCIM 3547 in PBR
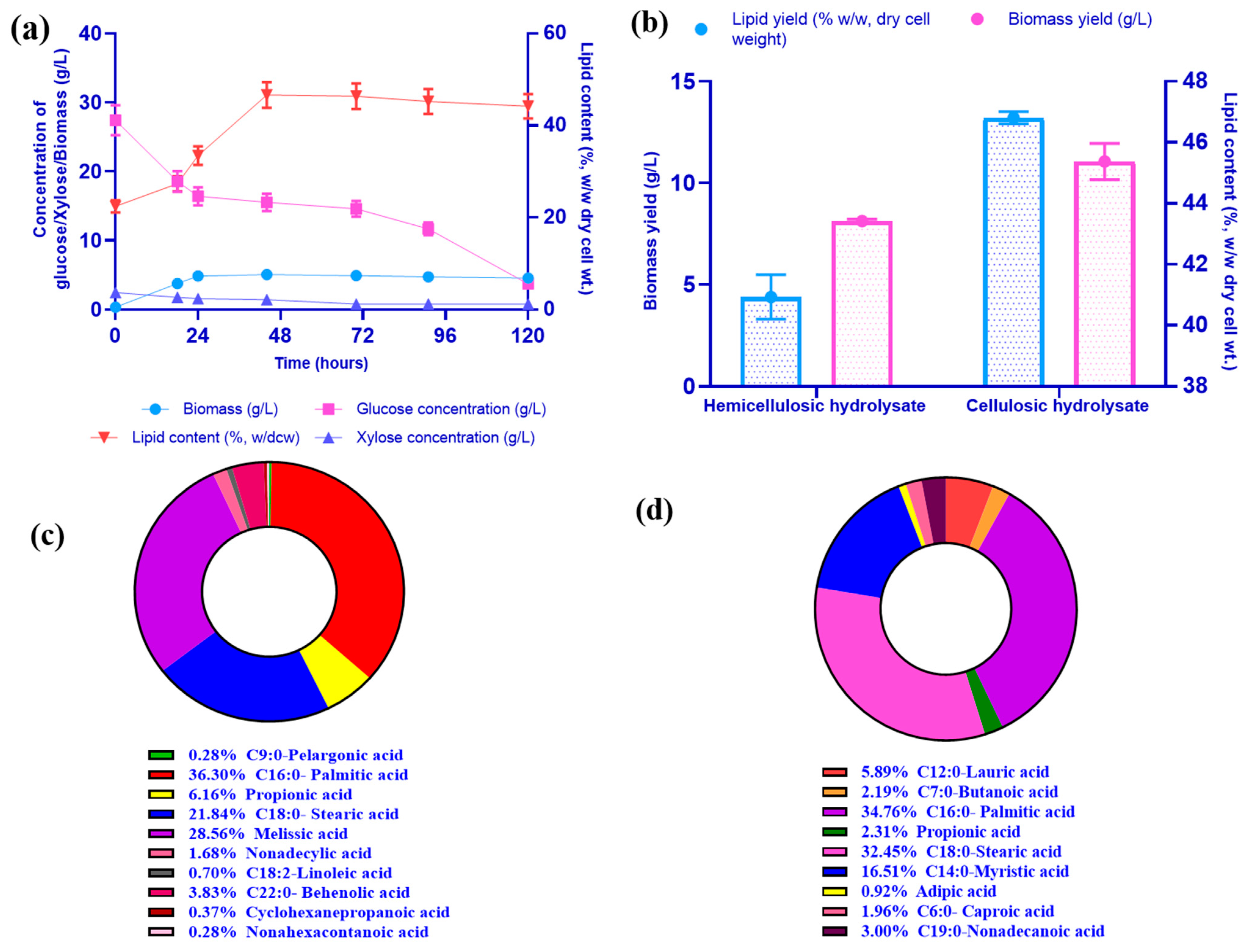
3.9. Production of Lipids from Cellulosic Hydrolysate by R. toruloides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vymazal, J. Constructed Wetlands, Surface Flow. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 765–776. ISBN 978-0-08-045405-4. [Google Scholar]
- Pegg, J.; South, J.; Hill, J.E.; Durland-Donahou, A.; Weyl, O.L.F. Impacts of Alien Invasive Species on Large Wetlands. In Fundamentals of Tropical Freshwater Wetlands; Elsevier: Amsterdam, The Netherlands, 2022; pp. 487–516. ISBN 978-0-12-822362-8. [Google Scholar]
- Wang, L.; D’Odorico, P. Decomposition and Mineralization. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 838–844. ISBN 978-0-08-045405-4. [Google Scholar]
- Bai, F.; Chisholm, R.; Sang, W.; Dong, M. Spatial Risk Assessment of Alien Invasive Plants in China. Environ. Sci. Technol. 2013, 47, 7624–7632. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Maharaj, S.; Prabhu, G.N.; Bhowmik, D.; Marino, A.; Akbari, V.; Rupavatharam, S.; Sujeetha, J.A.R.P.; Anantrao, G.G.; Poduvattil, V.K.; et al. Monitoring the Spread of Water Hyacinth (Pontederia Crassipes): Challenges and Future Developments. Front. Ecol. Evol. 2021, 9, 631338. [Google Scholar] [CrossRef]
- Verified Market Research. Global Xylitol Market Size by Application (Chewing Gum, Confectionary, Food, Personal Care, Pharmaceuticals, Nutraceuticals), by Geographic Scope and Forecast; Verified Market Research (VMR): Pune, India, 2021; p. 202. [Google Scholar]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and Applications—A Review. Front. Sustain. 2022, 3, 826190. [Google Scholar] [CrossRef]
- Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods 2020, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, D.-J. Lignocellulosic Biomass Pretreatment by Deep Eutectic Solvents on Lignin Extraction and Saccharification Enhancement: A Review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Che, X.; Liu, S.; Tian, W. Multivariate Analysis of the Process of Deep Eutectic Solvent Pretreatment of Lignocellulosic Biomass. Ind. Crops Prod. 2020, 150, 112363. [Google Scholar] [CrossRef]
- Massayev, S.; Lee, K.M. Evaluation of Deep Eutectic Solvent Pretreatment towards Efficacy of Enzymatic Saccharification Using Multivariate Analysis Techniques. J. Clean. Prod. 2022, 360, 132239. [Google Scholar] [CrossRef]
- Rajeswari, G.; Arutselvy, B.; Jacob, S. Delignification of Aloe Vera Rind by Mild Acid Associated Microwave Pretreatment to Persuade Enhanced Enzymatic Saccharification. Waste Biomass Valorization 2020, 11, 5965–5975. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Hegde, K.; Ferreira, P.; Brar, S.K.; Kermanshahipour, A.; Soccol, C.R.; Avalos-Ramírez, A. Lipid Production in Rhodosporidium toruloides Using C-6 and C-5 Wood Hydrolysate: A Comparative Study. Biomass Bioenergy 2019, 130, 105355. [Google Scholar] [CrossRef]
- Pinheiro, M.J.; Bonturi, N.; Belouah, I.; Miranda, E.A.; Lahtvee, P.-J. Xylose Metabolism and the Effect of Oxidative Stress on Lipid and Carotenoid Production in Rhodotorula toruloides: Insights for Future Biorefinery. Front. Bioeng. Biotechnol. 2020, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Maiti, M.K. Lipid Production by Oleaginous Yeasts. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 116, pp. 1–98. ISBN 978-0-12-824594-1. [Google Scholar]
- Manaf, S.F.A.; Luthfi, A.A.I.; Tan, J.P.; Abdul, P.M.; Jamali, N.S. Kinetic Study and Model of Fermentation Parameters Affected Growth and Xylitol Production in Bioreactor by Kluyveromyces marxianus ATCC 36,907. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Sasidharan, manekkara. EBird, Hotspot Report. Available online: https://ebird.org/hotspot/L3078680 (accessed on 25 September 2022).
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive Laboratory Evolution Principles and Applications in Industrial Biotechnology. Biotechnol. Adv. 2022, 54, 107795. [Google Scholar] [CrossRef] [PubMed]
- Salimiyan, K.; Saberi, D. Choline Chloride/Urea as an Eco-Friendly Deep Eutectic Solvent for TCT-Mediated Amide Coupling at Room Temperature. Chem. Select 2019, 4, 3985–3989. [Google Scholar] [CrossRef]
- Karlsson, H.; Ahlgren, S.; Sandgren, M.; Passoth, V.; Wallberg, O.; Hansson, P.-A. A Systems Analysis of Biodiesel Production from Wheat Straw Using Oleaginous Yeast: Process Design, Mass and Energy Balances. Biotechnol. Biofuels 2016, 9, 229. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep Eutectic Solvent Pretreatment and Subsequent Saccharification of Corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Hussain, M.A.; Emdadul Hu, M.; Matiur Rah, S.; Ahmed, Z. Estimation of Lignin in Jute by Titration Method. Pak. J. Biol. Sci. 2002, 5, 521–522. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Ricci, P.; Karvonen, V.; Ottolina, G.; Liimatainen, H. Acidic and Alkaline Deep Eutectic Solvents in Delignification and Nanofibrillation of Corn Stalk, Wheat Straw, and Rapeseed Stem Residues. Ind. Crops Prod. 2020, 145, 111956. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Narisetty, V.; Castro, E.; Durgapal, S.; Coulon, F.; Jacob, S.; Kumar, D.; Kumar Awasthi, M.; Kishore Pant, K.; Parameswaran, B.; Kumar, V. High Level Xylitol Production by Pichia fermentans Using Non-Detoxified Xylose-Rich Sugarcane Bagasse and Olive Pits Hydrolysates. Bioresour. Technol. 2021, 342, 126005. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Pham, P.J.; Hernandez, R.; French, W.T.; Estill, B.G.; Mondala, A.H. A Spectrophotometric Method for Quantitative Determination of Xylose in Fermentation Medium. Biomass Bioenergy 2011, 35, 2814–2821. [Google Scholar] [CrossRef]
- Singh, G.; Sinha, S.; Bandyopadhyay, K.K.; Lawrence, M.; Prasad, R.; Paul, D. Correction to: Triauxic Growth of an Oleaginous Red Yeast Rhodosporidium toruloides on Waste ‘Extract’ for Enhanced and Concomitant Lipid and Β-carotene Production. Microb. Cell Factories 2019, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Alankar, S.S.L.; Sajesh, N.; Rastogi, S.; Sakhuja, S.; Rajeswari, G.; Kumar, V.; Chandel, A.K.; Jacob, S. Bioprocessing of Fermentable Sugars Derived from Water Hyacinth into Microbial Lipids and Single Cell Proteins by Oleaginous Yeast Rhodosporidium toruloides NCIM 3547. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Sánchez, J. Colorimetric Assay of Alditols in Complex Biological Samples. J. Agric. Food Chem. 1998, 46, 157–160. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Xu, P. Analytical Solution for a Hybrid Logistic-Monod Cell Growth Model in Batch and Continuous Stirred Tank Reactor Culture. Biotechnol. Bioeng. 2020, 117, 873–878. [Google Scholar] [CrossRef]
- Nigam, J.N. Bioconversion of Water-Hyacinth (Eichhornia crassipes) Hemicellulose Acid Hydrolysate to Motor Fuel Ethanol by Xylose–Fermenting Yeast. J. Biotechnol. 2002, 97, 107–116. [Google Scholar] [CrossRef]
- Rathod, V.P.; Bhale, P.V.; Mehta, R.S.; Harmani, K.; Bilimoria, S.; Mahida, A.; Champaneri, H. Biogas Production from Water Hyacinth in the Batch Type Anaerobic Digester. Mater. Today Proc. 2018, 5, 23346–23350. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Madhavan, A.; Alphonsa, J.A.; Vivek, N.; Gnansounou, E.; Castro, E.; Faraco, V. Water Hyacinth a Potential Source for Value Addition: An Overview. Bioresour. Technol. 2017, 230, 152–162. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, K.; Zhou, W.; Zhu, D.; Zhou, Y.; Yuan, Y.; Xiao, N.; Wan, X.; Hua, Y.; Zhao, J. Analysis of Utilization Technologies for Eichhornia crassipes Biomass Harvested after Restoration of Wastewater. Bioresour. Technol. 2017, 223, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, J.L.; Kumari, S.; Das, D. Improvement of Energy Recovery from Water Hyacinth by Using Integrated System. Int. J. Hydrog. Energy 2018, 43, 1303–1318. [Google Scholar] [CrossRef]
- Wauton, I.; Ogbeide, S.E. Characterization of Pyrolytic Bio-Oil from Water Hyacinth (Eichhornia crassipes) Pyrolysis in a Fixed Bed Reactor. Biofuels 2021, 12, 899–904. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The Role of Pretreatment in Improving the Enzymatic Hydrolysis of Lignocellulosic Materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S. Densities and Viscosities of (Choline Chloride + Urea) Deep Eutectic Solvent and Its Aqueous Mixtures in the Temperature Range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C.-Y. Synthesis of Citric Acid Monohydrate-Choline Chloride Based Deep Eutectic Solvents (DES) and Characterization of Their Physicochemical Properties. J. Mol. Liq. 2019, 288, 111081. [Google Scholar] [CrossRef]
- Park, J.H.; Oh, K.W.; Choi, H.-M. Preparation and Characterization of Cotton Fabrics with Antibacterial Properties Treated by Crosslinkable Benzophenone Derivative in Choline Chloride-Based Deep Eutectic Solvents. Cellulose 2013, 20, 2101–2114. [Google Scholar] [CrossRef]
- Wang, W.; Kong, Y.; Peng, J.; Li, B.; Xu, H. Probing the Mechanism of Green Solvent Solubilization of Hemicellulose Based on Molecular Dynamics Simulations. Ind. Crops Prod. 2022, 186, 115159. [Google Scholar] [CrossRef]
- Smink, D.; Juan, A.; Schuur, B.; Kersten, S.R.A. Understanding the Role of Choline Chloride in Deep Eutectic Solvents Used for Biomass Delignification. Ind. Eng. Chem. Res. 2019, 58, 16348–16357. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.-J.; Xue, Z.; Sun, Z.; Yuan, T.-Q. Structure–Function Relationships of Deep Eutectic Solvents for Lignin Extraction and Chemical Transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]
- Sai, Y.W.; Lee, K.M. Enhanced Cellulase Accessibility Using Acid-Based Deep Eutectic Solvent in Pretreatment of Empty Fruit Bunches. Cellulose 2019, 26, 9517–9528. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Hong, S.; Wen, J.; Ma, C.-Y.; Tang, L.; Jiang, H.; Chen, J.-J.; Li, S.; Shen, X.-J.; Yuan, T.-Q. Lewis Acid-Facilitated Deep Eutectic Solvent (DES) Pretreatment for Producing High-Purity and Antioxidative Lignin. ACS Sustain. Chem. Eng. 2020, 8, 1050–1057. [Google Scholar] [CrossRef]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural Deep Eutectic Solvents (DES) for Fractionation of Waste Lignocellulosic Biomass and Its Cascade Conversion to Value-Added Bio-Based Chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Lee, K.M.; Quek, J.D.; Tey, W.Y.; Lim, S.; Kang, H.-S.; Quen, L.K.; Mahmood, W.A.W.; Jamaludin, S.I.S.; Teng, K.H.; Khoo, K.S. Biomass Valorization by Integrating Ultrasonication and Deep Eutectic Solvents: Delignification, Cellulose Digestibility and Solvent Reuse. Biochem. Eng. J. 2022, 187, 108587. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents for Pretreatment, Extraction, and Catalysis of Biomass and Food Waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Zhang, P.P.; Tong, D.S.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Yu, W.H.; Wang, H.; Zhou, C.H. Effects of Acid Treatments on Bamboo Cellulose Nanocrystals: Effects of Acid Treatments on Bamboo Cellulose Nanocrystals. Asia-Pac. J. Chem. Eng. 2014, 9, 686–695. [Google Scholar] [CrossRef]
- Chi, X.; Liu, C.; Bi, Y.-H.; Yu, G.; Zhang, Y.; Wang, Z.; Li, B.; Cui, Q. A Clean and Effective Potassium Hydroxide Pretreatment of Corncob Residue for the Enhancement of Enzymatic Hydrolysis at High Solids Loading. RSC Adv. 2019, 9, 11558–11566. [Google Scholar] [CrossRef]
- Piqueras, S.; Füchtner, S.; Rocha de Oliveira, R.; Gómez-Sánchez, A.; Jelavić, S.; Keplinger, T.; de Juan, A.; Thygesen, L.G. Understanding the Formation of Heartwood in Larch Using Synchrotron Infrared Imaging Combined with Multivariate Analysis and Atomic Force Microscope Infrared Spectroscopy. Front. Plant Sci. 2020, 10, 1701. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, G.; Deng, J.; Dong, M.; Murugadoss, V.; Liu, C.; Shao, Q.; Wu, S.; Guo, Z. Structural Characterization of Lignin from D. sinicus by FTIR and NMR Techniques. Green Chem. Lett. Rev. 2019, 12, 235–243. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Ray, P.; Gogate, P.R. Intensification of Delignification and Subsequent Hydrolysis for the Fermentable Sugar Production from Lignocellulosic Biomass Using Ultrasonic Irradiation. Ultrason. Sonochem. 2018, 40, 140–150. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Application of Deep Eutectic Solvents in Biomass Pretreatment and Conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar] [CrossRef]
- Tian, D.; Chandra, R.P.; Lee, J.-S.; Lu, C.; Saddler, J.N. A Comparison of Various Lignin-Extraction Methods to Enhance the Accessibility and Ease of Enzymatic Hydrolysis of the Cellulosic Component of Steam-Pretreated Poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef]
- Fu, D.; Mazza, G. Aqueous Ionic Liquid Pretreatment of Straw. Bioresour. Technol. 2011, 102, 7008–7011. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.A.; Orr, V.C.A.; Luque, L.; Nagendra, V.; Berruti, F.; Rehmann, L. High-Throughput Screening of Inhibitory Compounds on Growth and Ethanol Production of Saccharomyces Cerevisiae. BioEnergy Res. 2015, 8, 423–430. [Google Scholar] [CrossRef]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D.S. Dissolution of Wood in Ionic Liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodríguez, H.; Rogers, R.D. Complete Dissolution and Partial Delignification of Wood in the Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate. Green Chem. 2009, 11, 646. [Google Scholar] [CrossRef]
- Kogje, A.B.; Ghosalkar, A. Xylitol Production by Genetically Modified Industrial Strain of Saccharomyces cerevisiae Using Glycerol as Co-Substrate. J. Ind. Microbiol. Biotechnol. 2017, 44, 961–971. [Google Scholar] [CrossRef]
- Ariyan, M.; Uthandi, S. Xylitol Production by Xylose Reductase over Producing Recombinant Escherichia coli M15. Madras Agric. J. 2019, 106, 205–209. [Google Scholar] [CrossRef]
- Bura, R.; Vajzovic, A.; Doty, S.L. Novel Endophytic Yeast Rhodotorula mucilaginosa Strain PTD3 I: Production of Xylitol and Ethanol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Qi, K.; Chen, X.; Miao, X.; Zhong, J.-J. Favorable Effect of Very Low Initial KLa Value on Xylitol Production from Xylose by a Self-Isolated Strain of Pichia guilliermondii. J. Biosci. Bioeng. 2010, 109, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Yewale, T.; Panchwagh, S.; Rajagopalan, S.; Dhamole, P.B.; Jain, R. Enhanced Xylitol Production Using Immobilized Candida tropicalis with Non-Detoxified Corn Cob Hemicellulosic Hydrolysate. 3 Biotech 2016, 6, 75. [Google Scholar] [CrossRef]
- Fillet, S.; Ronchel, C.; Callejo, C.; Fajardo, M.-J.; Moralejo, H.; Adrio, J.L. Engineering Rhodosporidium toruloides for the Production of Very Long-Chain Monounsaturated Fatty Acid-Rich Oils. Appl. Microbiol. Biotechnol. 2017, 101, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.S.; Rao, C.V. Production of D-Arabitol from d-Xylose by the Oleaginous Yeast Rhodosporidium toruloides IFO0880. Appl. Microbiol. Biotechnol. 2018, 102, 143–151. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Kim, S.R.; Cate, J.H.D.; Jin, Y.-S. Enhanced Biofuel Production through Coupled Acetic Acid and Xylose Consumption by Engineered Yeast. Nat. Commun. 2013, 4, 2580. [Google Scholar] [CrossRef]


| Components | Parameters | Value |
|---|---|---|
| Substrate - WH | Moisture content (%, w/w) a | 94.20 ± 0.75 |
| Total solids (%, w/w) a | 5.80 ± 0.75 | |
| Ash content (%, w/w) b | 21.50 ± 0.20 | |
| Volatile solids (%, w/w) b | 78.44 ± 0.20 | |
| Cellulose (%, w/w) b | 26.90 ± 1.76 | |
| Hemicellulose (%, w/w) b | 24.50 ±1.71 | |
| Lignin (%, w/w) b | 5.68 ± 0.24 | |
| DES (Choline chloride: urea) | Density (g/mL) | 1.116 ± 0.02 |
| Solubility | Miscible Acetic acid, Ethanol, Water Immiscible Toluene, Chloroform | |
| Enzyme activity(Aspergillus niger) | Carboxy methyl cellulase (CMC-ase) (IU/mL) | 6.23 |
| Filter paper units (FPU/mL) | 0.77 | |
| Xylanase (IU/mL) | 2.21 |
| Run Order | Biomass:DES Ratio | Temperature (°C) | Time (h) | Delignification% * |
|---|---|---|---|---|
| 1 | 15 | 90 | 5 | 58.57 ± 2.63 |
| 2 | 10 | 85 | 5 | 45.92 ± 2.06 |
| 3 | 10 | 80 | 4 | 60.33 ± 2.77 |
| 4 | 15 | 80 | 3 | 64.33 ± 3.85 |
| 5 | 10 | 90 | 4 | 69.22 ± 3.11 |
| 6 | 20 | 85 | 3 | 52.33 ± 2.35 |
| 7 | 15 | 85 | 4 | 50.25 ± 2.26 |
| 8 | 15 | 90 | 3 | 65.37 ± 1.94 |
| 9 | 15 | 85 | 4 | 50.36 ± 2.11 |
| 10 | 10 | 85 | 3 | 47.67 ± 1.90 |
| 11 | 20 | 85 | 5 | 51.33 ± 2.56 |
| 12 | 20 | 80 | 4 | 50.37 ± 1.91 |
| 13 | 15 | 80 | 5 | 46.33 ± 1.38 |
| 14 | 15 | 85 | 4 | 49.62 ± 2.48 |
| 15 | 15 | 85 | 4 | 50.88 ± 3.05 |
| 16 | 20 | 90 | 4 | 56.77 ± 2.27 |
| 17 | 15 | 85 | 4 | 50.37 ± 2.01 |
| Source | Sum of Squares | Degree of freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 791.39 | 11 | 71.94 | 273.61 | <0.0001 | significant |
| A—Biomass:DES | 25.35 | 1 | 25.35 | 96.41 | 0.0002 | |
| B—Temperature | 102.03 | 1 | 102.03 | 388.03 | <0.0001 | |
| C—Time | 153.76 | 1 | 153.76 | 584.76 | <0.0001 | |
| AB | 1.55 | 1 | 1.55 | 5.89 | 0.05 | |
| BC | 31.36 | 1 | 31.36 | 119.26 | 0.0001 | |
| A2 | 0.2237 | 1 | 0.2237 | 0.8508 | 0.3987 | |
| B2 | 349.21 | 1 | 349.21 | 1328.07 | <0.0001 | |
| A2C | 60.78 | 1 | 60.78 | 231.13 | <0.0001 | |
| AB2 | 131.87 | 1 | 131.87 | 501.50 | <0.0001 | |
| Residual | 1.31 | 5 | 0.2629 | |||
| Lack of Fit | 0.5050 | 1 | 0.5050 | 2.49 | 0.1894 | not significant |
| Pure Error | 0.8097 | 4 | 0.2024 | |||
| Cor Total | 792.71 | 16 |
| Parameters | Fermentation | |||
|---|---|---|---|---|
| Hemicellulosic Hydrolysate, Xylose-Rich Fraction | Cellulosic Hydrolysate, Glucose-Rich Fraction | Hemicellulosic Hydrolysate, Xylose-Rich Fraction in PBR | ||
| Cycle 1 | Cycle 2 | |||
| Maximum biomass yield (Xm), g/L (Based on Luedeking–Piret kinetic model) | 7.06 | 10.98 | - | - |
| Maximum specific growth rate (h−1) (Based on Luedeking –Piret kinetic model) | 0.075 | 0.20 | - | - |
| Yield coefficient for biomass (YX/S) (g/g) | 0.311 | 0.45 | - | - |
| Yield coefficient for product (YP/S) (g/g) | 0.57 (xylitol) | 0.21 (lipid) | 1.50 (xylitol) | 1.80 (xylitol) |
| Rate of substrate consumption (qs) g/L.h (Based on first-order kinetic model) | 0.012 | 0.014 | Xylose–0.84 Glucose–0.35 | Xylose–0.95 Glucose–0.33 |
| Rate of product formation (qp) g/L.h | 0.02 | 0.005 | 1.31 | 1.29 |
| Substrate uptake efficiency | 79.22% (xylose) 88.42% (glucose) | 86.42% (glucose) 66.78% (xylose) | 71.56% (xylose) 87.31% (glucose) | 82.92% (xylose) 80.98% (glucose) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umai, R.D.; Jacob, S.; Kumar, V. Deep Eutectic Solvent Pretreatment of Water Hyacinth for Improved Holocellulosic Saccharification and Fermentative Co-Production of Xylitol and Lipids Using Rhodosporidium toruloides NCIM 3547. Fermentation 2022, 8, 591. https://doi.org/10.3390/fermentation8110591
Umai RD, Jacob S, Kumar V. Deep Eutectic Solvent Pretreatment of Water Hyacinth for Improved Holocellulosic Saccharification and Fermentative Co-Production of Xylitol and Lipids Using Rhodosporidium toruloides NCIM 3547. Fermentation. 2022; 8(11):591. https://doi.org/10.3390/fermentation8110591
Chicago/Turabian StyleUmai, Ramachandran Devasena, Samuel Jacob, and Vinod Kumar. 2022. "Deep Eutectic Solvent Pretreatment of Water Hyacinth for Improved Holocellulosic Saccharification and Fermentative Co-Production of Xylitol and Lipids Using Rhodosporidium toruloides NCIM 3547" Fermentation 8, no. 11: 591. https://doi.org/10.3390/fermentation8110591
APA StyleUmai, R. D., Jacob, S., & Kumar, V. (2022). Deep Eutectic Solvent Pretreatment of Water Hyacinth for Improved Holocellulosic Saccharification and Fermentative Co-Production of Xylitol and Lipids Using Rhodosporidium toruloides NCIM 3547. Fermentation, 8(11), 591. https://doi.org/10.3390/fermentation8110591