Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Inoculant
2.2. Incubation Substrates
2.3. Experimental Design and In Vitro Incubation
2.4. Sample Collection, Measurement and Calculation
2.5. DNA Extraction and Determination
2.6. Statistical Methods
3. Results
3.1. pH, Volatile Fatty Acid and NH3-N Concentration
3.2. Gas Production and Fermentation Kinetics Parameter
3.3. CH4 and CO2 Composition
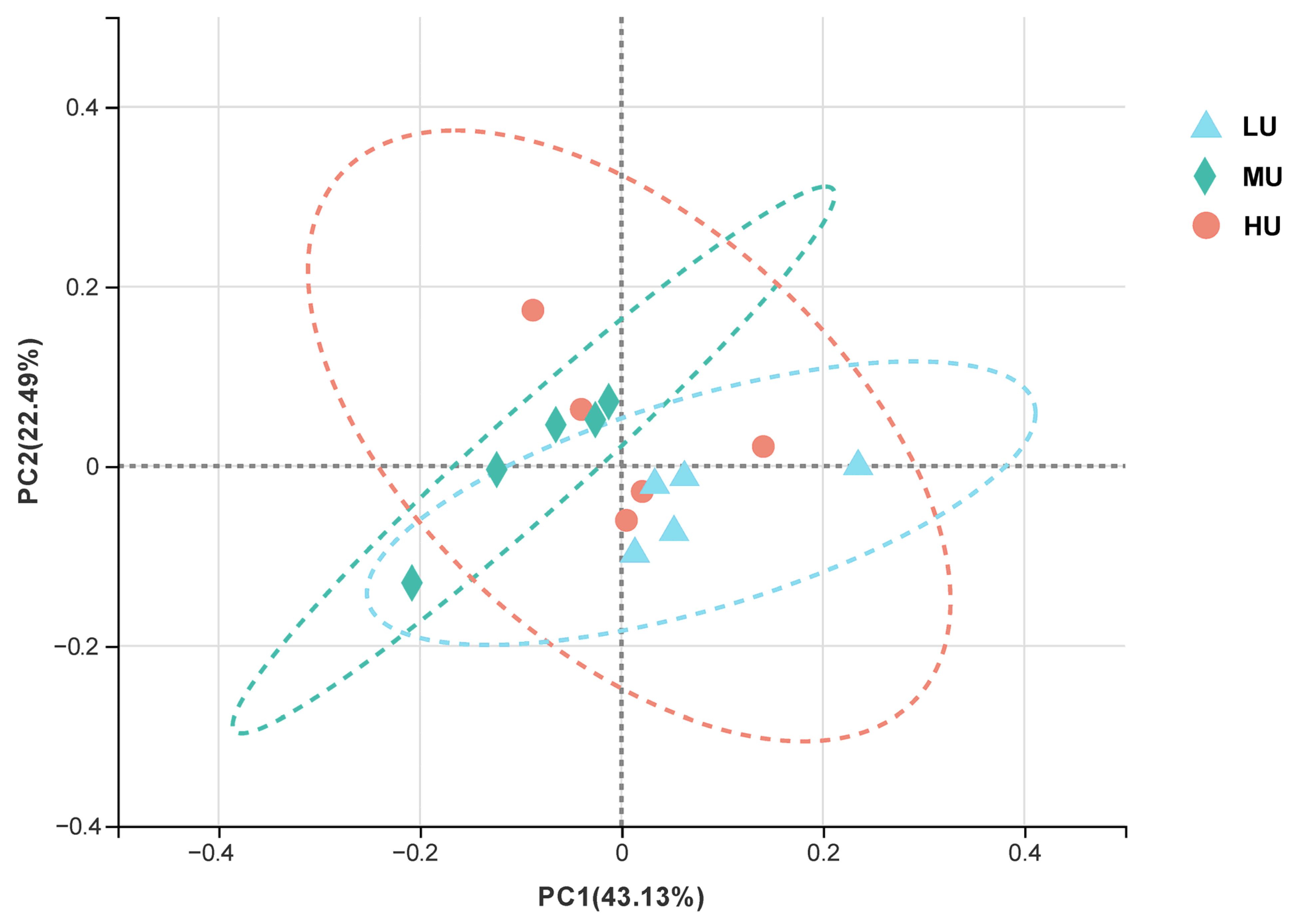
3.4. Microbiota Diversity
3.5. Microbiota Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savoini, G.; Omodei Zorini, F.; Farina, G.; Agazzi, A.; Cattaneo, D.; Invernizzi, G. Effects of Fat Supplementation in Dairy Goats on Lipid Metabolism and Health Status. Animals 2019, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotechnol. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Calder, P.C. N-3 polyunsaturated fatty acids and allergic disease. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 123–129. [Google Scholar] [CrossRef]
- Moallem, U. Invited review: Roles of dietary n-3 fatty acids in performance, milk fat composition, and reproductive and immune systems in dairy cattle. J. Dairy Sci. 2018, 101, 8641–8661. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Cheema, S.; Field, C.J.; Garg, M.L.; Venkatraman, J.; Clandinin, T.R. Dietary fat: Exogenous determination of membrane structure and cell function. FASEB J. 1991, 5, 2761–2769. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Rabiee, A.R.; Breinhild, K.; Scott, W.; Golder, H.M.; Block, E.; Lean, I.J. Effect of fat additions to diets of dairy cattle on milk production and components: A meta-analysis and meta-regression. J. Dairy Sci. 2012, 95, 3225–3247. [Google Scholar] [CrossRef]
- Brask, M.; Lund, P.; Weisbjerg, M.R.; Hellwing, A.L.F.; Poulsen, M.; Larsen, M.K.; Hvelplund, T. Methane production and digestion of different physical forms of rapeseed as fat supplements in dairy cows. J. Dairy Sci. 2013, 96, 2356–2365. [Google Scholar] [CrossRef]
- Sun, X.G.; Wang, Y.; Xie, T.; Yang, Z.T.; Wang, J.D.; Zheng, Y.H.; Guo, C.; Zhang, Y.; Wang, Q.Q.; Wang, Z.H.; et al. Effects of High-Forage Diets Containing Raw Flaxseeds or Soybean on In Vitro Ruminal Fermentation, Gas Emission, and Microbial Profile. Microorganisms 2021, 9, 2304. [Google Scholar] [CrossRef]
- Giger-Reverdin, S.; Morand-Fehr, P.; Tran, G. Literature survey of the influence of dietary fat composition on methane production in dairy cattle. Livest. Prod. Sci. 2003, 82, 73–79. [Google Scholar] [CrossRef]
- Grainger, C.; Beauchemin, K.A. Can enteric methane emissions from ruminants be lowered without lowering their production? Anim. Feed Sci. Technol. 2011, 166–167, 308–320. [Google Scholar] [CrossRef]
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- NRC. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2012; pp. 1048–1049, 1995. [Google Scholar]
- Loor, J.J.; Herbein, J.H. Alterations in blood plasma and milk fatty acid profiles of lactating Holstein cows in response to ruminal infusion of a conjugated linoleic acid mixture. Anim. Res. 2001, 50, 463–476. [Google Scholar] [CrossRef]
- Zhang, D.F.; Yang, H.J. In vitro ruminal methanogenesis of a hay-rich substrate in response to different combination supplements of nitrocompounds; pyromellitic diimide and 2-bromoethanesulphonate. Anim. Feed Sci. Technol. 2011, 163, 20–32. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Q.; Yang, Z.; Xie, T.; Wang, Z.; Li, S.; Wang, W. Altering Methane Emission, Fatty Acid Composition, and Microbial Profile during In Vitro Ruminant Fermentation by Manipulating Dietary Fatty Acid Ratios. Fermentation 2022, 8, 310. [Google Scholar] [CrossRef]
- Cui, J.H.; Yang, H.J.; Yu, C.Q.; Bai, S.; Wu, T.T.; Song, S.S.; Sun, W.; Shao, X.M.; Jiang, L.S. Effect of urea fertilization on biomass yield, chemical composition, in vitro rumen digestibility and fermentation characteristics of straw of highland barley planted in Tibet. J. Agric. Sci. 2016, 154, 151–164. [Google Scholar] [CrossRef]
- Verdouw, H.; Vanechteld, C.J.A.; Dekkers, E.M.J. Ammonia Determination Based on Indophenol Formation with Sodium Salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Yang, H.J.; Tamminga, S.; Williams, B.A.; Dijkstra, J.; Boer, H. In vitro gas and volatile fatty acids production profiles of barley and maize and their soluble and washout fractions after feed processing. Anim. Feed Sci. Technol. 2005, 120, 125–140. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Kong, F.; Liu, Y.; Wang, S.; Zhang, Y.; Wang, W.; Yang, H.; Lu, N.; Li, S. Nutrient Digestibility, Microbial Fermentation, and Response in Bacterial Composition to Methionine Dipeptide: An In Vitro Study. Biology 2022, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. A Place for DNA-DNA Reassociation and 16s Ribosomal-RNA Sequence-Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Wanapat, M.; Mapato, C.; Pilajun, R.; Toburan, W. Effects of vegetable oil supplementation on feed intake, rumen fermentation, growth performance, and carcass characteristic of growing swamp buffaloes. Livest. Sci. 2011, 135, 32–37. [Google Scholar] [CrossRef]
- Martínez Marín, A.L.; Gómez-Cortés, P.; Gómez Castro, A.G.; Juárez, M.; Pérez Alba, L.; Pérez Hernández, M.; de la Fuente, M.A. Short communication: Linear discriminant analysis and type of oil added to dairy goat diets. J. Dairy Sci. 2012, 95, 4045–4049. [Google Scholar] [CrossRef] [PubMed]
- Jalc, D.; Potkanski, A.; Szumacher-Strabel, M.; Kowalczyk, J.; Cieslak, A. The effect of a high forage diet and different oil blends on rumen fermentation in vitro. J. Anim. Feed Sci. 2006, 15, 141–144. [Google Scholar] [CrossRef]
- Adeyemi, K.D.; Sazili, A.Q.; Ebrahimi, M.; Samsudin, A.A.; Alimon, A.R.; Karim, R.; Karsani, S.A.; Sabow, A.B. Effects of blend of canola oil and palm oil on nutrient intake and digestibility, growth performance, rumen fermentation and fatty acids in goats. Anim. Sci. J. 2016, 87, 1137–1147. [Google Scholar] [CrossRef]
- Kadkhoday, A.; Riasi, A.; Alikhani, M.; Dehghan-Banadaky, M.; Kowsar, R. Effects of fat sources and dietary C-18:2 to C-18:3 fatty acids ratio on growth performance, ruminal fermentation and some blood components of Holstein calves. Livest. Sci. 2017, 204, 71–77. [Google Scholar] [CrossRef]
- Ueda, K.; Ferlay, A.; Chabrot, J.; Loor, J.J.; Chilliard, Y.; Doreau, M. Effect of linseed oil supplementation on ruminal digestion in dairy cows fed diets with different forage:concentrate ratios. J. Dairy Sci. 2003, 86, 3999–4007. [Google Scholar] [CrossRef]
- McCabe, M.S.; Cormican, P.; Keogh, K.; O’Connor, A.; O’Hara, E.; Palladino, R.A.; Kenny, D.A.; Waters, S.M. Illumina MiSeq Phylogenetic Amplicon Sequencing Shows a Large Reduction of an Uncharacterised Succinivibrionaceae and an Increase of the Methanobrevibacter gottschalkii Clade in Feed Restricted Cattle. PLoS ONE 2015, 10, e0133234. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.H.; Hungate, R.E. Succinic acid turnover and propionate production in the bovine rumen. Appl. Microbiol. 1963, 11, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.P.; McCormick, C.A.; Suen, G. Fibrobacter communities in the gastrointestinal tracts of diverse hindgut-fermenting herbivores are distinct from those of the rumen. Environ. Microbiol. 2017, 19, 3768–3783. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, S.; Zhao, Y.; Li, S. Effect of Cassava Residue Substituting for Crushed Maize on In Vitro Ruminal Fermentation Characteristics of Dairy Cows at Mid-Lactation. Animals 2020, 10, 893. [Google Scholar] [CrossRef]
- Pi, Y.; Ma, L.; Pierce, K.M.; Wang, H.R.; Xu, J.C.; Bu, D.P. Rubber seed oil and flaxseed oil supplementation alter digestion, ruminal fermentation and rumen fatty acid profile of dairy cows. Animal 2019, 13, 2811–2820. [Google Scholar] [CrossRef]
- Scollan, N.D.; Dhanoa, M.S.; Choi, N.J.; Maeng, W.J.; Enser, M.; Wood, J.D. Biohydrogenation and digestion of long chain fatty acids in steers fed on different sources of lipid. J. Agric. Sci. 2001, 136, 345–355. [Google Scholar] [CrossRef]
- Broudiscou, L.; Pochet, S.; Poncet, C. Effect of Linseed Oil Supplementation on Feed Degradation and Microbial Synthesis in the Rumen of Ciliate-Free and Refaunated Sheep. Anim. Feed Sci. Technol. 1994, 49, 189–202. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, M.; Yu, Q.; Ma, Z.Y.; Beauchemin, K.A.; Wang, R.; Wen, J.N.; Lukuyu, B.A.; Tan, Z.L. Liquid hot water treatment of rice straw enhances anaerobic degradation and inhibits methane production during in vitro ruminal fermentation. J. Dairy Sci. 2020, 103, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Rouel, J.; Jouany, J.P.; Doreau, M.; Chilliard, Y. Methane output and diet digestibility in response to feeding dairy cows crude linseed, extruded linseed, or linseed oil. J. Anim. Sci. 2008, 86, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Ferlay, A.; Mosoni, P.; Rochette, Y.; Chilliard, Y.; Doreau, M. Increasing linseed supply in dairy cow diets based on hay or corn silage: Effect on enteric methane emission, rumen microbial fermentation, and digestion. J. Dairy Sci. 2016, 99, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Schoenhals, K.E.; Brady, P.A.; Estill, C.T.; Perumbakkam, S.; Craig, A.M. Flaxseed supplementation decreases methanogenic gene abundance in the rumen of dairy cows. Animal 2012, 6, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tominaga, K.; Aoki, H.; Murayama, M.; Oishi, K.; Hirooka, H.; Yoshida, T.; Kumagai, H. Calcium salts of long-chain fatty acids from linseed oil decrease methane production by altering the rumen microbiome in vitro. PLoS ONE 2020, 15, e0242158. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.K. The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: A meta-analysis. Livest. Sci. 2013, 155, 244–254. [Google Scholar] [CrossRef]
- Kong, F.; Lu, N.; Liu, Y.; Zhang, S.; Jiang, H.; Wang, H.; Wang, W.; Li, S. Aspergillus oryzae and Aspergillus niger Co-Cultivation Extract Affects In Vitro Degradation, Fermentation Characteristics, and Bacterial Composition in a Diet-Specific Manner. Animals 2021, 11, 1248. [Google Scholar] [CrossRef]




| Items 1 | Contents |
|---|---|
| Ingredients | |
| Corn silage | 30.58 |
| Alfalfa hay | 11.75 |
| Steam-flaked corn | 15.87 |
| Corn flour | 11.26 |
| Soybean meal | 13.51 |
| Soybean hulls | 4.64 |
| Whole cottonseeds | 1.95 |
| Corn gluten meal | 2.78 |
| Fat powder | 1.47 |
| Yeast culture | 0.11 |
| Sodium bicarbonate | 0.64 |
| Premix 2 | 2.75 |
| Molasses | 2.68 |
| Total | 100 |
| Nutrient levels | |
| NEL (MJ/kg) | 7.45 |
| CP, % | 16.98 |
| NDF, % | 22.86 |
| ADF, % | 13.47 |
| EE, % | 4.22 |
| Ingredients | LU | MU | HU |
|---|---|---|---|
| C6:0 | 0.02 | 0.02 | 0.02 |
| C8:0 | 0.02 | 0.02 | 0.02 |
| C10:0 | 0.04 | 0.03 | 0.01 |
| C12:0 | 0.42 | 0.33 | 0.25 |
| C13:0 | 0.14 | 0.14 | 0.13 |
| C14:0 | 0.81 | 0.68 | 0.56 |
| C15:0 | 0.11 | 0.10 | 0.08 |
| C16:0 | 50.88 | 38.02 | 25.27 |
| C16:1 | 0.22 | 0.21 | 0.20 |
| C17:0 | 0.13 | 0.12 | 0.11 |
| C18:0 | 3.65 | 2.70 | 1.75 |
| C18:1n9c | 12.71 | 12.50 | 12.30 |
| C18:2n6c | 26.15 | 25.99 | 25.83 |
| C18:3n3 | 3.23 | 17.71 | 32.05 |
| C20:0 | 0.30 | 0.29 | 0.28 |
| C20:1 | 0.13 | 0.13 | 0.13 |
| C21:0 | 0.05 | 0.05 | 0.05 |
| C20:2 | 0.03 | 0.03 | 0.03 |
| C22:0 | 0.25 | 0.25 | 0.25 |
| C22:1n9 | 0.08 | 0.08 | 0.08 |
| C22:2 | 0.02 | 0.02 | 0.02 |
| C23:0 | 0.08 | 0.08 | 0.08 |
| C24:0 | 0.29 | 0.29 | 0.28 |
| C24:1 | 0.22 | 0.22 | 0.22 |
| ΣUFA 1 | 42.80 | 56.90 | 70.9 |
| Items 1 | Treatment 2 | SEM 3 | p Value | ||
|---|---|---|---|---|---|
| LU | MU | HU | |||
| A, mL/g DM | 67.89 | 71.29 | 75.89 | 2.155 | 0.35 |
| B | 1.18 | 1.17 | 1.26 | 0.037 | 0.56 |
| C, h | 2.82 | 2.78 | 2.90 | 0.120 | 0.92 |
| GP48, mL/g DM | 65.45 | 68.68 | 73.42 | 1.968 | 0.28 |
| RmaxG, mL/h | 17.73 | 18.45 | 18.19 | 0.813 | 0.95 |
| RmaxS, mL/h | 0.29 | 0.29 | 0.28 | 0.010 | 0.84 |
| TRmaxG, h | 0.35 | 0.34 | 0.37 | 0.059 | 0.99 |
| TRmaxS, h | 0.67 | 0.66 | 0.70 | 0.110 | 0.99 |
| Item | Treatment 1 | Time | SEM 2 | p-Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LU | MU | HU | 0.5 | 1 | 2 | 3 | 6 | Treat | Time | INT | ||
| CH4, % | 0.41 a | 0.38 ab | 0.37 b | 0.15 e | 0.21 d | 0.28 c | 0.52 b | 0.79 a | 0.030 | 0.02 | <0.01 | 0.31 |
| CO2, % | 23.88 a | 22.09 b | 21.19 b | 14.61 e | 16.65 d | 19.07 c | 27.16 b | 34.44 a | 0.961 | <0.01 | <0.01 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Liu, S.; Xie, T.; Wang, Q.; Wang, Z.; Yang, H.; Li, S.; Wang, W. Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile. Fermentation 2022, 8, 540. https://doi.org/10.3390/fermentation8100540
Yang Z, Liu S, Xie T, Wang Q, Wang Z, Yang H, Li S, Wang W. Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile. Fermentation. 2022; 8(10):540. https://doi.org/10.3390/fermentation8100540
Chicago/Turabian StyleYang, Zhantao, Siyuan Liu, Tian Xie, Qianqian Wang, Zhonghan Wang, Hongjian Yang, Shengli Li, and Wei Wang. 2022. "Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile" Fermentation 8, no. 10: 540. https://doi.org/10.3390/fermentation8100540
APA StyleYang, Z., Liu, S., Xie, T., Wang, Q., Wang, Z., Yang, H., Li, S., & Wang, W. (2022). Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile. Fermentation, 8(10), 540. https://doi.org/10.3390/fermentation8100540




