Abstract
Hispidin (6-(3,4-dihydroxystyrl)-4-hydroxy-2-pyrone) production in submerged cultured mycelia of the basidiomycete Inonotus hispidus was doubled in shake flasks through irradiation with white light. The daily addition of 1 mM hydrogen peroxide as a chemical stressor and a repeated supplementation of the shake flask cultures with 2 mM caffeic acid, a biogenetic precursor, further increased the hispidin synthesis. These cultivation conditions were combined and applied to parallel fermentation trials on the 4 L scale using a classical stirred tank bioreactor and a wave bag bioreactor. No significant differences in biomass yield and colorant production were observed. The hispidin concentration in both bioreactors reached 5.5 g·L−1, the highest ever published. Textile dyeing with hispidin was successful, but impeded by its limited light stability in comparison to industrial dyes. However, following the idea of sustainability and the flawless toxicity profile, applications in natural cosmetics, other daily implements, or even therapeutics appear promising.
1. Introduction
Inonotus hispidus, a basidiomycete from the class of Agaricomycetes, is popular in Asian folk medicine and supposed to cure many kinds of illnesses. Modern pharmacological studies have confirmed various bioactivities, which were frequently attributed to the occurrence of the yellow pigment hispidin [1,2,3,4,5]. While it is dominant in the metabolite spectrum, a number of other compounds seem to contribute to the observed antimicrobial, antiviral, antioxidant, anti-inflammatory, immunomodulatory, antiproliferative, and cytotoxic activities of extracts from this species [6,7,8]. Keto–enol tautomerism may result in an equilibrium between the lactone and the 4-pyrone form (isohispidin), a reaction giving rise to the formation of further, possibly bioactive oligomer compounds [6]. Much less work has been devoted to the use of hispidin as a natural colorant, with few sources reporting a potential application for dyeing [9]. The fruiting bodies of I. hispidus were recently used for textile dyeing, thus confirming earlier findings [10].
Natural dyes have been used since ancient times for the coloring of art and everyday objects of all kinds. The commencing industrialization in the 19th century changed the focus to the new synthetic dyes with an enormous potential for different hues combined with affordable prices. Currently, toxicity and environmental issues caused by synthetic dye production and the dyeing process, as well as a newfound appreciation for natural and sustainable products by consumers, have revived the search for natural dyes. Colorants extracted from plants or fruiting bodies of mushrooms provide alternatives, but suffer from numerous imponderabilities and can, with few exceptions, only be gained once per season [11,12]. Fruiting bodies from higher fungi (Basidiomycota) display a full color spectrum, but most fungal cells stop to produce the colorants when transferred to submerged cultivation, where they form cell clusters.
As hispidin is synthesized in both the fruit body and the mycelium, cultures of I. hispidus were chosen as a prototype to study its production more closely [7,13]. Hispidin is partly derived from acetate via the polyketide pathway [14,15] and, as I. hispidus decays wood, from lignin degradation products [2]. Perrin et al. suggested already 50 years ago that hispidin was synthesized from cinnamic, p-coumaric, or caffeic acid [16]. In the closely related Inonotus obliquus, the addition of hydrogen peroxide led to a higher yield of hispidin derivates [17]. It was further found that hispidin synthesis was light-dependent, with blue light and a 30 min light period per day stimulating biosynthesis best [16,18,19]. Various cell culture models convincingly demonstrated the protective effects of hispidin, acting as a scavenger of reactive oxygen species (ROS) [20,21,22].
The purpose of this study was to fuse the findings detailed above to set up a bioprocess producing high yields of hispidin using I. hispidus. A comparative, parallel fermentation in a stirred tank and wave bag reactor was explored, as our previous work on the shear stress sensitive basidiomycetes showed that cultivation in a wave bag reactor resulted in highly dispersed mycelia, followed by higher biomass production and a doubled formation of peptidases [23]. The established bioprocess would present a sustainable cell factory to be run anywhere, anytime, and, in contrast to solid-state fermentation, on any scale without using arable land or jeopardizing endangered wild specimens.
2. Materials and Methods
2.1. Cultivation Parameters and Media
Inonotus hispidus (DSMZ 8658, verified by ITS sequencing after [24]) was incubated for 10 days at 24 °C on standard nutrient liquid agar plates (SNL; 30 g of d-glucose monohydrate, 4.5 g of l-asparagine monohydrate, 3 g of yeast extract, 1.5 g of KH2PO4, 0.5 g of MgSO4, 5 μg of CuSO4∙5H2O, 80 μg of FeCl3∙6H2O, 30 μg of MnSO4∙H2O, 90 μg of ZnSO4∙7 H2O, 400 μg of EDTA, and 20 g of agar per liter, pH 6.0). All chemicals were purchased in pro analysis grade (Sigma Aldrich, Seelze, Germany; Merck, Darmstadt, Germany; Carl Roth, Karlsruhe, Germany), if not stated otherwise. Then, 1 cm3 of the overgrown agar plate was transferred into 150 mL of Moser b medium (30 g of d-glucose monohydrate, 10 g of malt extract, 2 g of peptone, 0.15 g of K2HPO4, 0.35 g of KH2PO4, 1 g of NH4NO3, 0.3 g of NaNO3, 0.5 g of MgSO4∙7H2O, 0.1 g of CaCl2∙2 H2O, 1 mg of biotin, 50 mg of myo-inositol, 1.8 mg of ZnSO4∙7 H2O, 10 mg of FeCl3, 5.6 mg of MnSO4∙H2O, and 50 mg of thiamine hydrochloride per liter) and homogenized (MiniBatch D-9 with DS-20/PF-SMIR, Miccra GmbH, Heiterheim, Germany) at 11,000 min−1 for 5 s. The preparatory culture was incubated on an orbital shaker (Multitron, Infors AG, Bottmingen, Switzerland) for 10 days at 24 °C and 150 rpm and used subsequently to inoculate the main cultures (10% v/v, 250 mL culture volume in total) after homogenization. All fermentations were carried out in triplicates. Bioreactors were sampled in triplicate.
2.2. Investigation of Different Influences on Pigment Yield
To test the influence of different light conditions, the window of an orbital shaker was obscured and equipped with LED stripes (1 m long, 60 LEDs, 4 W·m−1, PUR-LED GmbH & Co. KG), emitting light of different wavelengths. Wavelengths given by the manufacturer (400 nm, 470 nm, white light/full visible spectrum) were verified with a spectrometer (OceanOptics HR4000, wavelength range 200–1100 nm, resolution ~240 pm). The LED stripe labeled as “400 nm” exhibited a sharp intensity maximum measured at 394 nm, that labeled as “470 nm” exhibited a maximum at 464 nm, and “white light” exhibited a broad range from 420 to 740 nm with a maximum at around 590 nm. The agar plate, pre-culture, and main culture were kept in the dark. The main cultures were irradiated every day for 30 min, as described by Nambudiri et al. [13,18], for 6 days a week. The control culture was cultivated in an orbital shaker with windows, as were the cultures for the determination of the influence of hydrogen peroxide and the precursors.
Hydrogen peroxide was added from a 30% stock bottle to the cultures as described by Kavitha and Chandra [25]. Briefly, 10 or 25 mM final concentration was added once on day 4 of the cultivation. Alternatively, 1 mM hydrogen peroxide was added on days 4, 5, 7, 8, 9, 10, 11, 13, and 14 of the cultivation (a total of 252 μL 30% H2O2). For supplementation of precursors, l-tyrosine, l-phenylalanine, cinnamic acid, p-coumaric acid, and caffeic acid were dissolved in 1 mL of 70% ethanol and added to the cultures on days 4, 7, 9, and 11 to give a final concentration of 2 mM. For all shake flask experiments, 10 mL samples were taken on days 1, 5, 10, and 15.
2.3. Fermentation Experiments
For parallel fermentation experiments, I. hispidus was cultivated in a 6 L stirred tank bioreactor (STR) (Minifors 2, Infors AG, CH) and a 10 L single-use cultivation bag (ReadyToProcess Wave 25, Cytiva, Marlborough, MA, USA; utilized without lid) simultaneously, both filled with 4 L of Moser b medium and inoculated with 5% (v/v) starter culture (see Scheme S1). For both systems, cultivation temperature was 24 °C with a constant air flow of 0.5 L·min−1 and no specific light regulation. The agitation rate was set to 20–40 rpm at 9° platform angle for the bag cultivation and 150–500 rpm for the STR, controlled via the minimum dissolved oxygen value, which was set to 20% DO. For the bag cultivation, the DO was measured via optical sensors outside the bag; for STR cultivation, the reactor was equipped with an internal DO sensor (VisiFerm DO Sensor, Hamilton Bonaduz AG, Bonaduz, Switzerland). The pH value was measured externally for the bag cultivation; for STR, an Easyferm 325 pH electrode (Hamilton Bonaduz AG) was used. Moreover, the STR was equipped with a Rushton turbine. Then, 10 mL of rapeseed oil was added to both systems on day 2 of the cultivation as an antifoam agent. To dampen excessive foam formation, 30 mL of rapeseed oil was added to the STR on day 7. Caffeic acid and hydrogen peroxide (0.25 and 1 mM) were added on a daily basis after cultivation day 4, as the preceding shake flask experiments suggested a yield improvement. A sample was taken every day and measured in triplicate (3 × 5 mL).
2.4. Analytics
Samples taken in triplicate during the cultivation were centrifuged (15 min, 4 °C, 5000× g; Rotina 380 R, Hettich GmbH & Co KG. Tuttlingen, Germany) to separate biomass and supernatant. The supernatant was used for pH measurement (pH 211, Hanna Instruments, Vöhringen, Germany), and the total reducing sugars were determined after filtration with a 0.45 μm filter according to the method developed by Miller [26]. The filtered supernatant was analyzed by HPLC, but only negligible hispidin concentrations, presumably from cell lysis, were found. The biomass was washed with 5 mL of demineralized water once and freeze-dried (Alpha 1–4, Christ, Osterode am Harz, Germany). The dried biomass was weighed, and the concentration was determined. The dry matter was then extracted exhaustively with methanol (Carl Roth, Karlsruhe, Germany), and the extract was concentrated. After filtration, it was measured via HPLC, equipped with a system control unit (Shimadzu CBM-20A, Kyoto, Japan), degasser (Shimadzu DGU-20A5R), autosampler (Shimadzu SIL-20ACHT), column (Chromolith® Performance, RP-18 endcapped, 100–4.6 mm), and DAD (Shimadzu Nexera X2 SPD-M30A) using the following conditions: flow 1.5 mL·min−1, injection volume 10 μL, detector 220–450 nm, oven 35 °C. The eluent gradient consisted of A (acetonitrile) and B (water + 0.1% formic acid) according to the following scheme: 0 min 98% B, 8 min 80% B, 13 min 0% B, 15 min 0% B, 16 min 98% B, and 17 min 98% B. Hispidin was measured at 9.4 min at λmax = 369 nm. Hispidin concentration was calculated via external calibration with a hispidin standard (≥98%, Sigma Aldrich Chemie GmbH, Germany) using eight concentrations between 4 and 240 mg·L−1 (regression: y = 18564x − 83881, R2 = 0.9896). Cinnamic acid (Ret. 11.0 min, λmax = 277 nm), p-coumaric acid (Ret. 7.2 min, λmax = 309 nm), and caffeic acid (Ret. 5.7 min, λmax = 323 nm) were measured with the same method, using external standards for quantification. l-Tyrosine and l-phenylalanine concentrations were determined as described by Rottmann et al. [27], and the identity of hispidin was confirmed by mass spectrometry using conditions as previously detailed.
2.5. Application of Cultivation Extracts
An accelerated light stability test was performed in triplicate with dyed silk. Samples were dyed with I. hispidus culture broth and tested against silk samples dyed with Dyer’s madder (WEJA Färbeset Resedgelb, Livos Pflanzenchemie, Germany) and commercially available textile dyes (Simplicol Textilfarbe expert–Mais Gelb, Brauns-Heitmann GmbH & Co. KG; Marabu Easy Color gelb, Marabu GmbH & Co. KG; Dylon Sunflower Yellow, Henkel AG & Co. KGaA, Germany). Samples were prepared by heating 100 g of silk or wool at 90 °C for 1 h in the respective liquor. Samples were not treated with mordants or fixing agents other than those present in the commercial products. The samples were radiated for 48 h with 400 W·m−2 at 15 °C in an Atlas Suntest XLS+, equipped with a Xenon lamp and window glass filter (Atlas Material Testing Technology GmbH, Linsengericht-Altenhaßlau, Germany). The color was measured after 1, 6, 12, 24, and 48 h with a Konica Minolta CM-600d (Geometry 8°, measurement area 8 mm, reflection mode, specular component excluded) and analyzed with the program Colibri, version 3.8.12. The given ΔE values, as defined by the International Commission on Illumination, reflect the color difference of a sample (tx) compared to a reference (t0). For documentation, pictures were taken with a Canon EOS 800D with an 18–55 mm EF-S zoom lens, in a PackshotStart Mark II (Sysnext, Levallois-Perret, France) (see Table S1).
3. Results
In an early stage of the research, it was observed that I. hispidus cultures grew morphologically different when cultivated in Moser b medium as compared to standard nutrient liquid (SNL). While both cultures yielded around 100 g of wet biomass per liter after 11 days (SNL 110 ± 19 g·L−1, Moser b 91 ± 6 g·L−1), absorbance of the supernatant at λ = 365 nm differed significantly, indicating a much stronger color formation in Moser b medium. Thus, all subsequent experiments were performed using Moser b medium.
3.1. Influence of Light
I. hispidus was cultivated under different light conditions. Changes in irradiation slightly delayed biomass production compared to the control setup (incubator with windows, but without extra illumination), but had no effect on the final dry matter concentration of about 9 g·L−1 after 15 days of cultivation (Figure 1). Hispidin generation started around day 10, when biomass production ceased. Pigment yields were dramatically reduced in the dark. Illumination with λ = 400 nm restored them to roughly the same amount as under control conditions (49.1 and 60.2 mg·g−1, respectively). An increase of about 50% on day 15 was obtained by illumination with 470 nm (89.8 mg·g−1), and white light more than doubled the yield to a final 131.6 mg·g−1 hispidin.
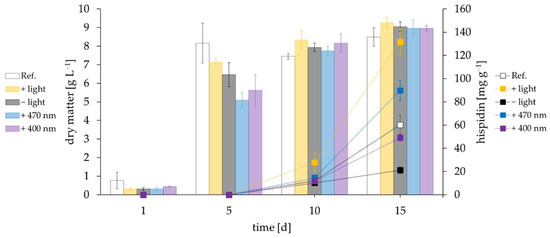
Figure 1.
Biomass (bars, left ordinate) and hispidin yields (squares, right ordinate) of differently irradiated cultures of I. hispidus. Cultures were either kept in the dark (−light, depicted in black/gray) or irradiated with LEDs, specified as 400 nm (purple), 470 nm (blue), and white light (+light, depicted in yellow). The reference culture (Ref., depicted in white) was cultivated in an incubator with a glass window, but without specific light regulation.
3.2. Influence of Oxidative Stress
Different concentrations of hydrogen peroxide were added either daily (1 mM) or once on day 4 to determine the influence of oxidative stress. Both 10 and 25 mM slightly reduced biomass generation (Figure 2). As seen before (Figure 1), pigment generation started again on day 10. The single dosages of H2O2 decreased the hispidin yield on day 15, but daily addition of 1 mM H2O2 increased the yield by 45% up to 87.8 mg·g−1 when compared to the control.
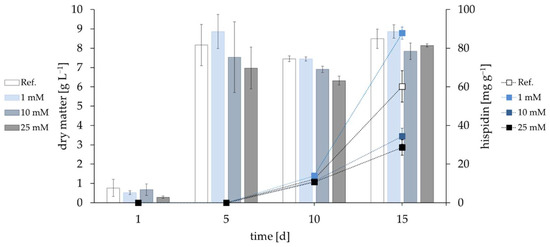
Figure 2.
Biomass (bars, left ordinate) and pigment yields (squares, right ordinate) of cultures of I. hispidus exposed to oxidative stress through addition of hydrogen peroxide. The indicated concentration of H2O2 was added either daily (1 mM) or once during the cultivation on day 4 (10 mM and 25 mM). As the cultivations evaluating the influences of light and oxidative stress were performed in parallel, the control in Figure 1 and Figure 2 was identical.
3.3. Influence of Precursors
Following earlier biogenetic work [2,13] (Scheme 1), 2 mM l-tyrosine, l-phenylalanine, cinnamic (CiA), p-coumaric (CuA), or caffeic acid (CaA) was supplemented to I. hispidus cultures on cultivation day 4, 7, 9 and 11. All cultures developed equally until the precursors were added on day 4 (Figure 3). Both cinnamic and p-coumaric acid completely inhibited pigment formation, but also negatively affected both biomass generation and sugar consumption, with cinnamic acid having a more severe effect. This was also reflected by the pH of the cultures, which was slightly (2.7, p-coumaric acid) and significantly (3.6, cinnamic acid) increased compared to all other cultivations (pH~2.5).
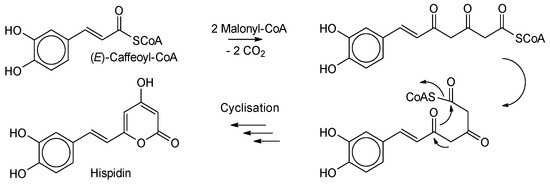
Scheme 1.
Proposed biogenesis of hispidin in I. hispidus after [2]. Caffeic acid is elongated by two molecules (malonyl-CoA) with the release of carbon dioxide. After cyclization, the target molecule hispidin is formed. A detailed portrayal of the proposed pathways can be found in Lee and Yun (2011).
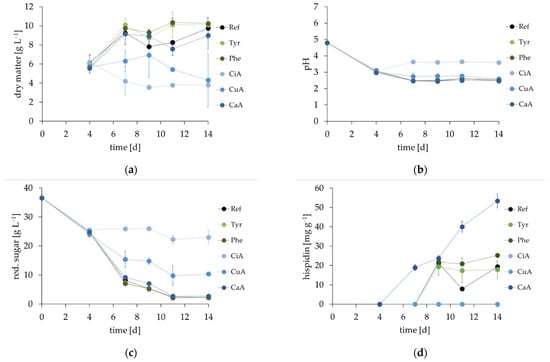
Figure 3.
Cultivation of I. hispidus with precursors (2 mM supplemented on days 4, 7, 9, and 11). The control (Ref.) is depicted in black, l-tyrosine (Tyr) in light green, l-phenylalanine (Phe) in dark green, cinnamic acid (CiA) in light blue, p-coumaric acid (CuA) in blue, and caffeic acid (CaA) in dark blue. Comparison of (a) biomass yield, (b) pH value, (c) total content of reducing sugars, and (d) hispidin concentration.
Cultures supplemented with l-tyrosine, l-phenylalanine, and caffeic acid showed no significant differences to the reference (Ref.) regarding biomass generation, pH, or consumption of reducing sugars. All of them reached final dry matter concentrations of around 10 g·L−1. l-Phenylalanine slightly raised pigment yield to 25.2 mg·g−1 on day 14 compared to the control (19.4 mg·g−1). Caffeic acid enhanced pigment synthesis significantly, yielding 53.4 mg hispidin per gram dry matter on day 14.
3.4. Comparative Cultivation in Two Types of Bioreactors
Full-spectrum white light and the addition of 1 mM H2O2 (daily) and 2 mM caffeic acid on days 4, 7, 9, and 11 increased hispidin biosynthesis when applied separately (see above). To verify if the effects could be combined to increase the overall pigment yield and to compare two different bioreactor types, parallel cultivations were set up in a stirred tank (STR) and a bag bioreactor (Wave). The same preculture was used, and every effort was made to operate the two bioreactors under identical biological and chemical conditions to filter out merely the effects of the different bioreactor constructions (Scheme S1).
Initially, the mycelium grew slightly better in the wave as indicated by the higher biomass and lower pH around day 4, but this was not reflected by the consumption of reducing sugars (Figure 4). From day 7 on and, thus, during the active synthesis of hispidin, the STR showed slightly quicker biomass and hispidin generation. Both reactor types reached similar final concentrations of 12.8 and 12.6 g·L−1 dry matter, and 5.5 and 5.2 g·L−1 hispidin for the STR and wave, respectively (Figure 4a). The slightly better growth in the STR matched the more pronounced decrease in the content of reducing sugars (Figure 4b). In comparison to shake flask cultivations (9 to 10 g·L−1, Figure 1 and Figure 2), the cultivation in both bioreactors led to a slight increase of the final biomass (>12 g·L−1). The hispidin yields were significantly improved: The g·L−1 data indicated in Figure 4a correspond to 428.5 (STR) and 410.9 mg hispidin·g−1 dry matter (wave), respectively, an increase by a factor of seven compared to the control (Figure 1 and Figure 2) and by a factor of three compared to the best single-parameter change in shake flasks, with full illumination (Figure 1). Both biomass and hispidin generation kinetics were comparable to those observed in the shake flasks.
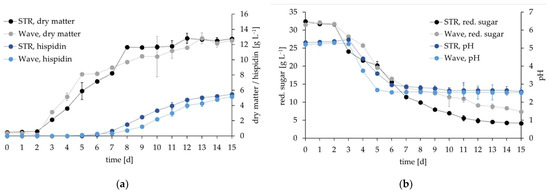
Figure 4.
Parallel cultivation of I. hispidus in a stirred tank (STR, depicted in black and dark blue) and cell-bag bioreactor (wave, depicted in gray and light blue). (a) Pigment and biomass yield, (b) consumption of reducing sugars, and pH over the course of cultivation.
3.5. Application of Hispidin as a Colorant
For a long time, extracted fruiting bodies of I. hispidus have been used for textile dyeing. Any large-scale application, however, would require a permanent and economical hispidin source. Submerged cultures serve this purpose. Wool and silk were dyed with I. hispidus culture broth (for silk samples see Figure S1), and a preliminary stability test against commercial textile dyes was conducted with the silk samples. The initially obtained coloring on silk appeared easy on the eye, but accelerated light stability tests demonstrated that hispidin was not as lightfast as the plant-based or synthetic dyes. Changes in color appearance noticeable for the human eye occurred in all samples (even in the noncolored reference). Control samples stayed below a ΔE of 5 during the 48 h of illumination, while I. hispidus samples faded more quickly. They reached ΔE values over 5 within 6 h and of about 17 after 48 h (Figure 5).
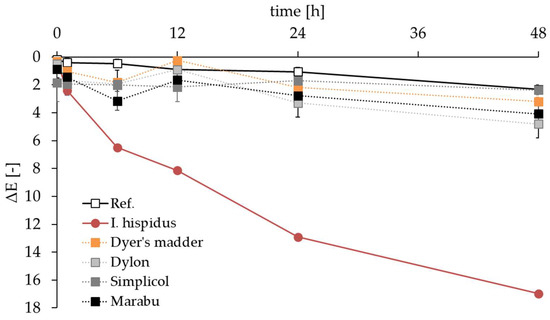
Figure 5.
Comparison of the light stability of silk samples dyed with I. hispidus (red) and commercially available plant-based (Dyer’s madder, depicted in orange) and synthetic textile dyes (Dylon, Simplicol, and Marabu depicted in gray, dark gray, and black, respectively). The noncolored reference (Ref.) is shown in white squares.
4. Discussion
In plants, the key step from l-phenylalanine to phenylpropenoid acids is catalyzed by the blue-light dependent phenylalanine/tyrosine ammonia-lyase (PAL). A maximum promotor activity in carrot was observed using UV-B light [28], which agreed with earlier findings for hispidin formation in I. rheades. This species accumulated hispidin in mycelium under blue light, yielding 8.1 mg·styrylpyrones g−1 dry matter [29]. In this work, a final concentration of 131.6 mg hispidin·g−1 dry matter was obtained under white light (Figure 1). Comparison of chromatographic and mass spectrometry data (ESI-MS: M + H+ = m/z 247, M − H+ = 245; a pair of signals indicating (E/Z)-isomers in similar concentration) with the reference compound confirmed the identity [29].
As the laboratory glass of the Erlenmeyer flasks and the stirred tank used for the submerged cultures in this study showed a blue-light cutoff at around 400 nm, only wavelengths >400 nm were examined. White light with an emission maximum at around 590 nm gave the best results (Figure 1). At the same time, the best biomass yields were recorded, indicating that the observed improvement was less attributable to PAL induction, but rather to a nonspecific stimulation of the cell metabolism, perhaps to protect against ROS [20,21,22]. Illumination of the cell cultures with UV light would be possible by mounting waterproof UV lamps inside the bioreactor, but at the risk of promoting the mutation rate of the cultures. Nevertheless, such an experiment, perhaps with an intermittent illumination regime, seems appealing, especially to prepare for an upscaling of the bioprocess.
Hispidin generation started around day 10, when biomass production was nearly finished, suggesting a shift to secondary metabolism in the stationary phase of the culture. This poses the question for the ecological role of hispidin. The discovery of luminous fungi could provide an answer. Fruiting bodies of Mycena chlorophos, Omphalotus japonicus, and Neonothopanus gardneri shared a common mechanism of bioluminescence using hispidin as a luciferin precursor [30]. Hydroxylation of hispidin to 3-hydroxyhispidin, the “fungal luciferin”, may also occur in I. hispidus and related hispidin-producing fungi [31].
Inspired by the work of Zheng et al. on I. obliquus, hydrogen peroxide was applied as a chemical stressor (Figure 2) [17]. The use of this chemical was preferred over others not only because basidiomycetes, as is commonly known, generate this compound through secreted hexose and aryl oxidases and dispose a surplus of the oxidant via catalase activity, but also because heavy-metal ions or fungicidal compounds would not have fitted the idea of a sustainable and natural process. Furthermore, the phenolic structure of hispidin confers the known antioxidative capacity and, in turn, a defense mechanism against exogenous hydrogen peroxide [20,21,22,32]. Despite the small size of the dataset, it can be concluded that higher concentrations of the stressor (10 and 25 mM) exceeded the tolerance of the cells resulting in growth retardation. The subliminal stress exerted by the daily application of 1 mM hydrogen peroxide, however, gave a significantly higher hispidin yield, confirming the earlier results [17].
The hispidin molecule is built from a shikimate derived phenylpropenoid unit by a polyketide-type chain elongation [7,13,14,15], as proven by 14C-tracer studies [16] and the detection of hispolon, the intermediate after elongation by one malonate unit, in some extracts [4]. It was hypothesized that the acetate pool of well-growing cells should be filled up, while mycelia growing in vitro should lack the typical phenylpropenoid lignin degradation products liberated by a parasitizing fruiting body growing on wood. The parallel cultures thrived well until 2 mM l-tyrosine, l-phenylalanine, cinnamic (CiA), p-coumaric (CuA), or caffeic acid (CaA) was supplemented on day 4 (Figure 3). Both CiA and CuA immediately inhibited growth and hispidin formation. Although known as fungicides, it was hypothesized that I. hispidus would possess enough hydroxylase activities to convert them to nontoxic caffeic acid, but this could not be confirmed as the mycelia did not recover until the end of cultivation. Both aromatic amino acids showed negligible effects compared to the control culture, another piece of evidence for the missing involvement of a PAL activity. Only caffeic acid, the phenylpropenoic acid structure closest to the product structure, had a pronounced effect, lifting the hispidin yield from around 19 mg·g−1 (control) to 53 mg·g−1 after 2 weeks (Figure 3). With the principle of circular bioeconomy in mind, the residual caffeic acid present in coffee grounds could serve as a source of caffeic acid in the future. Our data showed that around 1.8 of the 7 g·kg−1 of caffeic acid remained in spent grounds after a common hot-water extraction of coffee powder. The decrease in hispidin yield of the reference experiment in comparison to the reference in Figure 1 and Figure 2 (19.4 vs. 60.2 mg·g−1, respectively) was most likely due to the addition of the precursors solved in 70% ethanol (final 0.28% ethanol in the culture on the day of addition). Further experiments with final 1.4% ethanol in the cultures strongly inhibited pigment production (data not shown). For future applications, care has to be taken to remove or minimize this effect by choosing another solvent.
The combined effects of illumination and addition of 1 mM H2O2 and 2 mM caffeic acid were compared in parallel cultivations using a STR and a wave bioreactor (Figure 4). The wave was originally developed for the cultivation of shear sensitive animal cells. The thin cells of fungal mycelia could equally benefit from this cultivation mode as confirmed previously for the production of peptidases [23]. In contrast to expectations, this was not observed. Both bioreactors produced similar concentrations of biomass (STR vs. wave: 12.8 vs. 12.6 g·L−1), and hispidin (STR vs. Wave: 5.5 vs. 5.2 g·L−1) (Figure 4a). For comparison, the overall highest yield was reported by Park et al. (2004), who achieved a maximum of 2.5 g·L−1 hispidin yield using Phellinus linteus cultivated in shake flasks [8]. The more controlled cultivation in the bioreactors resulted in a better biomass yield compared to the shake flask cultivations (12.8 vs. 9 g·L−1, Figure 1). The hispidin yields increased even more significantly when compared to shake flask cultures, from 131.6 to 428.5 mg·g−1 dry matter. Earlier experiments with the wave bioreactor without addition of caffeic acid and H2O2 reached 3.9 g·L−1 hispidin on cultivation day 13 (data not shown). These values are not fully comparable to the data presented in this manuscript, as different precultures were used. Nonetheless, the addition of both inducers resulted in 4.3 g·L−1 hispidin on day 13 (Figure 4a), which amounts to an increase of 10%.
The successful cultivation of basidiomycetes in an STR goes along with numerous problems; inactivation of probes due to adherence of cells, excessive foaming, and subsequent plugging of waste air filters are among the worst. As a result, it took several attempts to operate the STR properly. On the other hand, the wave is a single-use device, and scale-up is currently limited to 300 L. In solid-state fermentation trials using Phellinus linteus, 1.107 mg hispidin per gram was achieved after 6 weeks [33]. This corresponds to a roughly 390-fold increased yield compared to the highest yield obtained in the STR after 2 weeks in this study (428.5 mg·g−1). In addition, obstacles of the solid-state approach are the lack of fermentation control and the problematic scale-up. A 20 ton submerged fermentation was reported, but the yield of 3 mg·g−1 hispidin was low [34].
All colorants bleach when exposed to light (and oxygen) over time, because they absorb electromagnetic waves from the visible spectrum; otherwise, they would not appear colorful. The absorbed energy is usually converted into heat, but in part also responsible for the excitation of bonds to react with oxygen and, in the UV range, for the direct cleavage of covalent bonds, which results in colorless products. Natural colorants are well known to bleach faster, which was also the case for hispidin (Figure 5). However, it was stable for months in the refrigerator (data not shown). Neither the Ames test, in vitro chromosome aberration test, acute oral toxicity test, nor bone marrow micronucleus test detected toxicological properties of hispidin [34]. It may, thus, be favorably used to dye enclosed or opaque products, such as soap, shampoo, body lotion, or toothpaste with additional health benefits (see Section 1). Textile or wood dyeing [9,10] would require UV filters, perhaps mordants, or other fixing agents.
In conclusion, a combination of physical and chemical parameters combined in an optimized bioprocess may yield hispidin in concentrations that would allow to substitute petrochemical-based, toxicologically problematic synthetic dyes for a number of applications. In addition to its utilization for dyeing purposes, the reported bioprocess might also be used to generate hispidin to study or implement its plethora of previously reported bioactivities.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation8100541/s1: Scheme S1. Overview of the experimental steps to inoculate the two bioreactor types; Table S1. Accelerated aging of dyed silk samples; Figure S1. Dyed wool. Scheme S1 and the graphical abstract were created with BioRender.
Author Contributions
Conceptualization, P.B., A.W. and R.G.B.; methodology, P.B.; validation, P.B.; formal analysis, M.T., C.F. and P.B.; investigation, M.T., C.F. and P.B.; resources, R.G.B.; data curation, P.B. and F.E.; writing—original draft preparation, P.B., F.E. and R.G.B.; writing—review and editing, M.Z. and A.W.; visualization, C.F., M.T., and P.B.; supervision, F.E., M.Z., A.W. and R.G.B.; project administration, F.E. and R.G.B.; funding acquisition, A.W. and R.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry of Education and Research, grant numbers 031B0879 and 031B1079A. The publication of this article was funded by the Open Access Fund of Leibniz Universität Hannover.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Eva Maria Hubbermann from Oterra A/S, for providing equipment for light stability tests. The authors also thank David Zuber for lending the spectrometer, as well as Katharina Mundry and Daria Roggenbruck, who contributed through their work as student assistants. The technical support of Carmen Theobald and Ulrich Krings is highly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palkina, K.A.; Ipatova, D.A.; Shakhova, E.S.; Balakireva, A.V.; Markina, N.M. Therapeutic Potential of Hispidin-Fungal and Plant Polyketide. J. Fungi 2021, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Yun, B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Angelini, P.; Girometta, C.; Tirillini, B.; Moretti, S.; Covino, S.; Cipriani, M.; D’Ellena, E.; Angeles, G.; Federici, E.; Savino, E.; et al. A comparative study of the antimicrobial and antioxidant activities of Inonotus hispidus fruit and their mycelia extracts. Int. J. Food Prop. 2019, 22, 768–783. [Google Scholar] [CrossRef]
- Gründemann, C.; Arnhold, M.; Meier, S.; Bäcker, C.; Garcia-Käufer, M.; Grunewald, F.; Steinborn, C.; Klemd, A.M.; Wille, R.; Huber, R.; et al. Effects of Inonotus hispidus Extracts and Compounds on Human Immunocompetent Cells. Planta Med. 2016, 82, 1359–1367. [Google Scholar] [CrossRef]
- Smolskaitė, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT—Food Sci. Technol. 2015, 60, 462–471. [Google Scholar] [CrossRef]
- El Hassane, A.; Shah, S.A.A.; Hassan, N.B.; El Moussaoui, N.; Ahmad, R.; Zulkefeli, M.; Weber, J.-F.F. Antioxidant activity of hispidin oligomers from medicinal fungi: A DFT study. Molecules 2014, 19, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Bu’Lock, J.D.; Leeming, P.R.; Smith, H.G. 400. Pyrones. Part II. Hispidin, a new pigment and precursor of a fungus “lignin”. J. Chem. Soc. 1962, 2085–2089. [Google Scholar] [CrossRef]
- Park, I.-H.; Chung, S.-K.; Lee, K.-B.; Yoo, Y.-C.; Kim, S.-K.; Kim, G.-S.; Song, K.-S. An antioxidant hispidin from the mycelial cultures of Phellinus linteus. Arch. Pharm. Res. 2004, 27, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Tudor, D.; Zhang, W.R.; Ng, S.; Cooper, P.A. Ability of three yellow pigment producing fungi to colour wood under controlled conditions. Int. Wood Prod. J. 2014, 5, 103–107. [Google Scholar] [CrossRef]
- Friese, W.; Wähnert, V. Einfach färben mit Pilzen und Pflanzen; Freya Verlag GmbH: Engerwitzdorf, Austria, 2021; ISBN 9783990254141. [Google Scholar]
- Sundström, C.; Sundström, E. Mit Pilzen färben: Eine Fundgrube für Kunstgewerbler, Pilzsammler und Naturfreunde; Orell Füssli: Zürich, Switzerland; Schwäbisch Hall, Germany, 1984; ISBN 3280014735. [Google Scholar]
- Tegeler, K.; Deutsche Gesellschaft für Mykologie e. V. Leitfaden zum Färben mit Pilzen, 2nd ed.; Josef Maria Christan: München, Germany, 2016. [Google Scholar]
- Nambudiri, A.M.D.; Vance, C.P.; Towers, G.H.N. Styrylpyrone biosynthesis in Polyporus hispidus: II. Enzymic hydroxylation of p-coumaric acid and bis-noryangonin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1974, 343, 148–155. [Google Scholar] [CrossRef]
- Velíšek, J.; Cejpek, K. Pigments of higher fungi—A review. Czech J. Food Sci. 2011, 29, 87–102. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Liu, J.-K. Pigments of fungi (macromycetes). Nat. Prod. Rep. 2010, 27, 1531–1570. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.W.; Towers, G. Hispidin biosynthesis in cultures of Polyporus hispidus. Phytochemistry 1973, 12, 589–592. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Y.; Zhang, M.; Wei, Z.; Miao, K.; Sun, W. Oxidative stress response of Inonotus obliquus induced by hydrogen peroxide. Med. Mycol. 2009, 47, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Nambudiri, A.M.D.; Vance, C.P.; Towers, G.H.N. Effect of light on enzymes of phenylpropanoid metabolism and hispidin biosynthesis in Polyporus hispidus. Biochem. J. 1973, 134, 891–897. [Google Scholar] [CrossRef]
- Vance, C.P.; Tregunna, E.B.; Nambudiri, A.M.D.; Towers, G.H.N. Styrylpyrone biosynthesis in Polyporus hispidus: I. Action spectrum and photoregulation of pigment and enzyme formation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1974, 343, 138–147. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Chang, S.-F.; Chau, S.-F.; Chiu, S.-C. The Protective Effect of Hispidin against Hydrogen Peroxide-Induced Oxidative Stress in ARPE-19 Cells via Nrf2 Signaling Pathway. Biomolecules 2019, 9, 380. [Google Scholar] [CrossRef]
- Jang, J.S.; Lee, J.S.; Lee, J.H.; Kwon, D.S.; Lee, K.E.; Lee, S.Y.; Hong, E.K. Hispidin produced from Phellinus linteus protects pancreatic beta-cells from damage by hydrogen peroxide. Arch. Pharm. Res. 2010, 33, 853–861. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S.; Kim, Y.R.; Jung, W.C.; Lee, K.E.; Lee, S.Y.; Hong, E.K. Hispidin isolated from Phellinus linteus protects against hydrogen peroxide-induced oxidative stress in pancreatic MIN6N β-cells. J. Med. Food 2011, 14, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, P.; Takenberg, M.; Hartwig, S.; Beutel, S.; Berger, R.G.; Scheper, T. Cultivation of shear stress sensitive microorganisms in disposable bag reactor systems. J. Biotechnol. 2013, 167, 370–376. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 9780123721808. [Google Scholar]
- Kavitha, S.; Chandra, T.S. Oxidative stress protection and glutathione metabolism in response to hydrogen peroxide and menadione in riboflavinogenic fungus Ashbya gossypii. Appl. Biochem. Biotechnol. 2014, 174, 2307–2325. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Rottmann, E.; Hauke, K.F.; Krings, U.; Berger, R.G. Enzymatic acrylamide mitigation in French fries—An industrial-scale case study. Food Control 2021, 123, 107739. [Google Scholar] [CrossRef]
- Takeda, J.; Ozeki, Y.; Yoshida, K. Action spectrum for induction of promoter activity of phenylalanine ammonia-lyase gene by UV in carrot suspension cells. Photochem. Photobiol. 1997, 66, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Gornostai, T.G.; Borovskii, G.G.; Kashchenko, N.I.; Olennikov, D.N. Phenolic Compounds of Inonotus rheades (Agaricomycetes) Mycelium: RP-UPLC-DAD-ESI/MS Profile and Effect of Light Wavelength on Styrylpyrone Content. Int. J. Med. Mushrooms 2018, 20, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Suzuki, Y.; Martins, G.N.R.; Carvalho, R.P.; Pereira, T.A.; Waldenmaier, H.E.; Kanie, S.; Naito, M.; Oliveira, A.G.; Dörr, F.A.; et al. Identification of hispidin as a bioluminescent active compound and its recycling biosynthesis in the luminous fungal fruiting body. Photochem. Photobiol. Sci. 2017, 16, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Purtov, K.V.; Petushkov, V.N.; Baranov, M.S.; Mineev, K.S.; Rodionova, N.S.; Kaskova, Z.M.; Tsarkova, A.S.; Petunin, A.I.; Bondar, V.S.; Rodicheva, E.K.; et al. The Chemical Basis of Fungal Bioluminescence. Angew. Chem. Int. Ed Engl. 2015, 54, 8124–8128. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Lee, Y.-M.; Park, S.R.; Da Kim, H.; Lim, B.O. Anticancer activity of hispidin via reactive oxygen species-mediated apoptosis in colon cancer cells. Anticancer. Res. 2014, 34, 4087–4093. [Google Scholar]
- Liang, C.-H.; Wu, C.-Y.; Li, P.-H.; Liang, Z.-C. Optimal Liquid Inoculum Conditions and Grain Medium Enhanced Hispidin Production by Species of Genus Phellinus (Agaricomycetes) in Solid-State Fermentation. Int. J. Med. Mushrooms 2022, 24, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Li, I.-C.; Chen, C.C.; Sheu, S.-J.; Huang, I.-H.; Chen, C.-C. Optimized production and safety evaluation of hispidin-enriched Sanghuangporus sanghuang mycelia. Food Sci. Nutr. 2020, 8, 1864–1873. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).