1. Introduction
The poultry sector is an industry that depends on maximum efficiency and minimum profit [
1]. Significantly, feed is a restrictive factor for the development of the poultry industry, mainly due to the high cost of feeds, which impact industry profits [
2]. In addition, the conventional feed ingredients corn and soybeans are now used for human food and bioenergy, leading to feed–food–fuel competition [
2,
3]. This situation has led most farmers to try raising their own grazing animals to reduce feed cost [
4,
5,
6] or to supplemented feed by enriching or manipulating nutrients to improve feed deficiencies [
6,
7]. Nowadays, studies are being carried out to assess the potential of alternative feedstuffs from low-cost but highly nutritious agricultural waste required for poultry [
5,
8,
9,
10,
11].
Okara, or soybean curd residue, is an agro-industrial by-product from soybean extract produced during the processing of soy milk and tofu [
12,
13]. Due to the high moisture content (about 80%), okara is highly perishable and moldy in normal environments [
14,
15], it is often discarded and turned into waste. Although studies have shown that okara is high in crude fiber (35–55%), protein (30%), fat (10–11%) [
9,
16], polyunsaturated fatty acid (56% of total fat), and enriched isoflavone [
9], the application of okara in poultry feed is still limited due to several anti-nutritional factors, including phytates, tannins, trypsin inhibitors, and oligosaccharides [
17]. In addition, the carbohydrates contained in okara are considered to be generally unavailable non-starch polysaccharide (NSP) fiber, which are mainly composed of mannan, galactomannan, and cellulose [
18,
19,
20]; intact NSP present in the poultry digestive tract is known to inhibit the digestive process and interfere with intestinal balance, leading to problems associated with wet dropping (wet feces) and even intestinal diseases [
21].
Copra meal (CM), another readily available agro-industrial by-product derived from coconut oil meals [
10], has similar nutritional characteristics to okara (rich in protein (15–25%) and carbohydrate (60%)). With proper feed technology, CM can be used to reduce the cost of poultry feed [
8,
22]. However, due to the low content of amino acids (lysine and methionine), the use of CM in poultry diets is still rare [
23]. Similar to okara, CM contains a high-water content and harbors indigestible NSP [
19]. However, the addition of CM for broiler feed has some benefits since it contains beta-mannan as prebiotics and as mycotoxin binder [
24].
Many studies have attempted to improve the utilization rate and feed value of agro-industrial by-products through the addition of amino acids [
5,
25], enzymes [
26], physical modification [
27], and microbial biotransformation; solid-state fermentation (SSF) is one of the strategies. SSF refers to a fermentation process in which microbial growth and metabolic activities take place in a moist solid substrate with little flowing water [
28,
29]. The most commonly used solid substrates are cereals (such as rice, wheat, barley, and corn), legume seeds, wheat bran, lignocellulosic raw materials, and various animal or plant materials. These grains are insoluble or sparingly soluble in water, but most are inexpensive and readily available [
30,
31]. In the past, the main microorganisms used in SSF were fungi, which were considered to have optimal activity in a matrix with low moisture substrates. More recently, many bacteria and yeasts that require liquid media have also been used for SSF, showing the advantages of SSF over liquid-state fermentation [
32,
33]. Specifically, bacterial fermentation results in improved nutritional factors, including increasing the number of small-sized peptides and increasing the content of essential and non-essential amino acids [
34].
The gut microbiota of broilers plays an important role in the development and function of the digestive system, including the ability to ferment and utilize dietary fiber to enhance the immune system [
35]. As broilers grow, the competition for gut microbial colonization gradually slows and the bacterial transition phase stabilizes, thereby affecting the abundance of gut microbiota [
36,
37]. In addition, the development of intestinal epithelial cells, nutrient digestion, and improvement of intestinal mucosal integrity enable poultry to improve the transformation efficiency and growth performance after energy harvesting [
37].
Lactobacillus species (
Lactobacillus acidophilus,
Lactobacillus delbrueckii, and
Lactobacillus salivarius) [
38,
39] and
Clostridium butyricum [
40] are known to ferment okara and improve the nutritional status and growth performance of animals. Specifically,
Lactobacillus species are capable of producing bacteriocin and short-chain fatty acids (lactate and butyrate), which help lower pH and maintain acidity in the animal gut [
41]. Such bacteria are bile-resistant because they have the bile salt hydrolase (BSH) enzyme that helps hydrolyze bound bile and reduce its toxic effects [
42]. Although there are no reports of using
Clostridium butyricum alone to ferment okara,
Clostridium butyricum is commonly used in soybean meal fermentation along with
Lactobacillus [
43,
44,
45]. Compared to other probiotics,
Clostridium butyricum is heat-resistant and can be stored in dry form at room temperature through the pelleting process of feed products [
46]. In addition,
Clostridium butyricum is resistant to low pH and high bile concentrations; hence, it can reach and colonize the small intestine after ingestion in broilers.
Previous studies have investigated whether SSF can improve the nutritional quality of animal feed by increasing nutrient bioavailability and reducing anti-nutritional factors in single-feed ingredients [
47], such as palm kernel cake [
48] and rapeseed meal [
49]. The present study was conducted to investigate the ability of SSF in mixed ingredients, okara, and CM. The combination of three homologous
Lactobacillus species (
L. acidophilus,
L. delbrueckii, and
L. salivarius) and
C. butyricum were used to ferment okara and CM, and NSPases were used to reduce the NSP and anti-nutritional factors in the fermentation matrix to help the digestion and absorption of broilers. Therefore, this study investigated the optimal parameters of fermented okara and CM (FOCM) with probiotics and NSPases and evaluated its effect on broilers. The results provide valuable information regarding the fermentation conditions of mixed okara and CM by probiotics and enzymes. These conditions are suitable for use on an industrial scale to mass-produce functional fermented products.
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
Clostridium butyricum (C. butyricum; MIYAIRI 588) was purchased from Miyarisan Pharmaceutical (Tokyo, Japan). Lactobacillus acidophilus (L. acidophilus; BCRC 10695), Lactobacillus delbrueckii (L. delbrueckii; BCRC 10696), and Lactobacillus salivarius (L. salivarius; BCRC12574) were purchased from the Food Industry Research and Development Institute (Hsinchu, Taiwan). After thawing, C. butyricum was inoculated into an Erlenmeyer flask containing brain-heart-infusion broth (BHI; Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37 °C for 48 h with shaking. Lactobacillus acidophilus, L. delbrueckii, and L. salivarius were inoculated into an Erlenmeyer flask containing de Man, Rogosa, and Sharpe broth (MRS; Sigma-Aldrich) at 37 °C for 24 h with shaking.
2.2. Solid-State Fermentation (SSF)
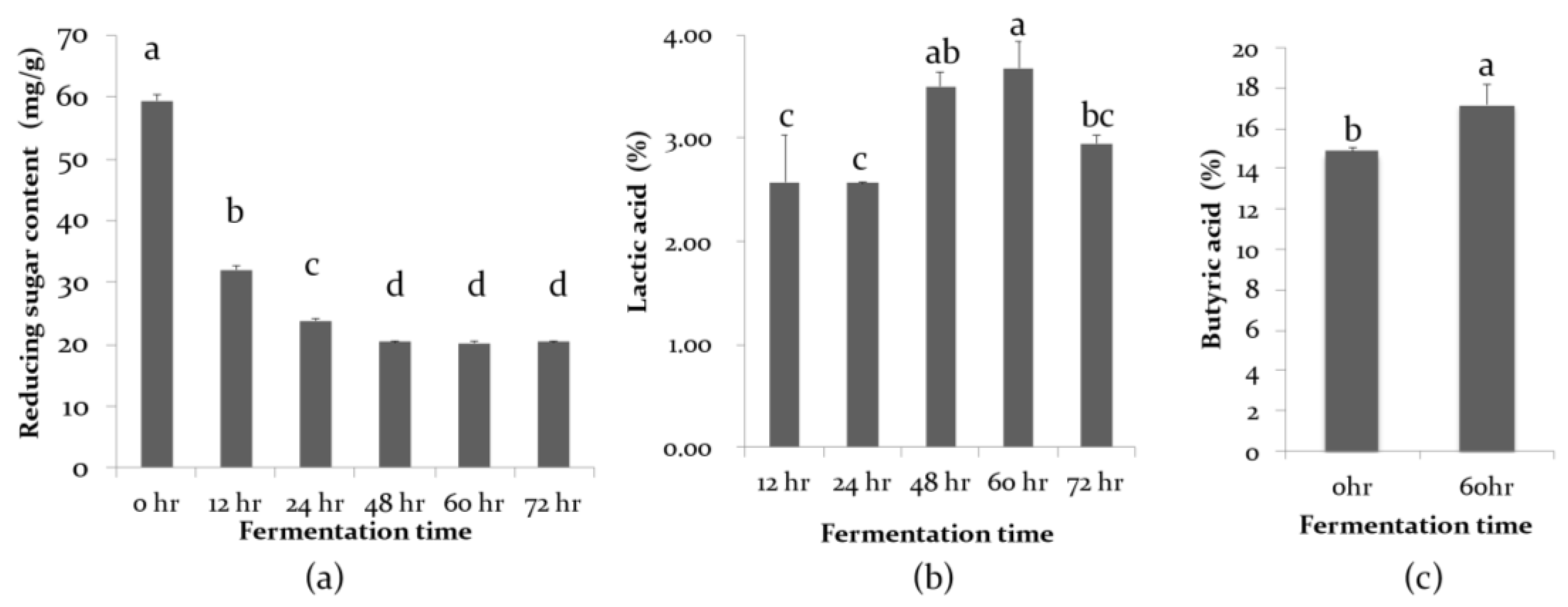
Okara was provided by Taiwan Jiaziyuan Stinky Tofu Factory and CM is imported from the Philippines. The procured substrates, including okara and CM, were mixed with water to the required relative moisture content in a space bag. The substrate mixtures were then inoculated with 3% (v/w) inoculums containing L. acidophilus, L. delbrueckii, L. salivarius, and C. butyricum (so that each bacterial solution was quantified beforehand as 1.0 × 108 CFU/mL), and carefully mixed under sterile conditions and incubated in 37 °C. To investigate the effects of initial moisture content and fermentation duration on SSF of mixed okara and CM, fermentations were performed with different initial moisture contents (50, 55 and 60%; w/w) and different fermentation durations (48, 60 and 72 h) at 37 °C.
The optimal initial moisture content (55%) and fermentation duration (60 h) of mixed okara and CM with the SSF method were further employed to reveal the effects of exogenous NSPases (VemoZyme®) on FOCM. The experimental groups were divided into (1) mixed okara and copra meal (control, CTRL), (2) mixed okara and copra meal plus exogenous NSPases (NSP enzyme), (3) mixed okara and copra meal plus probiotics (Probiotics), (4) mixed okara and copra meal plus probiotics and NSPases (mixed). All experiments were incubated at 37 °C and performed in triplicate. The FOCM were then dried at 50 °C for 24 h, homogenized through mechanical agitation, and stored at 4 °C prior to further analysis.
2.3. Determination of Total Viable Count
To determine total viable count of bacteria, 1 g of FOCM was diluted with 9 mL of 0.85% NaCl, shaken for 30 min, and then placed in a selective media for microbial enumeration. The MRS agar was used to measure all the Lactobacillus species; BHI agar was used to measure C. butyricum. The diluent was spread-plated and placed in an anaerobic bag in a 37 °C incubator for 24 h. The count of bacterial colonies was statistically analyzed and expressed as colony-forming units per gram (CFU/g).
2.4. Determination of Moisture, Neutral Detergent Fiber, Residual Reducing Sugars, Lactic Acid, and Butyric Acid Content
The moisture content of the FOCM was measured with the aid of a crucible under sequent drying conditions of 65 °C for 16 h and 105 °C for an hour. The content of neutral detergent fiber (NDF) was measured using neutral detergent. The content of reducing sugar was analyzed by incubating 1 g of FOCM in a 50 °C water bath for 30 min, and the reducing sugars in the supernatant were analyzed using the dinitrosalicylic acid method. The content of lactic acid and butyric acid was determined by high-performance liquid chromatography (HPLC). The FOCM was incubated at 50 °C in a water bath and ultrasonically shaken for 20 min. The homogenate was mixed in water and then filtered with a 0.22 μm filter membrane. The extract was then analyzed for lactic acid and butyric acid content using HPLC and compared with a standard. Lactic acid (Sigma-Aldrich) and butyric acid (Sigma-Aldrich) standards were prepared and analyzed a minimum of three times. By using a C18 column, which had the dimensions of 5 μm inner diameter 4.6 mm × 25 cm long, the lactic acid mobile phase was 0.01 mol/L phosphoric acid solution, and the butyric acid mobile phase was 80% acetonitrile and 0.02% phosphoric acid. HPLC analysis of lactic acid and butyric acid was performed at the same flow rate of 1.0 mL/min and wavelengths of 210 nm and 206 nm. The concentration of lactic acid and butyric acid were determined based on the slope of the standard curve.
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
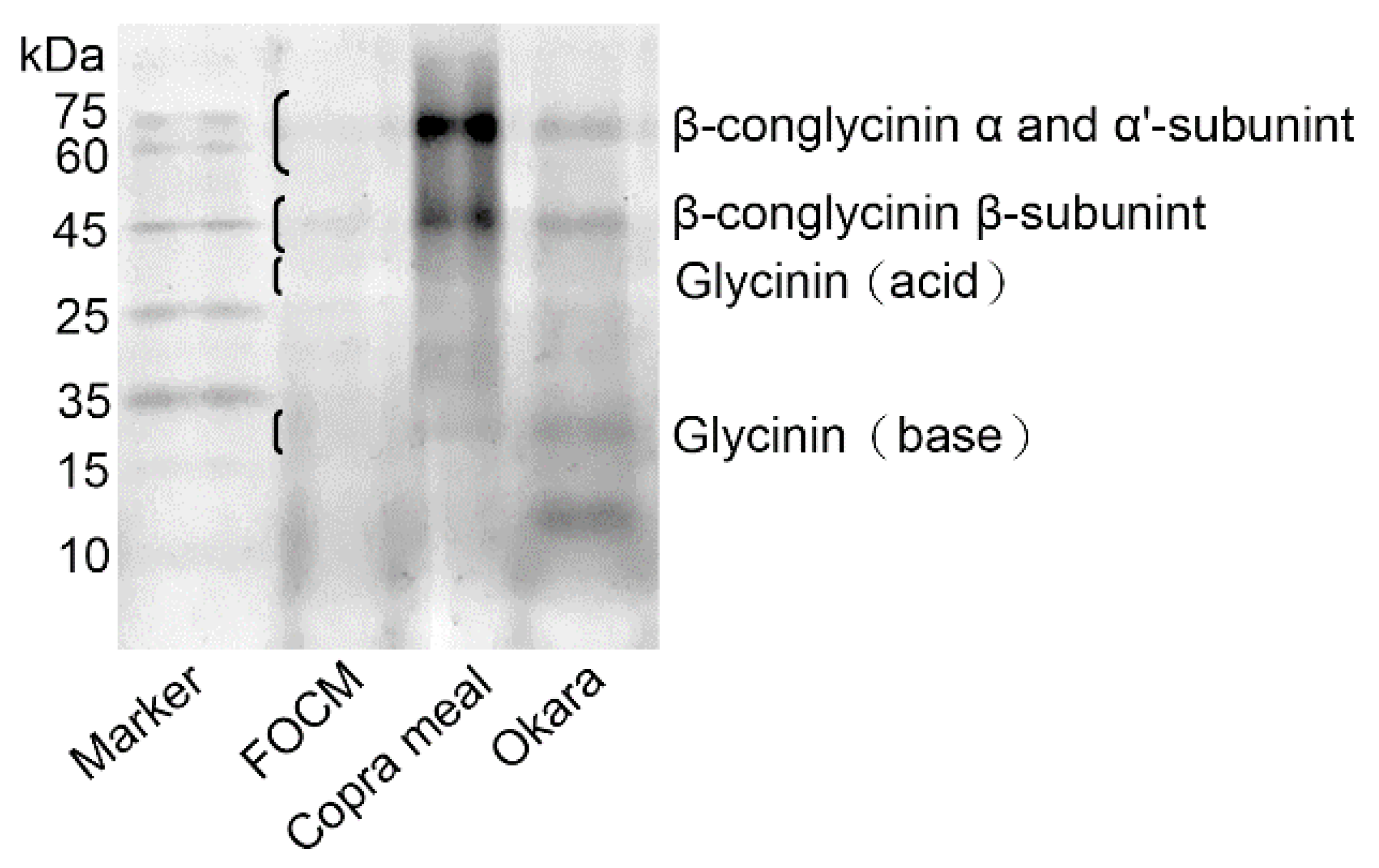
The extracted crude protein from FOCM was quantified using the Bradford method, with bovine serum albumin used as the protein standard. For the determination of soy globulin contents, total proteins from pre-fermented and FOCM treated with sodium dodecyl sulfate (SDS) were separated using polyacrylamide gel electrophoresis (PAGE), and the soy globulins in the gel were visualized using Coomassie Brilliant Blue R-250 (Sigma-Aldrich) staining.
2.6. Animal Study
The FOCM was prepared as aforementioned above, and the optimal initial moisture content was 55% and fermentation duration was 60 h. The animal protocol was approved by the Institutional Animal Care and Use Committee of the National Ilan University (110-8). A total of 96 1-day-old unsexed broiler chicks (Arbor Acres) obtained from a local commercial hatchery were randomly assigned to one of four treatments in a completely randomized design. Each treatment was assigned to six replicate cages with four birds per cage. Broilers were reared in stainless-steel cages (89 cm × 56.5 cm × 60 cm). The experimental diets were (1) a basal diet without FOCM as control (control, CTRL), (2) a basal diet plus 1.25% FOCM (FOCM1.25), (3) a basal diet plus 2.5% FOCM (FOCM2.5), (4) a basal diet plus 5% FOCM (FOCM5.0). Broilers received the test diet from 1 to 35 days of age. The feeding program had two phases, spanning days 1–20 (starter diet) and 21–35 (finisher diet). No coccidiostats and antibiotics were included in the diets. The diets were formulated to meet or exceed the requirements of the birds according to the National Research Council recommendations (NRC, Nutrient Requirements for Poultry, 1994,
Table 1). Broilers were fed and watered ad libitum throughout the experiment. The mortality of broilers was monitored daily. On days 4 and 14 of the experiment, Newcastle disease (ND) and infectious bronchitis (IB) vaccines were administered through the eyes and nose. Broiler growth performance, including average daily gain (ADG), average daily feed intake (ADFI), feed conversion ratio (FCR), body weight, and feed intake were measured weekly. One broiler per replicate was sacrificed on days 21 and 35, and their blood and ileal contents were collected for subsequent analysis.
2.7. Blood Chemistry and Antibody Titer Analysis
The blood chemistry and antibody titer analysis were followed as described by previous studies [
50,
51]. For blood chemistry analysis, blood samples from broilers (one bird per replicate, six replicates were used per group (
n = 6)) were collected through heart puncture on the 21st and 35th day of age and separated by centrifugation at 1500 g for 10 min. An automatic biochemical analyzer (TOSHIBA TBA-80FR NEO2, Tokyo, Japan) to perform a total of 6 serum biochemical analyses, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), and glucose (GLU).
To analyze the antibody titer, blood samples were collected through cardiac puncture and centrifuged at 1500 g for 10 min. Serum was collected and stored at 20 °C. To evaluate the effect of feeding FOCM on the host’s immune response to ND and IB vaccines, we also cooperated with the Poultry Health Center of the Central Animal Husbandry Association to detect ND and IB titer antibody. The detection method of ND titer antibody used the hemagglutinin inhibition (HI) test; as has been noted, the HI test is a highly accurate measure of a herd’s immunological health and resistance to ND, particularly for evaluating the protective response to ND vaccination [
52]. The IB titer antibody was detected by enzyme-linked immunosorbent assay (ELISA) kit (BioChek, Gouda, The Netherlands).
2.8. Intestinal Morphology Analysis
On the 21st and 35th day of age, one bird per replicate was randomly chosen (six replicates were used per group (
n = 6)) and sacrificed with carbon dioxide, and three sections of duodenum, jejunum, and ileum were taken, as previously described [
53]. After washing the chyme on the intestinal mucosal surface with ice-cold PBS solution, this sample was put it in 10% formalin solution and fixed at room temperature for a week. Subsequently, using paraffin embedding procedures, fixed intestine samples (duodenum, jejunum, and ileum) were prepared. Three cross-sections from each sample were sectioned at a thickness of 5 μm, mounted on a glass slide, and stained with hematoxylin and eosin. Villus height and crypt depth were measured randomly for 20 villi per bird using a micrometer using an optical microscope (Olympus CKX41, Tokyo, Japan).
2.9. Bacterial Diversity Analysis
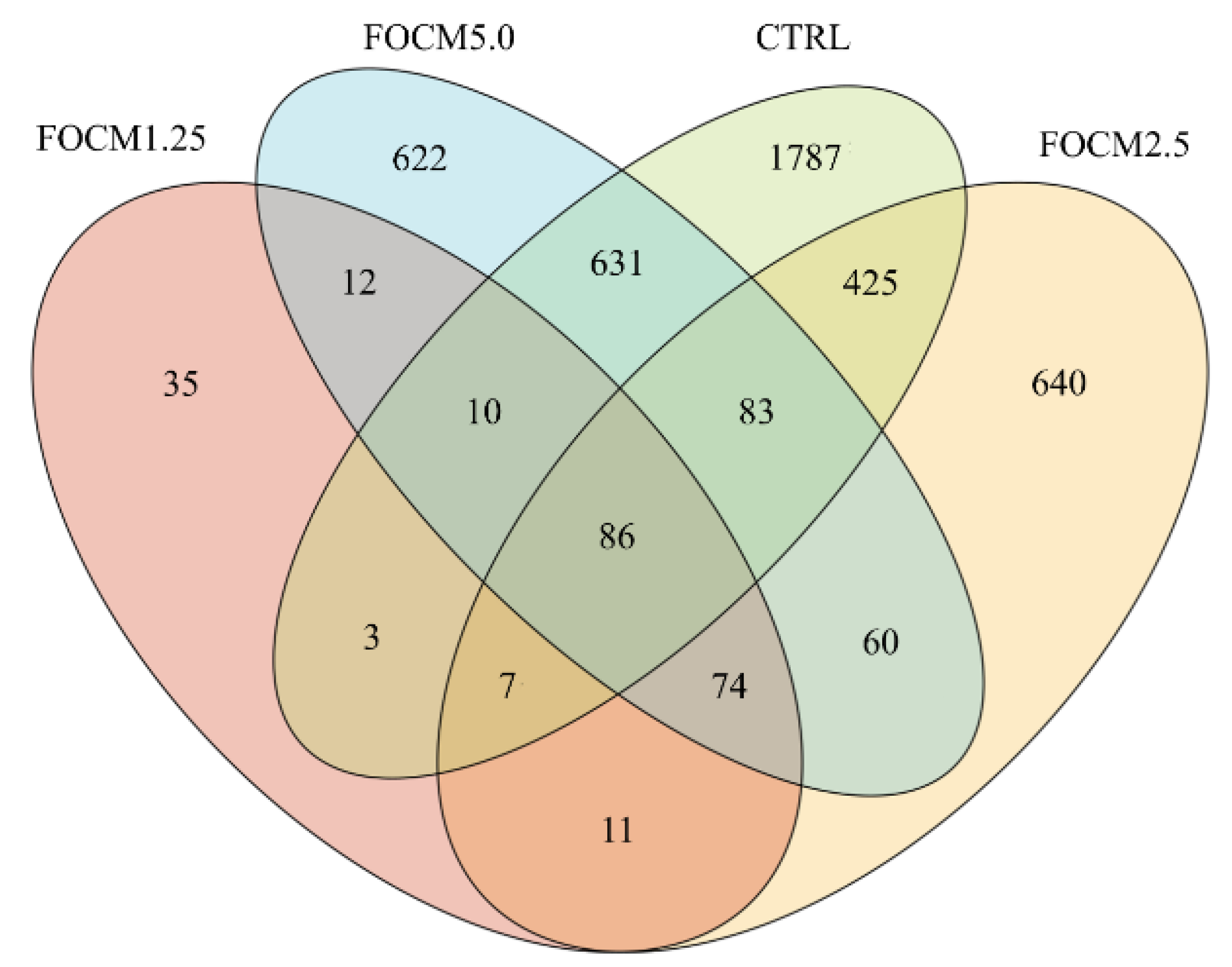
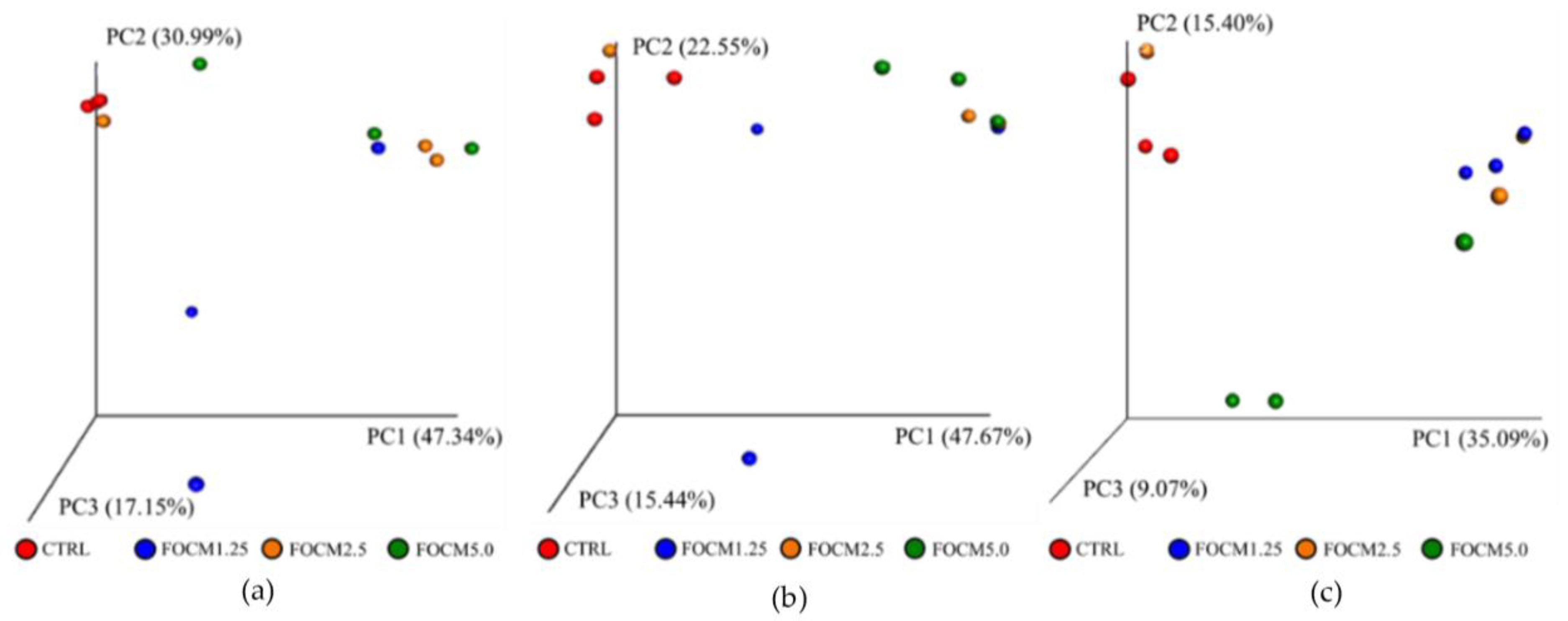
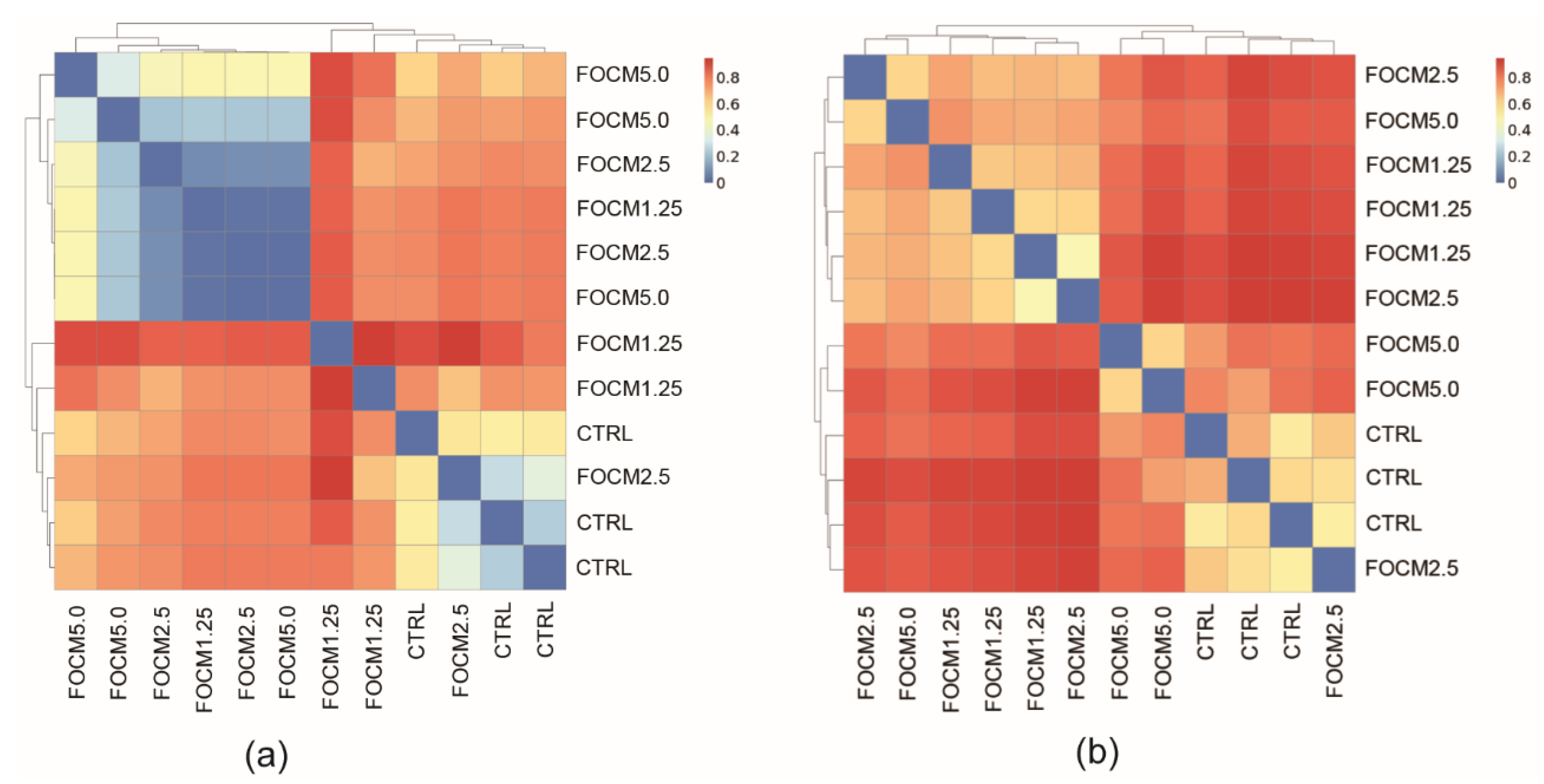
A 21-day-old broiler was randomly chosen from every two cages based on the average of the cage then euthanized by carbon dioxide inhalation, and a five-centimeter ileum fragment was taken for microbiota analysis (three replicates were used per group (n = 3)). The bacterial deoxyribonucleic acid (DNA) was extracted using the QIAmp Fast DNA Stool Mini kit (QIAGEN, Hilden, Germany), according to manufacturer’s instructions. The v3-v4 hypervariable regions of the bacterial 16S rRNA gene was performed by polymerase chain reaction (PCR) using specific 341F-805R primer (5′-CCTACGGGNGGCWGCAG-3′ and 5′-GACTACHVGGGTATCTAATCC-3′). Sequencing was conducted at a read length of 300 nucleotides by next-generation sequencing platform (Illumina, San Diego, CA, USA). For data sequence processing, the QIIME 2 software package (version 2017.4, GitHub, San Francisco, CA, USA) was used. High-quality reads were selected, and all of the effective reads from all samples were clustered into operational taxonomic units (OTUs) based on 97% sequence similarity using the UCHIME (version 4.2, GitHub) and mothur software (version 1.39.5, GitHub). In addition, the QIIME 2 software (version 2017.4, GitHub) and the naïve Bayesian classification approach were used to access alpha diversity (richness and evenness) and phylogenetic assignment, respectively. Furthermore, the R packages were used to display the differences in microbiota between groups using principal component analysis (PCA) and principal coordinate analysis (PCoA) based on the unweighted and weighted UniFrac distance matrices (version 3.5.0 and version 1.7.13, GitHub).
2.10. Statistical Analysis
All experimental data were analyzed by ANOVA using the General Linear Model (GLM) program of SAS (SAS Institute, Cary, NC, USA). Significant differences between each treatment were measured using Duncan’s new multiple range test. Values of p < 0.05 were considered statistically significant.