Immobilization of Aspergillus oryzae DSM 1863 for l-Malic Acid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Media
2.2. Immobilization
2.3. Preculture Conditions
2.4. Main Culture for Acid Production in Shake Flasks
2.5. Main Culture for Acid Production in Bioreactors
2.6. Analytics
3. Results
3.1. Malic Acid Production in Shake Flask Cultivations
3.2. Malic Acid Production in Bioreactor Cultivations
3.3. Shake Flask Cultivations without Carbon Source in the Preculture Medium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Substrate | Immobilization Matrix | Organic Acid [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| Malate | Succinate | Fumarate | Pyruvate | Ketoglutarate | Oxalate | Citrate | ||
| Acetic acid | Free biomass | 50.8 ± 0.7 | 41.8 ± 0.7 | 4.2 ± 0.5 | 0.8 ± 0.1 | 0.3 ± 0.1 | 1.3 ± 0.3 | 0.7 ± 0.3 |
| 3% Alginate | 52.6 ± 1.2 | 38.9 ± 0.7 | 4.6 ± 0.2 | 1.0 ± 0.3 | 0.4 ± 0.1 | 2.1 ± 0.8 | 0.5 ± 0.4 | |
| 4% Alginate | 48.4 ± 1.1 | 41.8 ± 1.0 | 5.1 ± 0.3 | 0.9 ± 0.3 | 0.5 ± 0.0 | 3.2 ± 1.9 | 0.1 ± 0.2 | |
| 5% Alginate | 45.1 ± 2.5 | 36.3 ± 4.4 | 4.6 ± 1.0 | 0.6 ± 0.6 | 0.4 ± 0.4 | 12.5 ± 9.1 | 0.4 ± 0.7 | |
| 6% Alginate | 34.4 ± 5.5 | 34.5 ± 1.8 | 3.1 ± 1.0 | 1.3 ± 0.2 | 0.3 ± 0.3 | 26.4 ± 8.1 | 0.0 ± 0.0 | |
| 1.5% Agar | 48.5 ± 0.7 | 41.4 ± 1.4 | 5.1 ± 0.4 | 1.3 ± 0.1 | 0.4 ± 0.1 | 2.9 ± 1.2 | 0.4 ± 0.3 | |
| 3% Agar | 48.5 ± 2.2 | 34.6 ± 5.7 | 5.6 ± 0.2 | 1.3 ± 0.2 | 0.3 ± 0.2 | 9.2 ± 2.6 | 0.6 ± 0.6 | |
| 0.75% κ-Carrageenan | 43.8 ± 2.1 | 41.0 ± 2.8 | 4.8 ± 0.3 | 1.0 ± 0.2 | 0.6 ± 0.2 | 8.0 ± 1.1 | 0.8 ± 0.9 | |
| 1.5% κ-Carrageenan | 49.0 ± 0.9 | 38.9 ± 1.0 | 5.4 ± 0.2 | 1.1 ± 0.1 | 0.4 ± 0.0 | 4.5 ± 0.6 | 0.7 ± 0.3 | |
| Glucose | Free biomass | 73.3 ± 2.7 | 18.4 ± 1.4 | 1.7 ± 0.2 | 1.5 ± 0.6 | 0.4 ± 0.0 | 0.2 ± 0.0 | 4.6 ± 1.8 |
| 1.5% Alginate | 69.6 ± 1.6 | 19.1 ± 1.9 | 1.1 ± 0.1 | 2.5 ± 0.3 | 0.4 ± 0.0 | 0.2 ± 0.0 | 7.1 ± 0.5 | |
| 3% Alginate | 72.3 ± 1.9 | 15.0 ± 1.8 | 1.7 ± 0.0 | 2.3 ± 0.4 | 0.3 ± 0.0 | 1.4 ± 0.5 | 7.0 ± 0.4 | |
| 4% Alginate | 72.8 ± 0.8 | 15.0 ± 0.5 | 1.5 ± 0.2 | 2.8 ± 0.3 | 0.3 ± 0.0 | 0.8 ± 0.2 | 6.8 ± 0.4 | |
| 5% Alginate | 70.2 ± 1.5 | 16.0 ± 0.7 | 1.9 ± 0.1 | 2.6 ± 1.0 | 0.3 ± 0.1 | 1.3 ± 0.7 | 7.7 ± 1.1 | |
| 6% Alginate | 72.0 ± 1.8 | 14.7 ± 1.8 | 2.0 ± 0.2 | 2.2 ± 1.7 | 0.2 ± 0.1 | 1.8 ± 1.0 | 7.0 ± 0.7 | |
| 1.5% Agar | 71.0 ± 2.1 | 16.3 ± 0.9 | 1.5 ± 0.2 | 3.0 ± 0.3 | 0.3 ± 0.0 | 0.8 ± 0.2 | 7.1 ± 1.6 | |
| 3% Agar | 73.8 ± 0.9 | 15.6 ± 1.6 | 1.4 ± 0.0 | 2.7 ± 0.5 | 0.3 ± 0.0 | 0.6 ± 0.1 | 5.6 ± 0.4 | |
| 1.5% κ-Carrageenan | 75.7 ± 0.5 | 13.8 ± 0.2 | 1.4 ± 0.1 | 2.9 ± 0.2 | 0.3 ± 0.1 | 0.5 ± 0.1 | 5.3 ± 0.4 | |
| Main Substrate | Immobilization Matrix | Organic Acid [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| Malate | Succinate | Fumarate | Pyruvate | Ketoglutarate | Oxalate | Citrate | ||
| Acetic acid | Free biomass | 23.1 ± 4.0 | 34.1 ± 3.9 | 1.4 ± 0.5 | 37.0 ± 10.5 | 2.1 ± 0.5 | 1.2 ± 0.9 | 1.1 ± 1.6 |
| 3% Alginate | 48.1 ± 2.0 | 35.0 ± 2.1 | 5.1 ± 0.6 | 10.6 ± 0.5 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.0 ± 0.0 | |
| 6% Alginate | 43.6 ± 2.0 | 36.5 ± 1.4 | 5.9 ± 1.3 | 10.6 ± 0.3 | 1.0 ± 0.7 | 2.4 ± 0.3 | 0.0 ± 0.0 | |
| 1.5% κ-Carrageenan | 37.9 ± 0.5 | 33.7 ± 0.0 | 5.1 ± 0.1 | 21.5 ± 0.7 | 1.1 ± 0.1 | 0.7 ± 0.1 | 0.0 ± 0.0 | |
| Glucose | Free biomass | 80.7 ± 1.0 | 15.2 ± 1.5 | 1.6 ± 0.0 | 2.2 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.3 ± 0.4 |
| 1.5% Alginate | 78.4 ± 6.3 | 17.7 ± 5.8 | 1.9 ± 0.1 | 1.2 ± 0.6 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.5 ± 0.8 | |
| 3% Alginate | 82.0 ± 0.6 | 13.7 ± 1.1 | 2.0 ± 0.1 | 1.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.7 ± 0.4 | |
| 1.5% κ-Carrageenan | 75.0 ± 1.9 | 21.1 ± 1.6 | 1.9 ± 0.2 | 1.5 ± 0.5 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.5 | |
| Substrate | Immobilization Matrix | Organic Acid [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| Malate | Succinate | Fumarate | Pyruvate | Ketoglutarate | Oxalate | Citrate | ||
| Acetic acid | 3% Alginate | 13.5 ± 23.4 | 12.3 ± 21.3 | 2.9 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 71.4 ± 44.7 | 0.0 ± 0.0 |
| 6% Alginate | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.9 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 99.1 ± 0.1 | 0.0 ± 0.0 | |
| 1.5% Agar | 56.6 ± 1.3 | 34.0 ± 1.3 | 5.0 ± 0.0 | 1.2 ± 0.1 | 0.6 ± 0.2 | 1.4 ± 0.6 | 1.3 ± 0.4 | |
| 1.5% κ-Carrageenan | 53.0 ± 2.4 | 35.8 ± 1.0 | 5.1 ± 0.2 | 1.2 ± 0.1 | 0.5 ± 0.2 | 2.1 ± 1.0 | 1.3 ± 0.5 | |
References
- Chi, Z.; Wang, Z.-P.; Wang, G.-Y.; Khan, I.; Chi, Z.-M. Microbial biosynthesis and secretion of L-malic acid and its applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kövilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2020, 95, 513–526. [Google Scholar] [CrossRef]
- Zou, X.; Yang, J.; Tian, X.; Guo, M.; Li, Z.; Li, Y. Production of polymalic acid and malic acid from xylose and corncob hydrolysate by a novel Aureobasidium pullulans YJ 6–11 strain. Process Biochem. 2016, 51, 16–23. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, Y.; Lin, M.; Wei, P.; Yang, S.-T. Polymalic acid fermentation by Aureobasidium pullulans for malic acid production from soybean hull and soy molasses: Fermentation kinetics and economic analysis. Bioresour. Technol. 2017, 223, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Kleineberg, W.; Sarikaya, E.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Enhanced malic acid production from glycerol with high-cell density Ustilago trichophora TZ1 cultivations. Biotechnol. Biofuels 2016, 9, 135. [Google Scholar] [CrossRef]
- Iyyappan, J.; Baskar, G.; Bharathiraja, B.; Saravanathamizhan, R. Malic acid production from biodiesel derived crude glycerol using morphologically controlled Aspergillus niger in batch fermentation. Bioresour. Technol. 2018, 269, 393–399. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Malic acid production from thin stillage by Aspergillus species. Biotechnol. Lett. 2011, 33, 2463–2467. [Google Scholar] [CrossRef]
- Kövilein, A.; Umpfenbach, J.; Ochsenreither, K. Acetate as substrate for L-malic acid production with Aspergillus oryzae DSM 1863. Biotechnol. Biofuels 2021, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Yoshimi, A.; Abe, K. The mechanisms of hyphal pellet formation mediated by polysaccharides, α-1,3-glucan and galactosaminogalactan, in Aspergillus species. Fungal Biol. Biotechnol. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Vaija, J.; Linko, Y.Y.; Linko, P. Citric acid production with alginate bead entrapped Aspergillus niger ATCC 9142. Appl. Biochem. Biotechnol. 1982, 7, 51–54. [Google Scholar] [CrossRef]
- Eikmeier, H.; Westmeier, F.; Rehm, H.J. Morphological development of Aspergillus niger immobilized in Ca-alginate and κ-carrageenan. Appl. Microbiol. Biotechnol. 1984, 19, 53–57. [Google Scholar] [CrossRef]
- Eikmeier, H.; Rehm, H.J. Production of citric acid with immobilized Aspergillus niger. Appl. Microbiol. Biotechnol. 1984, 20, 365–370. [Google Scholar] [CrossRef]
- Tsay, S.S.; To, K.Y. Citric acid production using immobilized conidia of Aspergillus niger TMB 2022. Biotechnol. Bioeng. 1987, 29, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Eikmeier, H.; Rehm, H.J. Stability of calcium-alginate during citric acid production of immobilized Aspergillus niger. Appl. Microbiol. Biotechnol. 1987, 26, 105–111. [Google Scholar] [CrossRef]
- Garg, K.; Sharma, C.B. Continuous production of citric acid by immobilized whole cells of Aspergillus niger. J. Gen. Appl. Microbiol. 1992, 38, 605–615. [Google Scholar] [CrossRef][Green Version]
- Bayraktar, E.; Mehmetoglu, Ü. Production of Citric Acid Using Immobilized Conidia of Aspergillus niger. Appl. Biochem. Biotechnol. 2000, 87, 117–126. [Google Scholar] [CrossRef]
- Omar, S.; Honecker, S.; Rehm, H.-J. A comparative study on the formation of citric acid and polyols and on morphological changes of three strains of free and immobilized Aspergillus niger. Appl. Microbiol. Biotechnol. 1992, 36, 518–524. [Google Scholar] [CrossRef]
- Khare, S.K.; Jha, K.; Gandhi, A.P. Use of agarose-entrapped Aspergillus niger cells for the production of citric acid from soy whey. Appl. Microbiol. Biotechnol. 1994, 41, 571–573. [Google Scholar] [CrossRef]
- Horitsu, H.; Adachi, S.; Takahashi, Y.; Kawai, K.; Kawano, Y. Production of citric acid by Aspergillus niger immobilized in polyacrylamide gels. Appl. Microbiol. Biotechnol. 1985, 22, 8–12. [Google Scholar] [CrossRef]
- Horitsu, H.; Takahashi, Y.; Tsuda, J.; Kawai, K.; Kawano, Y. Production of itaconic acid by Aspergillus terreus immobilized in polyacrylamide gels. Appl. Microbiol. Biotechnol. 1983, 18, 358–360. [Google Scholar] [CrossRef]
- Kautola, H.; Vahvaselk, M.; Linko, Y.-Y.; Linko, P. Itaconic acid production by immobilized Aspergillus terreus from xylose and glucose. Biotechnol. Lett. 1985, 7, 167–172. [Google Scholar] [CrossRef]
- Evstatieva, Y.; Yordanova, M.; Chernev, G.; Ruseva, Y.; Nikolova, D. Sol-gel immobilization as a suitable technique for enhancement of α-amylase activity of Aspergillus oryzae PP. Biotechnol. Biotechnol. Equip. 2014, 28, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Nawaz, W. Biotransformation of L-tyrosine to Dopamine by a Calcium Alginate Immobilized Mutant Strain of Aspergillus oryzae. Appl. Biochem. Biotechnol. 2016, 179, 1435–1444. [Google Scholar] [CrossRef]
- Sapna; Singh, B. Free and immobilized Aspergillus oryzae SBS50 producing protease-resistant and thermostable phytase. 3 Biotech 2017, 7, 213. [Google Scholar] [CrossRef]
- Alexandri, M.; Papapostolou, H.; Stragier, L.; Verstraete, W.; Papanikolaou, S.; Koutinas, A.A. Succinic acid production by immobilized cultures using spent sulphite liquor as fermentation medium. Bioresour. Technol. 2017, 238, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Krisch, J.; Szajani, B. Ethanol and acetic acid tolerance in free and immobilized cells of Saccharomyces cerevisiae and Acetobacter aceti. Biotechnol. Lett. 1997, 19, 525–528. [Google Scholar] [CrossRef]
- Hanaki, K.; Hirunmasuwan, S.; Matsuo, T. Protection of methanogenic bacteria from low pH and toxic materials by immobilization using polyvinyl alcohol. Water Res. 1994, 28, 877–885. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Keweloh, H.; Rehm, H.J. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl. Environ. Microbiol. 1991, 57, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Takata, I.; Yamamoto, K.; Tosa, T.; Chibata, I. Immobilization of Brevibacterium flavum with carrageenan and its application for continuous production of L-malic acid. Enzym. Microb. Technol. 1980, 2, 30–36. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tosa, T.; Yamashita, K.; Chibata, I. Continuous production of L-malic acid by immobilized Brevibacterium ammoniagenes cells. Appl. Microbiol. Biotechnol. 1976, 3, 169–183. [Google Scholar] [CrossRef]
- Takata, I.; Tosa, T.; Chibata, I. Stability of fumarase activity of Brevibacterium flavum immobilized with κ-arrageenan and Chinese gallotannin. Appl. Microbiol. Biotechnol. 1984, 19, 85–90. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Costa, A.A.R.; Figueiredo, Z.M.B.; Carvalho, L.B. L-malic acid production by entrapped Saccharomyces cerevisiae into polyacrylamide gel beads. Appl. Biochem. Biotechnol. 1994, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, Z.M.B.; Carvalho, L.B. L-malic acid production using immobilized Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 1991, 30, 217–224. [Google Scholar] [CrossRef]
- Menegatti, T.; Žnidaršič-Plazl, P. Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor. Micromachines 2019, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Barratt, R.W.; Johnson, G.B.; Ogata, W.N. Wild-Type and Mutant Stocks of Aspergillus nidulans. Genetics 1965, 52, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.W.; Kafer, E. Improved protocols for Aspergillus minimal medium: Trace element and minimal medium salt stock solutions. Fungal Genet. Rep. 2001, 48, 20–21. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Gong, J.-S.; Xiong, L.; Lu, Z.-M.; Li, H.; Shi, J.-S.; Xu, Z.-H. Improving the catalytic potential and substrate tolerance of Gibberella intermedia nitrilase by whole-cell immobilization. Bioprocess Biosyst. Eng. 2015, 38, 189–197. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Sun, W.; Fan, S.; Feng, X.; Wang, K.; He, W.; Yang, Z. The whole-cell immobilization of d-hydantoinase-engineered Escherichia coli for d-CpHPG biosynthesis. Electron. J. Biotechnol. 2016, 21, 43–48. [Google Scholar] [CrossRef]
- Idris, A.; Suzana, W. Effect of sodium alginate concentration, bead diameter, initial pH and temperature on lactic acid production from pineapple waste using immobilized Lactobacillus delbrueckii. Process Biochem. 2006, 41, 1117–1123. [Google Scholar] [CrossRef]
- Galaction, A.-I.; Kloetzer, L.; Turnea, M.; Webb, C.; Vlysidis, A.; Caşcaval, D. Succinic acid fermentation in a stationary-basket bioreactor with a packed bed of immobilized Actinobacillus succinogenes: 1. Influence of internal diffusion on substrate mass transfer and consumption rate. J. Ind. Microbiol. Biotechnol. 2012, 39, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Wang, X.-T.; Lou, W.-Y.; Li, Y.; Wu, H.; Zong, M.-H.; Smith, T.J.; Chen, X.-D. Immobilization of Acetobacter sp. CCTCC M209061 for efficient asymmetric reduction of ketones and biocatalyst recycling. Microb. Cell Fact. 2012, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Prabhu, P.; Lee, J.-K. Alginate immobilization of recombinant Escherichia coli whole cells harboring L-arabinose isomerase for L-ribulose production. Bioprocess Biosyst. Eng. 2010, 33, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, W.; Zhang, A.; Cao, X.; Shen, J.; Li, Y.; Chen, K.; Ouyang, P. Enhancing catalytic stability and cadaverine tolerance by whole-cell immobilization and the addition of cell protectant during cadaverine production. Appl. Microbiol. Biotechnol. 2018, 102, 7837–7847. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Bouropoulos, N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef]
- Voo, W.-P.; Ravindra, P.; Tey, B.-T.; Chan, E.-S. Comparison of alginate and pectin based beads for production of poultry probiotic cells. J. Biosci. Bioeng. 2011, 111, 294–299. [Google Scholar] [CrossRef]
- Nordqvist, D.; Vilgis, T.A. Rheological Study of the Gelation Process of Agarose-Based Solutions. Food Biophys. 2011, 6, 450–460. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef] [PubMed]
- Şen, M.; Erboz, E.N. Determination of critical gelation conditions of κ-carrageenan by viscosimetric and FT-IR analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Schreferl-Kunar, G.; Wöhrer, W.; Röhr, M. Evidence for a cytoplasmic pathway of oxalate biosynthesis in Aspergillus niger. Appl. Environ. Microbiol. 1988, 54, 633–637. [Google Scholar] [CrossRef]
- Han, Y.; Joosten, H.-J.; Niu, W.; Zhao, Z.; Mariano, P.S.; McCalman, M.; van Kan, J.; Schaap, P.J.; Dunaway-Mariano, D. Oxaloacetate hydrolase, the C-C bond lyase of oxalate secreting fungi. J. Biol. Chem. 2007, 282, 9581–9590. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Krabben, P. Hyphal growth and fragmentation of Penicillium chrysogenum in submerged cultures. Biotechnol. Bioeng. 1995, 46, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; Rodríguez Porcel, E.M.; Chisti, Y. Pellet morphology, culture rheology and lovastatin production in cultures of Aspergillus terreus. J. Biotechnol. 2005, 116, 61–77. [Google Scholar] [CrossRef]
- Shoji, J.; Arioka, M.; Kitamoto, K. Vacuolar membrane dynamics in the filamentous fungus Aspergillus oryzae. Eukaryot. Cell 2006, 5, 411–421. [Google Scholar] [CrossRef]
- Ohneda, M.; Arioka, M.; Nakajima, H.; Kitamoto, K. Visualization of vacuoles in Aspergillus oryzae by expression of CPY–EGFP. Fungal Genet. Biol. 2002, 37, 29–38. [Google Scholar] [CrossRef]
- Paul, G.C.; Kent, C.A.; Thomas, C.R. Hyphal vocuolation and fragmentation in Penicillium chrysogenum. Biotechnol. Bioeng. 1994, 44, 655–660. [Google Scholar] [CrossRef]
- Papagianni, M.; Mattey, M.; Kristiansen, B. Hyphal vacuolation and fragmentation in batch and fed-batch culture of Aspergillus niger and its relation to citric acid production. Process Biochem. 1999, 35, 359–366. [Google Scholar] [CrossRef]
- Oswald, F.; Dörsam, S.; Veith, N.; Zwick, M.; Neumann, A.; Ochsenreither, K.; Syldatk, C. Sequential Mixed Cultures: From Syngas to Malic Acid. Front. Microbiol. 2016, 7, 891. [Google Scholar] [CrossRef]
- Arikan, E.B.; Isik, Z.; Bouras, H.D.; Dizge, N. Investigation of immobilized filamentous fungi for treatment of real textile industry wastewater using up flow packed bed bioreactor. Bioresour. Technol. Rep. 2019, 7, 100197. [Google Scholar] [CrossRef]
- Kautola, H.; Rymowicz, W.; Linko, Y.-Y.; Linko, P. Itaconic acid production by immobilized Aspergillus terreus with varied metal additions. Appl. Microbiol. Biotechnol. 1991, 35, 154–158. [Google Scholar] [CrossRef]
- Papagianni, M.; Mattey, M. Physiological aspects of free and immobilized Aspergillus niger cultures producing citric acid under various glucose concentrations. Process Biochem. 2004, 39, 1963–1970. [Google Scholar] [CrossRef]
- Papagianni, M.; Joshi, N.; Moo-Young, M. Comparative studies on extracellular protease secretion and glucoamylase production by free and immobilized Aspergillus niger cultures. J. Ind. Microbiol. Biotechnol. 2002, 29, 259–263. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, C.; Chang, H. Citric acid production by Aspergillus niger immobilized on polyurethane foam. Appl. Microbiol. Biotechnol. 1989, 30, 141–143. [Google Scholar] [CrossRef]

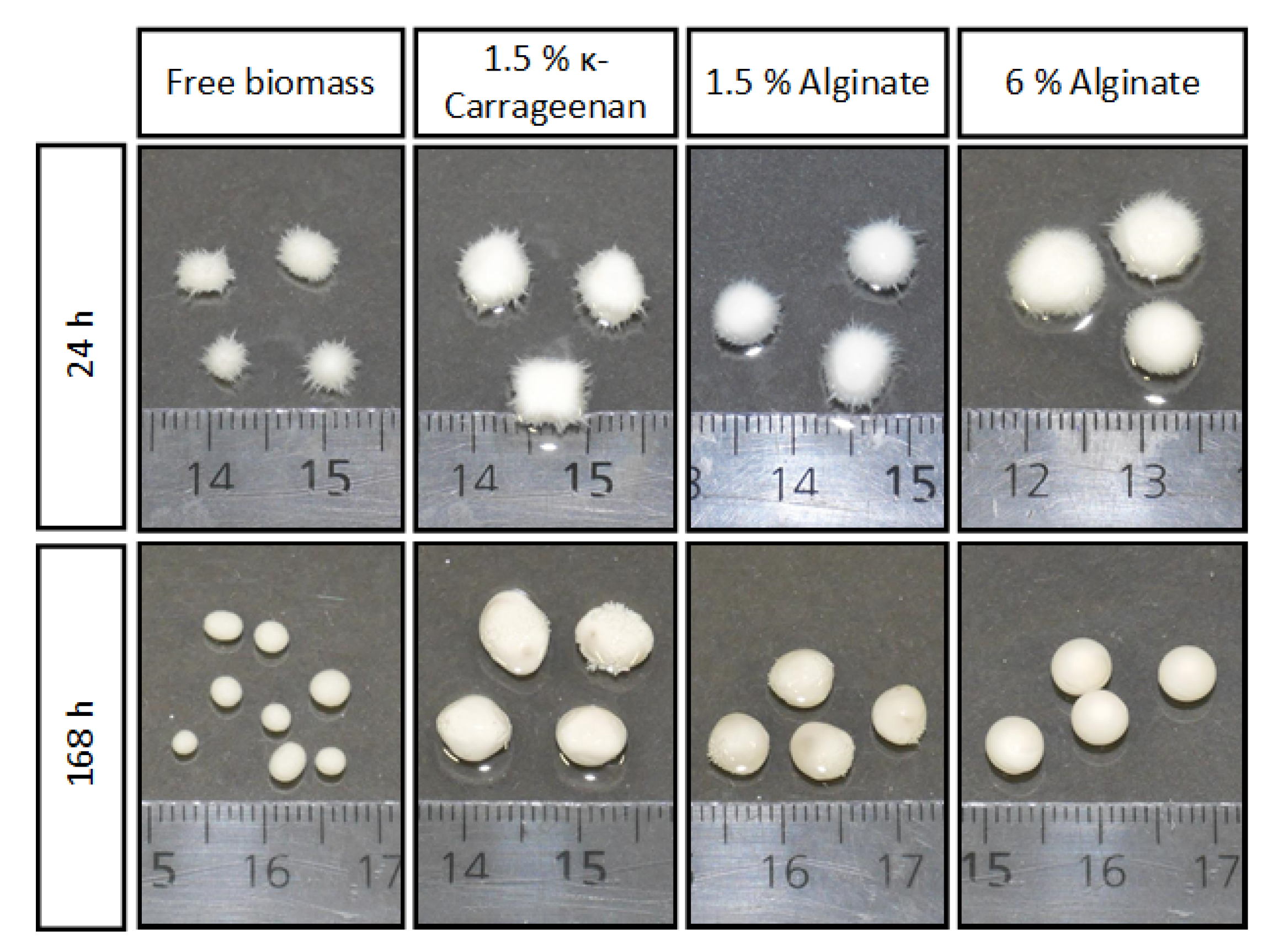

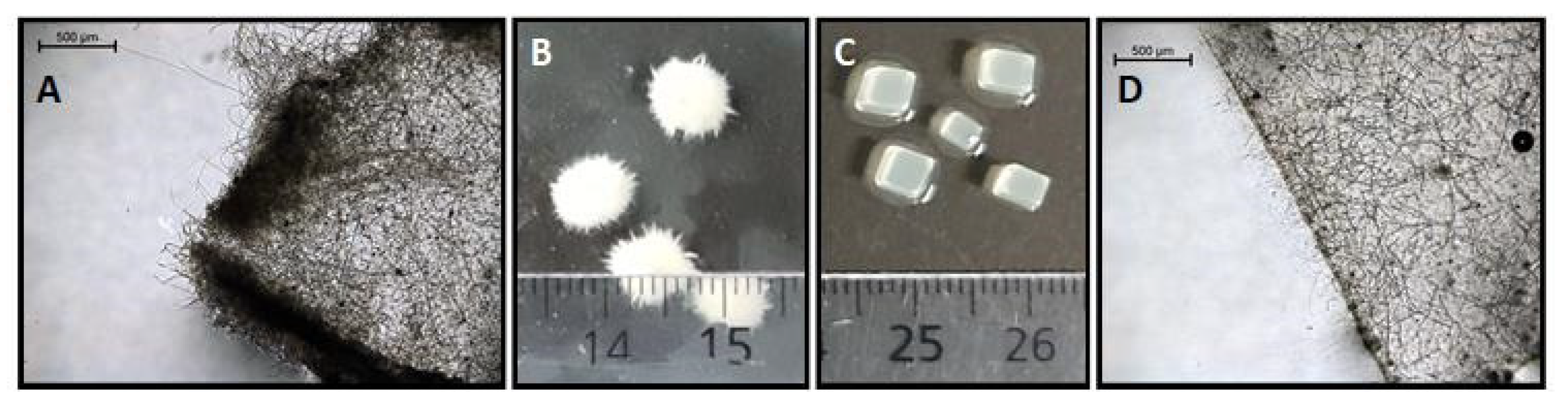


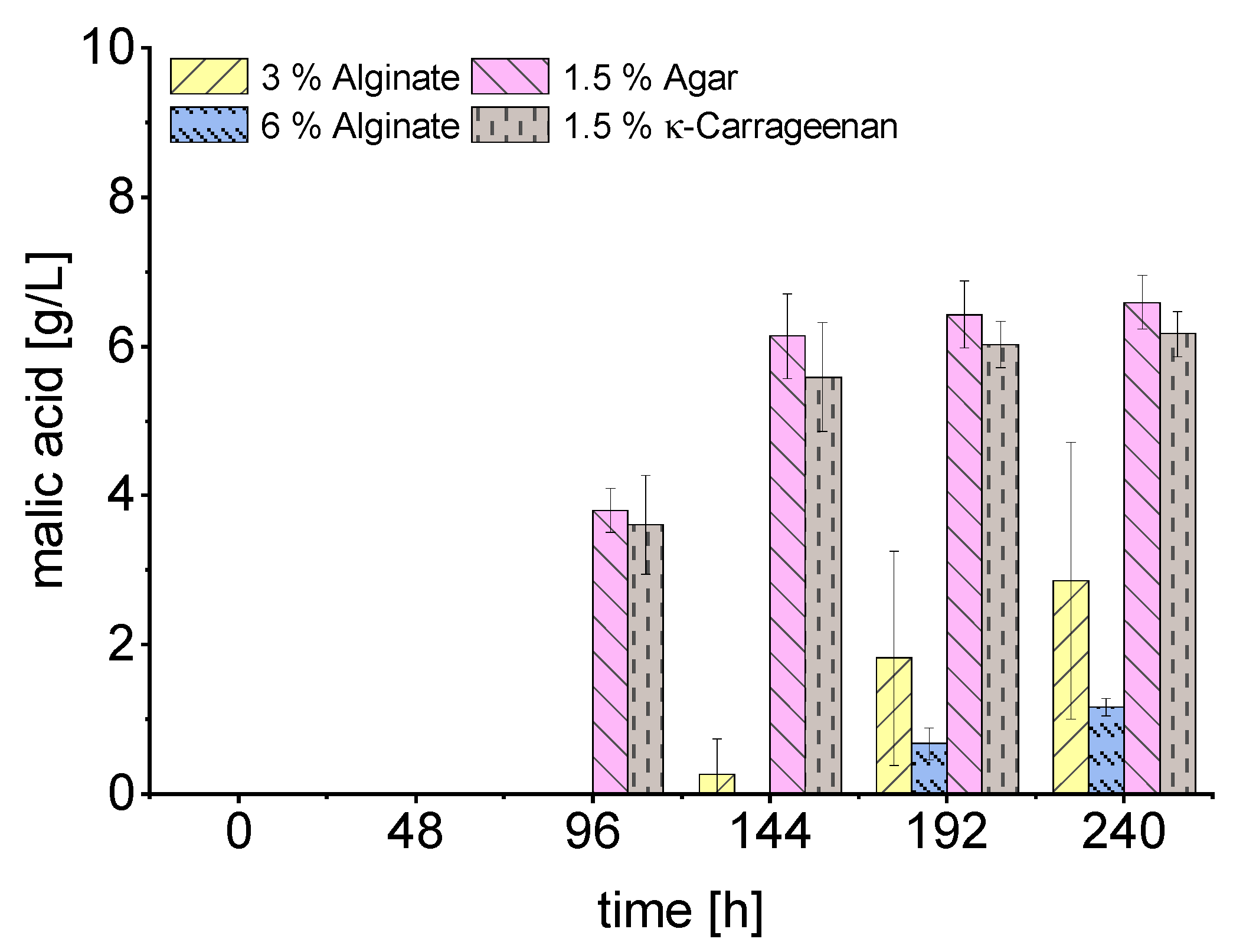
| Substrate | Immobilization Matrix | Consumed Substrate (g/L) | Malic Acid (g/L) | YP/S 1 (g/g) | Productivity (g/L*h) | Total Acids (g/L) |
|---|---|---|---|---|---|---|
| Acetic acid | Free biomass | 38.90 ± 1.09 | 7.96 ± 0.26 | 0.20 ± 0.01 | 0.055 ± 0.002 | 15.69 ± 0.67 |
| 3% Alginate | 32.15 ± 3.27 | 6.34 ± 0.72 | 0.19 ± 0.00 | 0.044 ± 0.005 | 12.03 ± 1.12 | |
| 4% Alginate | 26.91 ± 5.35 | 4.31 ± 1.13 | 0.16 ± 0.02 | 0.030 ± 0.008 | 8.88 ± 2.12 | |
| 5% Alginate | 20.30 ± 3.44 | 2.40 ± 0.82 | 0.12 ± 0.02 | 0.017 ± 0.006 | 5.26 ± 1.59 | |
| 6% Alginate | 16.56 ± 1.73 | 1.30 ± 0.43 | 0.08 ± 0.02 | 0.009 ± 0.003 | 3.71 ± 0.61 | |
| 1.5% Agar | 30.71 ± 1.28 | 5.53 ± 0.52 | 0.18 ± 0.01 | 0.038 ± 0.004 | 11.40 ± 1.00 | |
| 3% Agar | 25.72 ± 0.66 | 3.68 ± 0.21 | 0.14 ± 0.00 | 0.026 ± 0.001 | 7.62 ± 0.74 | |
| 0.75% κ-Carrageenan | 23.71 ± 2.50 | 2.98 ± 0.34 | 0.13 ± 0.02 | 0.021 ± 0.002 | 6.80 ± 0.48 | |
| 1.5% κ-Carrageenan | 28.92 ± 0.99 | 4.72 ± 0.39 | 0.16 ± 0.02 | 0.033 ± 0.003 | 9.64 ± 0.84 | |
| Glucose | Free biomass | 48.99 ± 0.97 | 28.69 ± 1.98 | 0.59 ± 0.05 | 0.199 ± 0.014 | 39.08 ± 1.26 |
| 1.5% Alginate | 69.80 ± 1.06 | 31.04 ± 1.95 | 0.45 ± 0.03 | 0.216 ± 0.014 | 44.63 ± 3.10 | |
| 3% Alginate | 64.96 ± 2.20 | 29.05 ± 1.03 | 0.45 ± 0.03 | 0.202 ± 0.007 | 40.18 ± 0.36 | |
| 4% Alginate | 66.81 ± 1.53 | 30.08 ± 1.66 | 0.45 ± 0.03 | 0.209 ± 0.012 | 41.31 ± 1.85 | |
| 5% Alginate | 64.83 ± 2.98 | 25.08 ± 2.40 | 0.39 ± 0.04 | 0.174 ± 0.017 | 35.68 ± 2.74 | |
| 6% Alginate | 59.29 ± 6.96 | 23.92 ± 4.30 | 0.40 ± 0.03 | 0.166 ± 0.030 | 33.34 ± 6.81 | |
| 1.5% Agar | 65.96 ± 2.43 | 28.96 ± 2.74 | 0.44 ± 0.05 | 0.201 ± 0.019 | 40.75 ± 2.76 | |
| 3% Agar | 64.96 ± 0.64 | 31.16 ± 0.63 | 0.48 ± 0.01 | 0.216 ± 0.004 | 42.21 ± 0.37 | |
| 1.5% κ-Carrageenan | 61.62 ± 1.63 | 31.63 ± 1.74 | 0.51 ± 0.02 | 0.220 ± 0.012 | 41.81 ± 2.41 |
| Main Substrate | Immobilization Matrix | Consumed Substrate 1 (g/L) | Malic Acid (g/L) | YP/S 2 (g/g) | Productivity (g/L*h) | Total Acids (g/L) |
|---|---|---|---|---|---|---|
| Acetic acid | Free biomass | HAc: 6.68 ± 4.91 Glc: 4.91 ± 0.54 | 0.73 ± 0.27 | 0.06 ± 0.00 | 0.005 ± 0.002 | 3.13 ± 0.64 |
| 3% Alginate | HAc: 7.38 ± 0.29 Glc: 4.90 ± 0.03 | 2.60 ± 0.14 | 0.21 ± 0.02 | 0.018 ± 0.001 | 5.39 ± 0.07 | |
| 6% Alginate | HAc: 7.91 ± 0.76 Glc: 5.04 ± 0.02 | 1.70 ± 0.05 | 0.13 ± 0.01 | 0.012 ± 0.000 | 3.91 ± 0.06 | |
| 1.5% κ-Carrageenan | HAc: 6.82 ± 0.55 Glc: 4.67 ± 0.05 | 1.60 ± 0.09 | 0.14 ± 0.00 | 0.011 ± 0.001 | 4.23 ± 0.19 | |
| Glucose | Free biomass | 39.09 ± 1.65 | 29.77 ± 2.70 | 0.76 ± 0.10 | 0.207 ± 0.019 | 36.91 ± 3.81 |
| 1.5% Alginate | 35.26 ± 1.36 | 25.23 ± 3.13 | 0.72 ± 0.12 | 0.175 ± 0.022 | 32.12 ± 1.42 | |
| 3% Alginate | 35.35 ± 2.90 | 27.62 ± 0.05 | 0.78 ± 0.06 | 0.192 ± 0.000 | 33.66 ± 0.30 | |
| 1.5% κ-Carrageenan | 42.40 ± 0.99 | 23.61 ± 4.68 | 0.56 ± 0.12 | 0.164 ± 0.033 | 31.42 ± 5.45 |
| Substrate | Immobilization Matrix | Consumed Substrate (g/L) | Malic Acid (g/L) | YP/S 1 (g/g) | Productivity (g/L*h) | Total Acids (g/L) |
|---|---|---|---|---|---|---|
| Acetic acid | 3% Alginate | 15.46 ± 1.25 | 0.27 ± 0.47 | 0.02 ± 0.03 | 0.002 ± 0.003 | 1.33 ± 0.59 |
| 6% Alginate | 15.37 ± 0.88 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.000 ± 0.000 | 1.06 ± 0.13 | |
| 1.5% Agar | 34.28 ± 1.67 | 6.14 ± 0.57 | 0.18 ± 0.01 | 0.043 ± 0.004 | 10.85 ± 0.94 | |
| 1.5% κ-Carrageenan | 32.04 ± 1.04 | 5.59 ± 0.72 | 0.17 ± 0.02 | 0.039 ± 0.005 | 10.51 ± 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kövilein, A.; Aschmann, V.; Hohmann, S.; Ochsenreither, K. Immobilization of Aspergillus oryzae DSM 1863 for l-Malic Acid Production. Fermentation 2022, 8, 26. https://doi.org/10.3390/fermentation8010026
Kövilein A, Aschmann V, Hohmann S, Ochsenreither K. Immobilization of Aspergillus oryzae DSM 1863 for l-Malic Acid Production. Fermentation. 2022; 8(1):26. https://doi.org/10.3390/fermentation8010026
Chicago/Turabian StyleKövilein, Aline, Vera Aschmann, Silja Hohmann, and Katrin Ochsenreither. 2022. "Immobilization of Aspergillus oryzae DSM 1863 for l-Malic Acid Production" Fermentation 8, no. 1: 26. https://doi.org/10.3390/fermentation8010026
APA StyleKövilein, A., Aschmann, V., Hohmann, S., & Ochsenreither, K. (2022). Immobilization of Aspergillus oryzae DSM 1863 for l-Malic Acid Production. Fermentation, 8(1), 26. https://doi.org/10.3390/fermentation8010026






