Abstract
Procyanidins are bioactive molecules with industrial and pharmaceutical relevance, they are present in recalcitrant agro-industrial wastes that are difficult to degrade. In this study, we evaluated the potential consumption of procyanidins from Aspergillus niger and Trichoderma harzianum strains in submerged fermentations. For this purpose, a culture medium containing salts, glucose, and procyanidins was formulated, where procyanidins were added to the medium after the near-total consumption of glucose. The submerged cultures were carried out in amber flasks at 30 °C and 120 rpm. The addition of procyanidins to the culture medium increased the formation of micellar biomass for all the strains used. The use of glucose affected the growth of A. niger GH1 and A. niger HS1, however, in these assays, a total consumption of procyanidins was obtained. These results show that the consumption of procyanidins by fungal strains in submerged fermentations was influenced by the pH, the use of glucose as the first source of carbon, and the delayed addition of procyanidins to the medium. The study showed that A. niger and T. harzianum strains can be used as a natural strategy for the consumption or removal of procyanidins present in recalcitrant residues of risk to the environment and human health.
1. Introduction
Procyanidins (PC) are antioxidant molecules of importance to human health and have been grouped into the condensed tannins group [1,2]. These compounds can be classified according to the number of monomeric molecules present in their structure, which can be catechins or epicatechins. In other words, when there are between one and ten monomeric molecules in the procyanidin structure, they are called oligomeric, and when there are more than 10 monomeric molecules, they are called polymeric. Research has reported that as the number of monomers in the structure increases the degree of polymerization increases, as well as its complexity and degradation [3,4,5]. These structural characteristics are determinant in the digestibility of these compounds, availability, and biological activity [6,7,8].
The occurrence of procyanidins in fruits and vegetables has stimulated the development of natural products for the prevention and treatment of chronic and degenerative diseases [9,10,11]. Recently, other platforms for their use have emerged, among them the use of agro-industrial residues with a high organic load. The coffee industry represents a target for the extraction of compounds with biological potential. One of the main residues generated during fermentation is coffee pulp. It contains high concentrations of carbohydrates (35%), and lignin (31.5%), and lower amounts of protein (10.8%). Currently, the large quantities of coffee pulp resulting from the fermentation process constitute an environmental and social problem, due to the recalcitrant characteristics of these residues, conferred by their components [12,13,14]. The chemical structure and properties of procyanidins are still under discussion, given that they have the capacity to interact with proteins, enzymes, and carbohydrates, and can also polymerize to molecules of greater complexity according to the plant matrix used. These features make procyanidins diverse molecules, of low degradation, and capable of inhibiting microbial growth. Therefore, exploring strategies for obtaining them is relevant and necessary in the discovery of new molecules of industrial and pharmaceutical interest [15,16,17,18].
In this sense, the degradation of highly polluting wastes represents a biotechnological challenge, since the industrial scale-up of these bioprocesses requires addressing some problems regarding the stability of fungal growth under non-sterile conditions. It is known that bacteria could inhibit the growth of fungal strains, and therefore the enzymes involved in the degradation processes [19,20]. In industrial applications, the growth rate of fungi is lower than that reported for bacteria, therefore, in submerged fermentations, it is necessary to favor fungal growth by adding substrates that allow optimal growth for the expression of enzymes involved in degradation processes of recalcitrant and toxic compounds [21,22,23].
The most economical and environmentally friendly way to assess the degradation of procyanidins is by fungal fermentation. Some fungal strains possess the ability to degrade procyanidins, including Penicillium, Trichoderma, and Aspergillus species [24,25,26,27,28]. Previously, authors evaluated the degradation of procyanidins extracted from coffee pulp by submerged fermentation with Aspergillus fumigatus using a basic medium consisting of salts and glucose (2 g/L). The possible pathways of degradation by the action of the enzyme dioxygenase were also reported [29]. Roopesh et al. [30] report enzymatic mechanisms interfering in the biotransformation of procyanidins in solid fermentations with A. fumigatus using a mineral medium with glucose (2 g/L). Wong-Paz et al. [31] extracted, purified, and characterized procyanidins from coffee pulp, and studied the oxidation of these compounds and possible structural modifications. Despite the advances, the literature still does not report studies on the consumption of procyanidins using fungal strains generally recognized as safe (GRAS), and the information on this subject is still unclear; various terms have been used to refer to the decrease in procyanidins in fermentations, including “disappearance”, “elimination”, “depletion”, “consumption” and “biotransformation”.
In this research, four strains of A. niger (03, PSH, GH1, and HS1) and one T. harzianum were selected for their ability to degrade condensed tannins and some characteristics reported in submerged fermentations, such as preference for acidic environments, natural growth at water–air interfaces, short growth periods (maximum 7 days), with the purpose of making the results obtained more useful and reproducible for future industrial applications [22,28]. To the best of our knowledge, the scientific literature on the microbial consumption of procyanidins from coffee pulp is limited [30]. The reported studies on the consumption of these compounds with Aspergillus strains in submerged fermentations do not sufficiently address the influence of pH, microbial growth, glucose consumption, and addition of procyanidins in the stationary phase of fungal growth. In this study, the concentrations of mycelial biomass, glucose, and procyanidins were determined. All tests were conducted in presence of glucose as the co-substrate for fungal biomass growth. It is known that growth is better in culture media with glucose than without glucose [32,33]. Therefore, it could be hypothesized that a good fungal growth would allow the consumption of procyanidins in the submerged culture.
According to the above, this research evaluated the potential of A. niger and T. harzianum in the consumption of coffee pulp procyanidins through submerged fermentations using glucose and procyanidins as carbon sources, being the procyanidin added once the microorganism has almost completely consumed the glucose.
2. Materials and Methods
2.1. Media, Chemicals and Vegetal Material
Fungi growth medium Potato dextrose agar (PDA) was obtained from Dibico (Cuautitlan Izcalli, Mexico). Solvents used were acetone, ethanol, and hexane, all reactive grade from Jalmek. Sephadex LH 20 was acquired from GE Healthcare. Salts and surfactant agent Tween® 80 were of analytical grade purchased from Jalmek (Nuevo León, Mexico). The glucose was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Coffee pulp was provided by farmers from San Pedro in Xilitla (SLP, Mexico) in February 2020. The pulp underwent natural drying for three days before being transported to the Food Analysis Laboratory of the Instituto Tecnológico de Ciudad Valles, (SLP, Mexico) The coffee pulp was stored at room temperature in dark conditions in sealable plastic bags [31]. Procyanidins were extracted from coffee pulp with 70% acetone in the Food Analysis laboratory of the Tecnológico Nacional de México, Ciudad Valles, México [31].
2.2. Fungal Strains
The A. niger fungal strains 03, PSH, GH1, and HS1 were provided by Food Research Department of UAdeC, Saltillo, Coahuila, México. A T. harzianum strain provided by the Food Analysis Laboratory of the Instituto Tecnológico de Ciudad Valles, México was also used. These strains were selected for their potential of degrading or biotransforming procyanidins. The microorganisms were stored in a freezer at −50 °C in a solution of milk and glycerol (20%) for preservation. The fungal strains were activated on PDA for 7 days at 30 °C, the time at which the spores were harvested with 0.1% Tween 80, adjusted the concentration to 5 × 107 spores/mL.
2.3. Preparation of Inoculum and Medium
The spore solution was prepared with a sterile Tween 80 solution (0.1%), which was added to the culture of each A. niger strain on PDA, previously incubated for 7 days under aseptic conditions. The fermentation medium was composed of a basic nutrient solution, glucose (10 g/L), and PCs (1 g/L).
Procyanidins were added aseptically once the glucose was almost completely consumed by the fungal strain, the medium was named WPC. The conidial suspension was used at a concentration of 5 × 107 spores per gram of carbon source (glucose and PCs), which was transferred to 10 mL to the basic sterile nutrient solution prepared with distilled water (g/L): NaNO3, 6.0; KCl, 0.5; KH2PO4, 1.5; MgSO4·7H2O, 0.5; FeCl3, 0.0085; ZnSO4, 0.001; and 1 mL of oligo-element solution (g/L): Na2B4O7·10H2O, 0.1; Na2MoO4·2H2O, 0.05; MnCl2·4H2O, 0.05; CuSO4·5H2O, 0.25. Before sterilizing the basal medium (basic solution and glucose), the pH was adjusted to 6 with 1 M NaOH.
Two controls were used: (1) culture medium without the addition of procyanidins WTPC to monitor the behavior of the microorganism; (2) culture medium without inoculation of the fungal strain to monitor the oxidation of procyanidins.
2.4. Submerged Fermentation
Submerged fermentations were conducted to explore the consumption of procyanidin using fungi strains. Submerged experiments were carried out on an incubator orbital shaker at 120 rpm and 30 °C in amber glass bottles with a capacity and working volume of 30 and 10 mL, respectively. The experiments were incubated aerobically between 168 and 192 h. Samples were collected every 24 h in Eppendorf tubes for subsequent use in the determination of mycelial biomass, total sugars, and procyanidins as per the methods described below. The controls corresponded to liquid culture prepared with basic nutrient solution and glucose, without the addition of procyanidins. The experiments were performed in triplicate.
2.5. Biomass Growth and pH
The culture liquid was separated from the mycelium by filtration using a vacuum pump and filter paper (Whatman N°. 1); the mycelium was dried in an oven at 60 °C until constant weight according to AOAC standard. Mycelial biomass concentration was determined as grams of dry weight per liter of liquid culture (g/L). During the fermentation period, 5 mL samples were collected, and subsequently the pH was measured with a pH meter (Oakton, Vernon Hills, IL, USA).
2.6. Glucose and Procyanidin Quantification
Samples of 5 mL collected each day of fermentation were centrifuged at 14,000× g for 15 min. The supernatant obtained was separated from the residual biomass at 4 °C. Glucose concentrations were determined by anthrone sulfuric acid [34] and 3,5-dinitrosalicylic acid (DNS) [35] methods. In the case of the anthrone method, the anthrone reagent was prepared with 200 mg of anthrone dissolved in 100 mL of concentrated sulfuric acid. After, 500 uL of the sample was boiled at 80 °C for 15 min, then it was placed into an ice bath for 10 min. Finally, the sample was cooled down for 1° min to determine the absorbance at 530 nm. The absorbance readings were compared with a standard curve, using glucose as standard.
For the DNS method, a fermentation sample was reacted with 1 mL of DNS reagent, which consisted of 1% DNS, 30% sodium potassium tartrate, and 2% NaOH. The resulting mixture was subjected to boiling at 100 °C for 5 min, and it was placed into an ice bath for 5 min. Finally, the sample was cooled to room temperature and then dissolved with distilled water. The absorbance was recorded at 540 nm. For both sugar content determinations, glucose was used as the standard.
The Procyanidin concentrations of the liquid culture were monitored every 24 h according to the methodology of Porter et al. [36]. In glass tubes 250 uL of supernatant were reacted with 1.5 mL of HCl-butanol (5%, v/v) and 50 μL of ferric reagent NH4Fe(SO4) previously prepared in 2 M HCl (2% w/v). The supernatants were then dissolved and subjected to heating at 95 °C for 40 min. The samples were allowed to cool and then absorbances were measured at 550 nm using a spectrophotometer. Procyanidin concentrations were calculated by comparison with a standard curve performed with procyanidin C1. Calculations were expressed in mg/L.
2.7. Kinetic Parameter Estimation
The kinetic parameters: specific growth rate μ (h−1) and maximum biomass Xmax were estimated using non-linear regression, fitting the data in Matlab 2015 software using the Verhulst-Pearl logistic model, where Xmax is the maximum biomass produced. Statistical analyses were performed by analysis of variance (ANOVA) with a confidence level of 95%.
The experimental data of micellar biomass production over time were fitted into the logistic model. The logistic model used in this study is presented in Equation (1).
Equation (2) relates cell growth (X), maximum biomass (Xmax), and Xo at times t and t→∞, and t = 0, respectively; specific growth rate (μ) is also indicated [37]. This equation allows comparing the experimental data with theoretical values.
The statistical accuracy of the model for all strains was tested through the determination coefficients (R2). This value is a measure between 0 and 1. Other parameters were calculated using the experimental data such as productivity P, yield Yx/s, and substrate consumption rate qs. The definition of experimental and calculated kinetic parameters are summarized in Table 1.

Table 1.
Definition of kinetic parameters for liquid fermentation according to Aranda et al. [38].
3. Results and Discussion
3.1. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger 03
The microorganisms were initially grown in the absence of procyanidins and in 10 g/L of glucose as the sole carbon source. Once the microorganism had almost completely consumed the glucose, procyanidins were added. Variation in glucose concentrations, changes in micellar biomass and procyanidin concentrations, a kinetic model for micellar biomass production, and evolution of pH of A. niger 03 vs. time are given in Figure 1a–d, respectively. To determine whether procyanidins had an inhibitory effect on the growth of the microorganism, the experiments included a control treatment named medium without procyanidin (WTPC). For this purpose, a comparison of growth kinetics with (WPC) and without (WTPC) procyanidins in the medium was performed for all experiments, as well as pH monitoring. A. niger 03 grew exponentially in WTPC medium for up to 48 h and kept stable until the end. The climb in biomass concentration was due to the drastic decrease in the initial glucose concentration to 92.5% (Figure 1a,b), i.e., the addition of procyanidins to the culture medium took place when the medium contained 2.9% residual sugar (reducing sugar). This residue is determinant of the consumption of procyanidins, because they have hydroxyl groups in their structure that make them capable of linking with other molecules, giving rise to more complex molecules [39,40]. Therefore, in this study, it is not only relevant that the tested microorganisms grow in a medium with procyanidins as a second carbon source, but also that they have the potential to disappear almost completely, allowing the degradation of molecules of smaller molecular size.
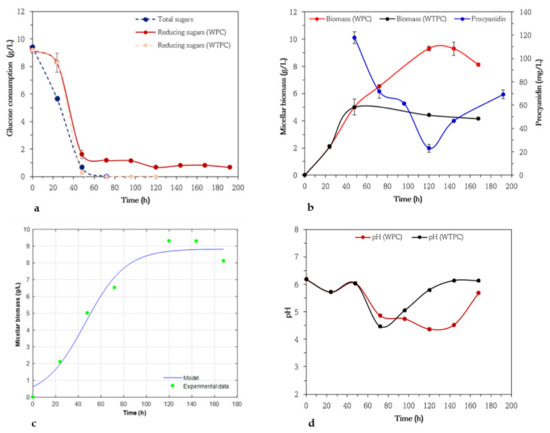
Figure 1.
Variation of glucose concentrations by anthrone and 3,5 dinitrosalicylic acid (DNS) methods (a), micellar biomass in culture medium with (WPC) and without addition of procyanidins (WTPC) when glucose was below 3.0 g/L (stationary phase), and changes in procyanidin concentrations (b) in submerged cultures of Aspergillus niger 03. Micellar biomass production by Aspergillus niger 03 in submerged cultures using basic medium of salts and glucose (10 g/L) and with addition of procyanidin when glucose was below 3.0 g/L (stationary phase). Symbols represent experimental data. Solid lines represent calculated data (c), Evolution of pH in submerged cultures of Aspergillus niger 03 in medium with (WPC) and without (WTPC) addition of procyanidins when glucose was below 3.0 g/L (stationary phase) (d).
In absence of procyanidins, the maximum biomass concentration of A. niger 03 was 5 g/L obtained at 48 h. With the addition of procyanidin, an increase in growth was observed, reaching a maximum biomass concentration of 9.3 g/L at 120 h. For A. niger 03 the growth data fit the regression model with a correlation coefficient (R2) of 0.970 (Figure 1c), whereby a specific growth rate and maximum biomass concentration of 0.059 h−1 and 8.83 g/L, respectively, were obtained. These results indicate that the logistic model fits the experimental data well. The addition of procyanidins to the medium had a stimulatory effect on mycelial growth with an increase of almost 50% when comparing the mycelial biomass of the control (4.4 g/L) and the procyanidin assay at hour 120 (highest biomass value). Some studies have explored the use of procyanidins and glucose as substrates in fermentations, which have been added from the beginning of fermentation to the culture medium. Wong-Paz et al. [31] reported that after 120 h, the degradation of procyanidins by A. niger 03 in submerged fermentations was 72%, using procyanidins as the sole carbon source at an initial concentration of 1 g/L. Contreras-Dominguez et al. [29] reported that A. fumigatus was able to degrade over 30% of procyanidins in liquid fermentations using glucose and procyanidins as carbon sources at an initial concentration of 2 g/L for both substrates.
The high concentrations of mycelial biomass in the WPC medium coincide with the highest levels of procyanidin consumption, where at 72 and 120 h there was a rapid consumption with percentages corresponding to 40 and 80%, respectively. After 12 h of fermentation, an increasing trend in the concentration of procyanidins was observed as shown in Figure 1b. These results may be since the microorganism is assimilating the procyanidins to metabolize them and stimulate the formation of cell biomass, i.e., the substrate is consumed for fungal growth. Another possible reason is the consumption or breakdown of procyanidins into monomers such as catechins or epicatechins [41]. Authors have cited the biotransformation of procyanidins into more complex polymeric molecules as another phenomenon related to these trends. Contreras-Dominguez et al. [29] reported that as fermentation with A. niger occurs, the degree of polymerization of procyanidins increases and therefore their structural complexity, favoring the formation of polymeric compounds, which are less biodegradable by species such as A. niger, compared to monomers or oligomers. In this study, they reported that once the degree of polymerization increases, the fungus enters its stationary phase of growth, attributed to the fact that the carbon source is less assimilable if compared to the degree of polymerization of the substrate at the beginning of fermentation.
The stationary phase was observed between 120th and 144th h after the biomass decreased rapidly due to the negative effect, and at the end of fermentation, a slight increase was observed. As shown in Figure 1d, the most relevant pH changes occur after the addition of procyanidin, with the most significant decrease in pH occurring between 48th h and 120th h (from 6.04 to 4.37). This decrease in the pH may be due to the production of metabolites derived from glucose consumption. These types of fungal strains can produce secondary metabolites such as organic acids and enzymes. Some enzymes such as son las cellulases, tannases, laccases, amylases, pectinases, proteases, amylases, xylanase, allow hydrolysis processes of complex compounds to monomer molecules [42,43,44].
3.2. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger GH1
The findings show decreasing procyanidin concentrations and comparisons with micro-organism growth, medium pH, and glucose consumption. Regarding the variations in glucose concentrations, changes in micellar biomass and procyanidin concentrations, a kinetic model for micellar biomass production, and evolution of pH in submerged cultures with A. niger GH1 are observed in Figure 2a–d.
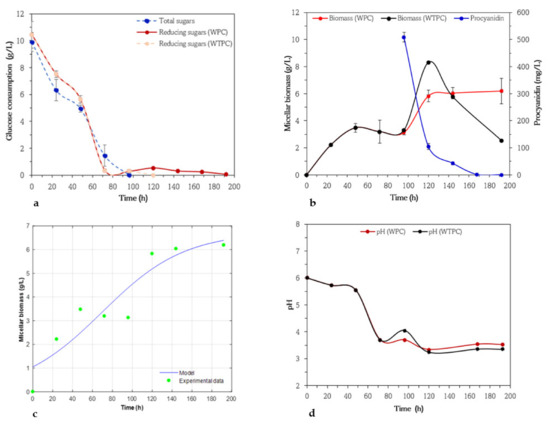
Figure 2.
Variation of glucose concentrations by anthrone and 3,5 dinitrosalicylic acid (DNS) methods (a), micellar biomass in culture medium with (WPC) and without addition of procyanidins (WTPC) when glucose was below 3.0 g/L (stationary phase), and changes in procyanidin concentrations (b) in submerged cultures of Aspergillus niger GH1. Micellar biomass production by Aspergillus niger GH1 in submerged cultures using basic medium of salts and glucose (10 g/L) and with addition of procyanidin when glucose was below 3.0 g/L (stationary phase). Symbols represent experimental data. Solid lines represent calculated data (c), Evolution of pH in submerged cultures of Aspergillus niger GH1 in medium with (WPC) and without (WTPC) addition of procyanidins when glucose was below 3.0 g/L (stationary phase) (d).
The results of the submerged fermentation using A. niger GH1 without procyanidins are shown in Figure 2a. It can be observed that the microorganism reached its maximum micellar biomass (3.5 g/L) after 48 h when half of the glucose had been consumed, confirming the results in both methods of measurement (total and reducing sugars). The remaining glucose fraction was consumed in the fungal stationary phase. These results indicate that glucose is not only used by the fungus for growth or respiration [45]. Once the glucose was almost completely consumed the fungal strain started to increase again the formation of micellar biomass up to 8.3 g/L at 120 h. These results indicate that using glucose at 10 g/L could favor the growth of A. niger GH1 at the onset of fermentation. However, as glucose concentrations decreased to 2 g/L (reducing sugars), the micellar biomass increased, and once the microorganism consumed all the substrate (reducing sugars) the micellar biomass dropped sharply between 120 and 192 h. In other words, concentrations above 3 g/L of glucose favor fungal growth and higher concentrations (10 g/L) inhibit its growth.
The addition of procyanidins to a basic medium led to a decrease in biomass micellar of A. niger GH1 with respect to the control medium (WTPC) between 96 and 120 h (Figure 2b), then these values became constant reaching concentrations of 6.19 g/L. In the last 48 h of incubation, the biomass was higher than that obtained in the control medium (WTPC). In this study, the maximum biomass and specific growth rate were recorded at 6.71 g/L and 0.039 h−1. Figure 2c presents the calculated data of fungal growth during a fermentation time, as well as the experimental values, where a correlation coefficient (R2) of 0.81 can be reached. This indicates that the logistic model does not fit the experimental data well. Therefore, it is necessary to review other factors that interfere with the growth of the microorganism.
The highest consumption of procyanidins (79%) occurs in the first 24 h after their addition, which coincides with increasing biomass production. During the total consumption of procyanidins the pH of the medium oscillated between 3.7 and 3.5 (Figure 2d). These values were close to those recorded in the control medium (4.1–3.4). At the beginning of the experiment, the pH gradually decreased from 6.0 to 3.7, at which stage the glucose had been mostly consumed. The greatest pH decrease occurs during the stationary growth phase (before the addition of procyanidins), probably due to the production of organic acids [46]. After the addition of procyanidins, the pH decreases slightly and then remained constant from 120 h. This observation can be correlated with the decrease and stability of the micellar biomass in the WPC and WTPC media, respectively.
In the micellar growth curves of A. niger GH1, two stationary phases were observed, one when 50% of the glucose was consumed and the second when 79% of the procyanidins disappeared. The decrease in micellar biomass in the WPC medium was presumably caused by the toxic effect of procyanidins. Leontopoulos et al. [47] and Roukas and Kotzekidou [48] report that phenolic compounds at high concentrations suppress the growth of A. niger species. Anttila et al. [49] attribute the inhibition of fungal growth due to high concentrations of tannins, which can form bonds with proteins and carbohydrates that are difficult to hydrolyze.
Our results showed that A. niger GH1 could grow in culture media supplemented with procyanidins in the stationary phase of fungal growth. Other authors have reported that A. niger GH1 is able to grow and degrade hydrolysable tannins, which is attributed to the ability of the fungus to produce tannin-degrading enzymes during submerged fermentations [50,51]. A previous study reports that the decrease in tannin concentrations in fungal fermentations is because of the carbon source and interactions of these compounds with proteins [52]. With respect to the consumption of condensed tannins, there are few reports on this subject, among them the reports of Contreras-Domínguez et al. [29] and Roopesh et al. [30] who describe possible reactions resulting in the degradation of procyanidins by the action of the enzyme dioxygenase in fermentations with A. fumigatus. However, this mechanism is not fully understood.
3.3. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger HS1
The effect of adding procyanidins to the culture medium (WPC) were assessed in fermentations with A. niger HS1, as well as the control test (WTPC), whose results are shown in Figure 3. Initially, in assays without procyanidins, fungal growth was favored by the presence of 10 g/L in the medium, reaching a maximum biomass of 7.32 g/L. Growth was rapid during the first 48 h of fermentation and reached a stationary phase at 50 h. In addition, the micellar biomass decreased after 72 h to 4.28 g/L, which was consistent with previous studies, where they correlate this with substrate consumption [53]. Alarid-García et al. [54] reported maximum biomass concentrations after 24 and 48 h of fermentation when using glucose and sucrose as carbon sources, respectively. Reginatto et al. [33] recorded maximum biomass concentrations after 64 and 41 h of fermentation using wheat bran in the presence and absence of glucose. During the growth phase, glucose was consumed over 77% of glucose by the fungus. The remaining glucose fraction was completely consumed in the stationary phase after 72 h of fermentation with a residual glucose amount of 2.7 g/L (reducing sugar) (Figure 3a). Then, procyanidins were added. It is important to note that the anthrone technique did not record glucose levels for the same period. In assays with procyanidins as substrate, the mycelial biomass increased smoothly between 96 and 144 h from 7.13 to 7.69 g/L. Procyanidins were completely disappeared at 168 h of fermentation. The microorganism utilized 61% of procyanidins when it had reached its stationary phase (Figure 3b). The initial pH values in culture were 6. The pH values decreased to 3.6 after 72 h of incubation (WTPC), attributed to the ability of these microorganisms to produce organic acids as a result of substrate consumption, in this case, glucose. In fermentations with A. niger, a bioconversion of carbon up to 95% into organic acids can be achieved [55]. At the end of fermentation, the pH values increased to 4.1, and when using media containing procyanidins the pH of samples varied within 3.98–3.71.
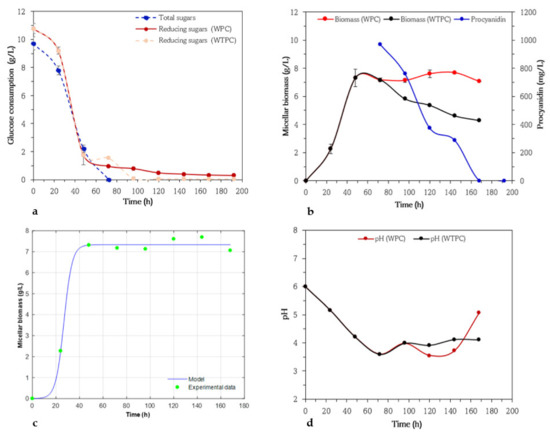
Figure 3.
Variation of glucose concentrations by anthrone and 3,5 dinitrosalicylic acid (DNS) methods (a), micellar biomass in culture medium with (WPC) and without addition of procyanidins (WTPC) when glucose was below 3.0 g/L (stationary phase), and changes in procyanidin concentrations (b) in submerged cultures of Aspergillus niger HS1. Micellar biomass production by Aspergillus niger HS1 in submerged cultures using basic medium of salts and glucose (10 g/L) and with addition of procyanidin when glucose was below 3.0 g/L (stationary phase). Symbols represent experimental data. Solid lines represent calculated data (c), Evolution of pH in submerged cultures of Aspergillus niger HS1 in medium with (WPC) and without (WTPC) addition of procyanidins when glucose was below 3.0 g/L (stationary phase) (d).
Figure 3c shows the experimental data of micellar biomass fitted to the logistic model. The predicted data agreed with the experimental data, achieving a coefficient of determination of 0.99. This value indicates that the model could efficiently describe the biomass growth during the submerged fermentation. Cell growth of A. niger HS1 in submerged cultures with procyanidin addition in stationary phase recorded a maximum biomass concentration and specific growth rate of 7.33 g/L and 0.047 h−1, respectively. To our knowledge, this is the first study on the consumption of procyanidins by submerged fermentations with A. niger and T. harzianum strains, where kinetic parameters are reported. Therefore, further research on this subject is necessary since the comparison with other authors would allow us to conclude on consumption capacity and affinity of the carbon source.
In addition, the data indicate that procyanidins do not affect fungal growth and maintain stable biomass formation at the end of the incubation period. The consumption of procyanidins seems not to be associated with the growth of the microorganism, but if the pH (Figure 3d). Authors indicate that pH is a critical factor in fermentations because it influences the growth of mycelia, fungal morphology (dispersed and pelleted), coagulation of spores, enzymatic production, pellet production, and transport phenomena across the cell membrane [56,57]. In the case of procyanidin consumption, it has been reported that it could be mediated by dioxygenase enzymes. Roopesh et al. [30] studied the fungal biodegradation of high molecular weight procyanidin fractions by fermentation with A. fumigatus MC 8, finding enzyme dioxygenase action in the degradation pathways. Despite this, there are no reports in the scientific literature on the effects of chemical factors on the activity of dioxygenases in procyanidins production. Although a previous study reported that fungal microorganisms can grow and secrete enzymes in a pH range between 4.5 and 5.5 [58]. Enzyme production is affected by mycelial morphology, for example, cellulase production is positively influenced when fungal morphology is dispersed [59], because of hydrogen ions, but when the fungus forms pellets (at high pH) it affects mass transport phenomena [60]. Fungal growth remained almost constant in the presence of procyanidins, while in their absence cell biomass decreased. Thus, in this case, fungal growth was not negatively influenced by the addition of procyanidins. Authors report that the addition of toxic compounds (phenols) in submerged fermentations can be used to improve the production of products of industrial interest, such as citric acid. Çevrimli et al. [61] observed in submerged fermentation of A. niger ATCC 16404 that the use of phenol concentrations between 20 and 25 mg/L in the culture medium resulted in increased citric acid and biomass concentrations. So far, there are no reports about the consumption of procyanidins in these experimental conditions, therefore, we consider this to be the first report on this subject.
3.4. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Aspergillus niger PSH
During the growth of A. niger, PSH in the basal medium with 10% of glucose and procyanidin differences in specific growth rates were observed. The mycelial biomass increased rapidly from 24 to 48 h. Then, it increased slowly from 48 to 72 with 7.3 g/L to 9.8 g/L, respectively, time lapse in which the 99% glucose was consumed (Figure 4a–d). Subsequently, the biomass decreased rapidly to 5.95 g/L and remained constant until the end of fermentation. Authors suggest that this decrease may be related to cell lysis due to a lack of some nutrients [62]. Moreover, glucose was often reported to be consumed by A. niger strains rapidly than other sources of carbon [54]. In fact, A. niger PSH obtains its maximum micellar biomass (WTPC) when glucose was mostly consumed. Once fungal growth has been stimulated, the second carbon source (procyanidin) is added to the bioprocess. Both carbon sources presented a decreasing trend, where glucose and procyanidins are consumed after 72 and 24 h, respectively, however, procyanidins were not totally consumed. This behavior could be associated with the increase in the degree of polymerization during fermentation [29].
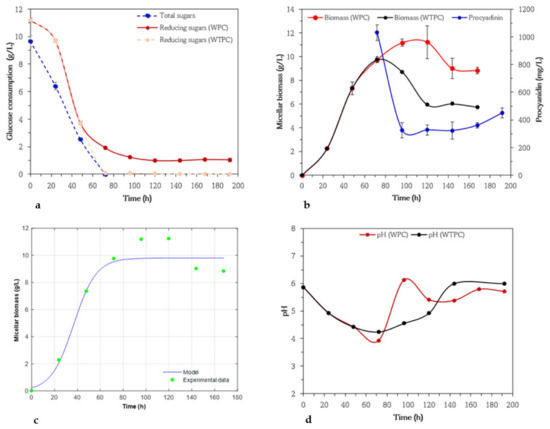
Figure 4.
Variation of glucose concentrations by anthrone and 3,5 dinitrosalicylic acid (DNS) methods (a), micellar biomass in culture medium with (WPC) and without addition of procyanidins (WTPC) when glucose was below 3.0 g/L (stationary phase), and changes in procyanidin concentrations (b) in submerged cultures of Aspergillus niger PSH. Micellar biomass production by Aspergillus niger PSH in submerged cultures using basic medium of salts and glucose (10 g/L) and with addition of procyanidin when glucose was below 3.0 g/L (stationary phase). Symbols represent experimental data. Solid lines represent calculated data (c), Evolution of pH in submerged cultures of Aspergillus niger PSH in medium with (WPC) and without (WTPC) addition of procyanidins when glucose was below 3.0 g/L (stationary phase) (d).
The decrease in procyanidin concentrations was accompanied by a slight increase in micellar biomass, reaching a concentration of 11.23 g/L and remaining stable between 96 and 120 h corresponding to the stationary growth phase (Figure 4b), while after 120 of fermentation, the content of procyanidins in the medium increased. This fact coincides with the consumption of more than 50% of the procyanidins after 24 h of addition to the culture medium. Papadaki et al. [63] in submerged fermentations with A. niger B60 report the degradation of phenolic compounds once the substrate is consumed, and the start of the stationary growth phase. In our study, the mycelial biomass was compared in culture media WPC and WTPC, where marked differences were observed. After 168 h of growth, 8.83 and 5.75 g/L of mycelial biomass was formed by A. niger HS1 in the mentioned culture mediums. These results can be compared with reports on the inhibitory effect of polyphenolic compounds on fungal growth [64]. Early investigations have reported the ability of A. niger PSH to degrade tannin-rich substrates [51,65].
The micellar biomass data fitted to the logistic model and the evolution of pH in WPC and WTPC are shown in Figure 4c,d. For the model used, the coefficient of determination was 0.922, showing that there is good agreement between the predicted and experimental data. In this case, the maximum biomass and specific growth rate were 9.8 g/L and 0.074 h−1, respectively (Table 2). It is noteworthy that so far there are no similar comparable studies, some of them discuss kinetic parameters as specific growth rate, yield, and productivity in the degradation of tannins from wastewater, olive mill wastewater, and green olive processing wastewaters for bioremediation purposes, but not in the topic of biodegradation studies of procyanidins and their metabolic pathways and discovery of new molecules [63,66,67,68]. On the other hand, the pH values moderately decreased from an initial 6.0 to 4.4 (72 h) in the control medium, while after 144 h the pH increased to 6 and then remained constant. The decrease in pH at the beginning of fermentation may be due to the production of organic acids such as citric, gluconic, and oxalic acids [69]. In the tests performed with procyanidins, during the first 24 h of their addition to the culture, pH values increased from 4.2 to 5.8, coinciding with the greatest decrease in procyanidin concentrations. Subsequently, pH dropped steadily to 5.41 after 48 h of the addition of these compounds (corresponding to 120 h of fermentation) and remained constant up to a pH of 6.

Table 2.
Estimation of experimental and calculated; fermentation kinetic parameters.
3.5. Kinetics of Biomass, Procyanidin, and Glucose Consumption of Trichoderma harzianum
The glucose variations and micellar biomass dynamics of Trichoderma harzianum WPC and WTPC medium are shown in Figure 5a,b to assess the effect of the addition of procyanidin. At the beginning for 2 days, the biomass increased smoothly hasta 1.64 g/L, then the microorganism entered the exponential phase between 42 and 72 h and reached 6 g/L biomass in WTPC. The concentrations of total and reducing sugars in WTPC decreased with increasing biomass formation giving rise to a maximum consumption of reducing sugars of 98% at 72 h of cultivation, total sugars were not recorded during this time. After the addition of procyanidins to the medium, reducing sugars increased slightly during the fermentation progress, while minimal amounts of reducing sugars were recorded in the WPC medium. The maximum consumption of glucose corresponds to the highest point of biomass, indicating that this microorganism used this carbon source for its growth. When procyanidins were added to the culture, the consumption of these compounds occurred with fluctuations in cell growth; first, the biomass decreased slightly and after 24 h of its addition there was an increase up to 6.61 g/L, then the biomass remained constant reaching a final biomass value of 6.76 g/L, time in which 66% of the procyanidins had disappeared. Which allowed us to achieve a maximum biomass and specific growth rate of 6.1 g/L and 0.051 h−1, respectively. In the stationary phase of the growth of the microorganism, low amounts of reducing sugars were detected in the medium as well as a marked consumption of procyanidins. The experimental data fitted to the model used in this study are presented in Figure 5c. The coefficient of correlation (R2) for this assay is estimated to be 0.95, indicating that this model could be adequate to describe the growth of A. niger PSH in submerged fermentations when the culture medium is supplemented with procyanidins after near-total glucose consumption.
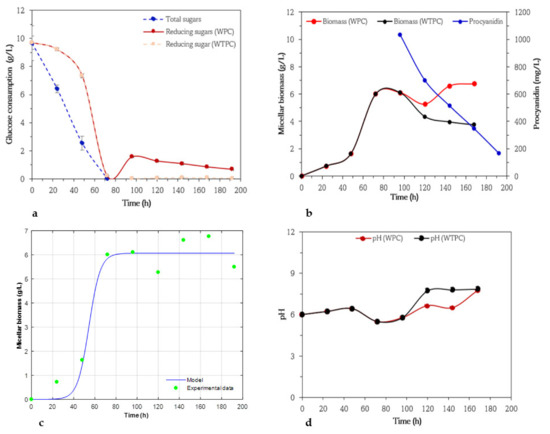
Figure 5.
Variation in glucose concentrations by anthrone and 3,5 dinitrosalicylic acid (DNS) methods (a), micellar biomass in culture medium with (WPC) and without addition of procyanidins (WTPC) when glucose was below 3.0 g/L (stationary phase), and changes in procyanidin concentrations (b) in submerged cultures of Trichoderma harzianum. Micellar biomass production by Trichoderma harzianum in submerged cultures using basic medium of salts and glucose (10 g/L) and with addition of procyanidin when glucose was below 3.0 g/L (stationary phase). Symbols represent experimental data. Solid lines represent calculated data (c), Evolution of pH in submerged cultures of Trichoderma harzianum in medium with (WPC) and without (WTPC) addition of procyanidins when glucose was below 3.0 g/L (stationary phase) (d).
A previous study has demonstrated that T. harzianum can degrade procyanidins at an initial concentration of 2 g/L in glucose and non-glucose cultures [28]. This study found that at 72 h of fermentation the consumption of procyanidins was 13 and 45%, respectively. The authors also report biomass concentrations of 0.12 and 0.1 mg/mL. Our results regarding procyanidin consumption and biomass formation were superior using a lower initial procyanidin concentration. This could be because of the concentration of procyanidins and the time of their addition to the medium.
Other reports on the effect of carbon source, nitrogen, and pH on the degradation of aromatic compounds have been published [70]. The plots of the evolution of pH are presented in Figure 5d. In general, the pH trend was stable during the lag phase, followed by a slight decrease during the exponential growth phase from 6.4 to 5.4. The cell death phase occurs when pH values increase from 5.8 to 7.8 and in the last 24 h remains stable, indicating that the basic conditions probably did not favor the growth of the fungus. As it can be observed in Figure 5d, when procyanidin was added to the culture, the pH oscillated between 5.8 and 6.7, this increase was lower when compared to the WTPC medium in the same time period. Between 120 and 144 h of fermentation, pH values decreased, fluctuating between 6.7 and 6.5. The authors suggested that this decrease in the pH values was related to the production of molecules such as peptides, pyrones, terpenoids, and aromatic heterocyclic compounds by T. harzianum. Particularly, the production of enzymes responsible for the degradation of polyphenolic compounds is related to the pH of the medium [71,72]. As noted in the previous sections, pH changes could be related to the positive effect of procyanidins on the production of micellar biomass formation, a similar behavior was observed for T. harzianum, reaching the highest micellar biomass concentrations of 6.8 g/L at 168 h. Reports indicate that the increase of pH positively influences the elimination of phenols in bioremediation assays with T. harzianum. However, the depletion of these compounds could be a consequence of their adsorption on the mycelium, since changes in the initial pH could affect the distribution of the charges on the fungal surfaces [73]. Some studies attribute or consider the depletion of polyphenolic compounds in bioprocesses as a variable related to mycelial adsorption [67,74,75]. However, further assays are needed to test this hypothesis.
The kinetic parameters observed for fungal growth, productivity, yields, and substrate consumption rate in submerged fermentations with all fungal strains are shown in Table 2. The specific growth rates obtained for A. niger HS1 and A. niger PSH had no significant differences, which were higher than those obtained for the other strains investigated. The maximum biomass for A. niger 03, A. niger GH1, and A. niger PSH were reached at 120 h of cultivation in WPC, while for WTPC this variable was obtained 48 h for the mentioned strains. This was not expected, as procyanidins can inhibit microbial growth, and there are no reports in the literature reviewed that are comparable to this study.
On the other hand, the procyanidin consumption times mentioned previously coincide with the highest consumption of procyanidins, except for A. niger HS1 and T. harzianum, which consumed procyanidins until the end of fermentation. Furthermore, the addition of procyanidins to the medium did not significantly influence biomass production compared to the other strains. The highest values productivity P and biomass yield per substrate consumed Yx/s were obtained for A. niger, being like those obtained for A. niger 03. Interestingly, the rate of substrate consumption qs was higher for A. niger HS1 and A. niger GH1, where total procyanidin consumption was also found, indicating that both strains are promising for use in future procyanidin degradation studies. It is important to note that both strains consumed more than 50% glucose during the first 48 h of fermentation, the remaining fraction of glucose was consumed in the stationary phase. These findings indicate that the strains studied differ in their ability to assimilate both carbon sources (glucose and procyanidins). The ability of the fungus to degrade glucose is a relevant factor for the consumption of procyanidins, as some strains showed a higher affinity for glucose.
4. Conclusions
Submerged fermentations under the chosen conditions allowed the cultivation of Aspergillus niger and Trichoderma harzianum strains. In some cases, the use of glucose at minimal concentrations facilitated the procyanidin degradation step. In this study, it was evident that the addition of procyanidins led to a significant increase in micellar biomass compared to the control trial. In addition, variables such as pH, the presence of glucose in the medium, and the delay in the addition of procyanidins were determinants in the bioprocess. However, further assays with procyanidin and glucose concentrations are needed to confirm these findings. This study provides further information on the potential of fungal strains to degrade recalcitrant compounds. In addition, the degradation of procyanidin complexes extracted from coffee pulp can be explored to obtain bioactive compounds of lower molecular weight that can allow the deepening of biodegradation pathways. The information presented here is also valuable for studies on the removal of toxic compounds present in agro-industrial wastes with environmental impact.
Author Contributions
Conceptualization, L.J.V.-H. and C.N.A.; methodology, L.J.V.-H., J.A.A.-V., J.E.W.-P., A.M.-P., G.C.-O. and C.N.A.; software, L.J.V.-H. and C.N.A.; validation, L.J.V.-H., J.A.A.-V., J.E.W.-P.; formal analysis, L.J.V.-H.; investigation, L.J.V.-H.; resources, M.L.C.-G., J.E.W.-P., J.C.C.-E. and C.N.A.; data curation, L.J.V.-H., J.A.A.-V., J.E.W.-P. and C.N.A.; writing—original draft preparation, L.J.V.-H.; writing—review and editing, L.J.V.-H., J.A.A.-V., J.E.W.-P., M.L.C.-G., J.C.C.-E. and C.N.A.; visualization, L.J.V.-H. and C.N.A.; supervision, J.A.A.-V. and J.E.W.-P.; project administration, M.L.C.-G. and C.N.A.; funding acquisition, C.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT, grant number FOSEC_CB 2017-18 A1-S-42515 and The APC was funded by project CONACYT-SEP-CB A1-S-42515.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thank National Council of Science and Technology (CONACYT, México) for the financial support given to this project through the program of Basic Science CONACYT-SEP 2017-18 CB-A1-S-42515 and the national program of scholarship of the PNPC-CONACYT through the financial support (CVU 1013484).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Girard, M.; Lehtimäki, A.; Bee, G.; Dohme-Meier, F.; Karonen, M.; Salminen, J.P. Changes in Feed Proanthocyanidin Profiles during Silage Production and Digestion by Lamb. Molecules 2020, 25, 5887. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wray, V.; Winterhalter, P. Isolation of dimeric, trimeric, tetrameric and pentameric procyanidins from unroasted cocoa beans (Theobroma cacao L.) using countercurrent chromatography. Food Chem. 2015, 179, 278–289. [Google Scholar] [CrossRef]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Bittner, K.; Rzeppa, S.; Humpf, H.U. Distribution and Quantification of Flavan-3-ols and Procyanidins with Low Degree of Polymerization in Nuts, Cereals, and Legumes. J. Agric. Food Chem. 2013, 61, 9148–9154. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) Using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Appeldoorn, M.M.; Vincken, J.P.; Gruppen, H.; Hollman, P.C.H. Procyanidin Dimers A1, A2, and B2 Are Absorbed without Conjugation or Methylation from the Small Intestine of Rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The Effects of Different Degrees of Procyanidin Polymerization on the Nutrient Absorption and Digestive Enzyme Activity in Mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins Should Be a Candidate in the Treatment of Cancer, Cardiovascular Diseases and Lipid Metabolic Disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef]
- Arvik, T.; Kim, H.; Seiber, J.; Yokoyama, W. Multiple effects of grape seed polyphenolics to prevent metabolic diseases. Front. Agric. Sci. Eng. 2018, 5, 351–361. [Google Scholar] [CrossRef]
- Lee, Y. Cancer Chemopreventive Potential of Procyanidin. Toxicol. Res. 2017, 33, 273–282. [Google Scholar] [CrossRef]
- Manasa, V.; Padmanabhan, A.; Anu Appaiah, K.A. Utilization of coffee pulp waste for rapid recovery of pectin and polyphenols for sustainable material recycle. Waste Manag. 2021, 120, 762–771. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Orozco, F.H.; Cegarra, J.; Trujillo, L.M.; Roig, A. Vermicomposting of coffee pulp using the earthworm Eisenia fetida: Effects on C and N contents and the availability of nutrients. Biol. Fertil. Soils 1996, 22, 162–166. [Google Scholar] [CrossRef]
- Birtic, S.; Régis, S.; Le Bourvellec, C.; Renard, C.M.G.C. Impact of air-drying on polyphenol extractability from apple pomace. Food Chem. 2019, 296, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Brandão, E.; Fernandes, A.; Guerreiro, C.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Soares, S. The effect of pectic polysaccharides from grape skins on salivary protein–procyanidin interactions. Carbohydr. Polym. 2020, 236, 116044. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Chen, J.; McClements, D.J.; Li, T.; Liu, C. Investigation the interaction between procyanidin dimer and α-glucosidase: Spectroscopic analyses and molecular docking simulation. Int. J. Biol. Macromol. 2019, 130, 315–322. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Gao, D.; Zeng, Y.; Wen, X.; Qian, Y. Competition strategies for the incubation of white rot fungi under non-sterile conditions. Process Biochem. 2008, 43, 937–944. [Google Scholar] [CrossRef]
- Rene, E.R.; Veiga, M.C.; Kennes, C. Biodegradation of gas-phase styrene using the fungus Sporothrix variecibatus: Impact of pollutant load and transient operation. Chemosphere 2010, 79, 221–227. [Google Scholar] [CrossRef]
- Bardi, A.; Yuan, Q.; Siracusa, G.; Chicca, I.; Islam, M.; Spennati, F.; Tigini, V.; Di Gregorio, S.; Levin, D.B.; Petroni, G.; et al. Effect of cellulose as co-substrate on old landfill leachate treatment using white-rot fungi. Bioresour. Technol. 2017, 241, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Spennati, F.; Ricotti, A.; Mori, G.; Siracusa, G.; Becarelli, S.; Di Gregorio, S.; Tigini, V.; Varese, G.C.; Munz, G. The role of cosubstrate and mixing on fungal biofilm efficiency in the removal of tannins. Environ. Technol. 2019, 41, 3515–3523. [Google Scholar] [CrossRef] [PubMed]
- Spennati, F.; La China, S.; Siracusa, G.; Di Gregorio, S.; Bardi, A.; Tigini, V.; Mori, G.; Gabriel, D.; Munz, G. Tannery wastewater recalcitrant compounds foster the selection of fungi in non-sterile conditions: A pilot scale long-term test. Int. J. Environ. Res. Public Health 2021, 18, 6348. [Google Scholar] [CrossRef] [PubMed]
- Badia-Fabregat, M.; Lucas, D.; Tuomivirta, T.; Fritze, H.; Pennanen, T.; Rodríguez-Mozaz, S.; Barceló, D.; Caminal, G.; Vicent, T. Study of the effect of the bacterial and fungal communities present in real wastewater effluents on the performance of fungal treatments. Sci. Total Environ. 2017, 579, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Bhoite, R.N.; Murthy, P.S. Biodegradation of coffee pulp tannin by Penicillium verrucosum for production of tannase, statistical optimization and its application. Food Bioprod. Process. 2015, 94, 727–735. [Google Scholar] [CrossRef]
- Chaudhary, P.; Chhokar, V.; Kumar, A.; Beniwal, V. Bioremediation of Tannery Wastewater. In Advances in Environmental Biotechnology; Springer: Singapore, 2017; pp. 125–144. [Google Scholar] [CrossRef]
- Prigione, V.; Trocini, B.; Spina, F.; Poli, A.; Romanisio, D.; Giovando, S.; Varese, G.C. Fungi from industrial tannins: Potential application in biotransformation and bioremediation of tannery wastewaters. Appl. Microbiol. Biotechnol. 2018, 102, 4203–4216. [Google Scholar] [CrossRef]
- Contreras-Dominguez, M.; Roopesh, K.; Coronel, A.R.; Oduardo, N.G.; Guyot, S.; Saucedo-Castaneda, G.; Perraud, I.G.; Roussos, S.; Augur, C. Use of fungal enzymes to study the degradation of specific plant polyphenols. Environ. Sci. 2008, 508–518. Available online: https://hal.ird.fr/ird-00865346/ (accessed on 1 December 2021).
- Contreras-Domínguez, M.; Guyot, S.; Marnet, N.; Le Petit, J.; Perraud-Gaime, I.; Roussos, S.; Augur, C. Degradation of procyanidins by Aspergillus fumigatus: Identification of a novel aromatic ring cleavage product. Biochimie 2006, 88, 1899–1908. [Google Scholar] [CrossRef]
- Roopesh, K.; Guyot, S.; Sabu, A.; Haridas, M.; Isabelle, P.G.; Roussos, S.; Augur, C. Biotransformation of procyanidins by a purified fungal dioxygenase: Identification and characterization of the products using mass spectrometry. Process Biochem. 2010, 45, 904–913. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Guyot, S.; Aguilar-Zárate, P.; Muñiz-Márquez, D.B.; Contreras-Esquivel, J.C.; Aguilar, C.N. Structural characterization of native and oxidized procyanidins (condensed tannins) from coffee pulp (Coffea arabica) using phloroglucinolysis and thioglycolysis-HPLC-ESI-MS. Food Chem. 2021, 340, 127830. [Google Scholar] [CrossRef]
- Lan, P.; Brosse, N.; Cui, J.Q.; Mao, H.Y.; Yang, R. Selective Biodegradation of Grape Pomace Tannins by Aspergillus niger and Application in Wood Adhesive. Bioresources 2018, 13, 894–905. [Google Scholar] [CrossRef]
- Reginatto, C.; Rossi, C.; Miglioranza, B.G.; dos Santos, M.; Meneghel, L.; da Silveira, M.M.; Malvessi, E. Pectinase production by Aspergillus niger LB-02-SF is influenced by the culture medium composition and the addition of the enzyme inducer after biomass growth. Process Biochem. 2017, 58, 1–8. [Google Scholar] [CrossRef]
- Datta, P.; Tiwari, S.; Pandey, L.M. Bioethanol Production from Waste Breads Using Saccharomyces cerevisiae. In Utilization and Management of Bioresources; Springer: Singapore, 2018; pp. 125–134. [Google Scholar] [CrossRef]
- Miller, G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Liu, J.Z.; Weng, L.P.; Zhang, Q.L.; Xu, H.; Ji, L.N. A mathematical model for gluconic acid fermentation by Aspergillus niger. Biochem. Eng. J. 2003, 14, 137–141. [Google Scholar] [CrossRef]
- Aranda, C.; Robledo, A.; Loera, O.; Contreras-Esquivel, J.C.; Rodríguez, R.; Aguilar, C.N.; Carranza, V.; Cárdenas, J. Fungal Invertase Expression in Solid-State Fermentation. Food Technol. Biotechnol. 2006, 44, 229–233. [Google Scholar]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Muñoz-Labrador, A.; Prodanov, M.; Villamiel, M. Effects of high intensity ultrasound on disaggregation of a macromolecular procyanidin-rich fraction from Vitis vinifera L. seed extract and evaluation of its antioxidant activity. Ultrason. Sonochem. 2019, 50, 74–81. [Google Scholar] [CrossRef]
- Yepes-Betancur, D.P.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M.; Martinez-Saldarriaga, J.; Torres-León, C.; Ascacio-Valdes, A.; Aguilar, C.N. Solid-state fermentation–assisted extraction of bioactive compounds from hass avocado seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, U. Acta Biologica Szegediensis. Acta Biol. Szeged. 2017, 61, 25–33. [Google Scholar]
- Infanzón-Rodríguez, M.I.; Ragazzo-Sánchez, J.A.; del Moral, S.; Calderón-Santoyo, M.; Gutiérrez-Rivera, B.; Aguilar-Uscanga, M.G. Optimization of Cellulase Production by Aspergillus niger ITV 02 from Sweet Sorghum Bagasse in Submerged Culture Using a Box–Behnken Design. Sugar Tech. 2019, 22, 266–273. [Google Scholar] [CrossRef]
- Mojsov, K.D. Aspergillus Enzymes for Food Industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 215–222. [Google Scholar] [CrossRef]
- Laothanachareon, T.; Bruinsma, L.; Nijsse, B.; Schonewille, T.; Suarez-Diez, M.; Tamayo-Ramos, J.A.; Martins Dos Santos, V.A.P.; Schaap, P.J. Global transcriptional response of Aspergillus niger to blocked active citrate export through deletion of the exporter gene. J. Fungi 2021, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.C.E.; Rivera, W.L.; Vital, P.G. Influences of carbohydrate, nitrogen, and phosphorus sources on the citric acid production by fungal endophyte Aspergillus fumigatus P3I6. Prep. Biochem. Biotechnol. 2019, 50, 292–301. [Google Scholar] [CrossRef]
- Leontopoulos, S.V.; Giavasis, I.; Petrotos, K.; Kokkora, M.; Makridis, C. ScienceDirect Effect of Different Formulations of Polyphenolic Compounds Obtained from OMWW on the Growth of Several Fungal Plant and Food Borne Pathogens. Studies in vitro and in vivo. Agric. Agric. Sci. Procedia 2015, 4, 327–337. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. Pomegranate peel waste: A new substrate for citric acid production by Aspergillus niger in solid-state fermentation under non-aseptic conditions. Environ. Sci. Pollut. Res. 2020, 27, 13105–13113. [Google Scholar] [CrossRef]
- Anttila, A.K.; Pirttilä, A.M.; Häggman, H.; Harju, A.; Venäläinen, M.; Haapala, A.; Holmbom, B.; Julkunen-Tiitto, R. Condensed conifer tannins as antifungal agents in liquid culture. Holzforschung 2013, 67, 825–832. [Google Scholar] [CrossRef]
- Aguilar, C.N.; Augur, C.; Favela-Torres, E.; Viniegra-González, G. Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: Influence of glucose and tannic acid. J. Ind. Microbiol. Biotechnol. 2001, 26, 296–302. [Google Scholar] [CrossRef]
- Hernández, M.C.; Esquivel, J.C.C.; Lara, F.; Rodríguez, R.; Aguilar, C.N. Isolation and Evaluation of Tannin-degrading Fungal Strains from the Mexican Desert. Z. Nat. C 2005, 60, 844–848. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Guyot, S.; Rodríguez-Herrera, R.; Prado-Barragán, A.; Aguilar, C.N. Production profiles of phenolics from fungal tannic acid biodegradation in submerged and solid-state fermentation. Process Biochem. 2014, 49, 541–546. [Google Scholar] [CrossRef]
- Said, A.; Leila, A.; Kaouther, D.; Sadia, B. Date wastes as substrate for the production of α-Amylase and invertase. Iran. J. Biotechnol. 2014, 12, 41–49. [Google Scholar] [CrossRef]
- Alarid-García, C.; Hernández-Calderón, O.M.; Rios-Iribe, E.Y.; González-Llanes, M.D.; Escamilla-Silva, E.M. Production of β-glucosidase by Aspergillus niger CDBB-H-175 on submerged fermentation. Can. J. Chem. Eng. 2021, 1–13. [Google Scholar] [CrossRef]
- Karaffa, L.; Kubicek, C.P. Aspergillus niger citric acid accumulation: Do we understand this well working black box? Appl. Microbiol. Biotechnol. 2003, 61, 189–196. [Google Scholar] [CrossRef]
- Galbraith, J.C.; Smith, J.E. Filamentous growth of Aspergillus niger in submerged shake culture. Trans. Br. Mycol. Soc. 1969, 52, 237–246. [Google Scholar] [CrossRef]
- Kapoor, M.; Nair, L.M.; Kuhad, R.C. Cost-effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem. Eng. J. 2008, 38, 88–97. [Google Scholar] [CrossRef]
- Latifian, M.; Hamidi-Esfahani, Z.; Barzegar, M. Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresour. Technol. 2007, 98, 3634–3637. [Google Scholar] [CrossRef] [PubMed]
- Kalra, M.K.; Sandhu, D.K. Optimal production of cellulolytic enzymes and their location in trichoderma pseudokonigii. Acta Biotechnol. 1986, 6, 161–166. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- Çevrimli, B.S.; Kariptaş, E.; Yaşar, A.; Yiǧitoǧlu, M. Influences of phenolic compounds on citric acid productivity by Aspergillus niger in stirred fermentor. Electron. J. Biotechnol. 2010, 13, 8–9. [Google Scholar] [CrossRef]
- Sandri, I.G.; Fontana, R.C.; Moura da Silveira, M. Influence of pH and temperature on the production of polygalacturonases by Aspergillus fumigatus. LWT-Food Sci. Technol. 2015, 61, 430–436. [Google Scholar] [CrossRef]
- Papadaki, E.; Tsimidou, M.Z.; Mantzouridou, F.T. Changes in Phenolic Compounds and Phytotoxicity of the Spanish-Style Green Olive Processing Wastewaters by Aspergillus niger B60. J. Agric. Food Chem. 2018, 66, 4891–4901. [Google Scholar] [CrossRef] [PubMed]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T.; et al. Recovery of Polyphenolic Fraction from Arabica Coffee Pulp and Its Antifungal Applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Medina, M.A.; Belmáres, R.E.; Aguilera-Carbo, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal culture systems for production of antioxidant phenolics using pecan nut shells as sole carbon source. Am. J. Agric. Biol. Sci. 2010, 5, 397–402. [Google Scholar] [CrossRef][Green Version]
- Hanafi, F.; Mountadar, M.; Etahiri, S.; Fekhaoui, M.; Assobhei, O. Biodegradation of Toxic Compounds in Olive Mill Wastewater by a Newly Isolated Potent Strain: Aspergillus niger van Tieghem. J. Water Resour. Prot. 2013, 2013, 768–774. [Google Scholar] [CrossRef]
- Ayed, L.; Chammam, N.; Asses, N.; Hamdi, M. Optimization of biological pretreatment of green table olive processing wastewaters using Aspergillus niger. J. Bioremediation Biodegrad. 2014, 5, 212. [Google Scholar] [CrossRef]
- Hamdi, M.; Garciab, J.L. Anaerobic Digestion of Olive Mill Wastewaters after Detoxification by Prior Culture of AspergiZhs niger. Process Biochem. 1993, 28, 155–159. [Google Scholar] [CrossRef]
- Šimonovičová, A.; Kupka, D.; Nosalj, S.; Kraková, L.; Drahovská, H.; Bártová, Z.; Vojtková, H.; Boturová, K.; Pangallo, D. Differences in metabolites production using the Biolog FF MicroplateTM system with an emphasis on some organic acids of Aspergillus niger wild type strains. Biologia 2020, 75, 1537–1546. [Google Scholar] [CrossRef]
- Hadibarata, T.; Syafiuddin, A.; Al-Dhabaan, F.A.; Elshikh, M.S. Rubiyatno Biodegradation of Mordant orange-1 using newly isolated strain Trichoderma harzianum RY44 and its metabolite appraisal. Bioprocess Biosyst. Eng. 2018, 41, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- Chalimah, N.; Soesanto, L.; Suharti, W.S. The Effect of Various pH Medium on the Secondary Metabollites Production from Trichoderma harzianum T10 to Control Damping Off on Cucumber Seedlings. J. Trop. Hortic. 2020, 3, 65–70. [Google Scholar] [CrossRef]
- Campaniello, D.; Carlucci, A.; Speranza, B.; Raimondo, M.L.; Cibelli, F.; Corbo, M.R.; Bevilacqua, A. A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water. Microorganisms 2020, 8, 878. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; BouHamed, H.; Ellouz, R. Optimization of the fermentation of olive mill waste-waters by Aspergillus niger. Appl. Microbiol. Biotechnol. 1991, 36, 285–288. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).