Screening and Identification of Yeasts from Fruits and Their Coculture for Cider Production
Abstract
:1. Introduction
2. Material and Methods
2.1. Strain Screening and Identification
2.2. Analysis of Yeast Flora
2.3. Sugar, Ethanol, and Glycerol Content of the Cider Samples
2.4. Optimization of Cider-Brewing Conditions
2.5. Inoculation Order for the Coculture System
2.6. Fermentation Duration Experiments
2.7. Fermentation Temperature Experiments
2.8. Initial Sugar Content Experiments
2.9. RSM Experiments
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Strain Selection and Identification
3.2. Optimization of Fermentation Conditions
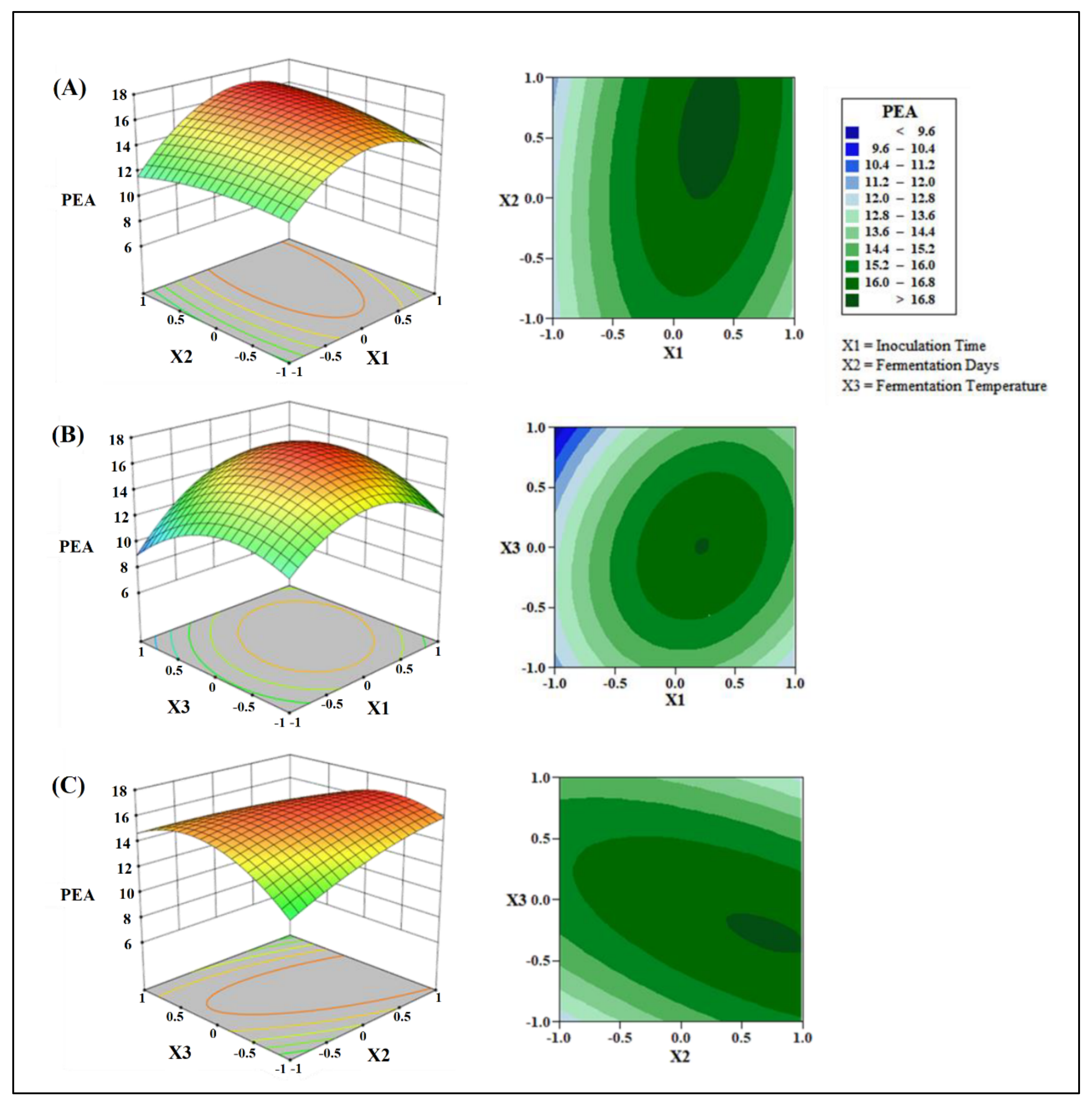
Effects of Inoculation Time, Fermentation Time, and Fermentation Temperature on PEA Production
3.3. Construction and Validation of the RSM Model
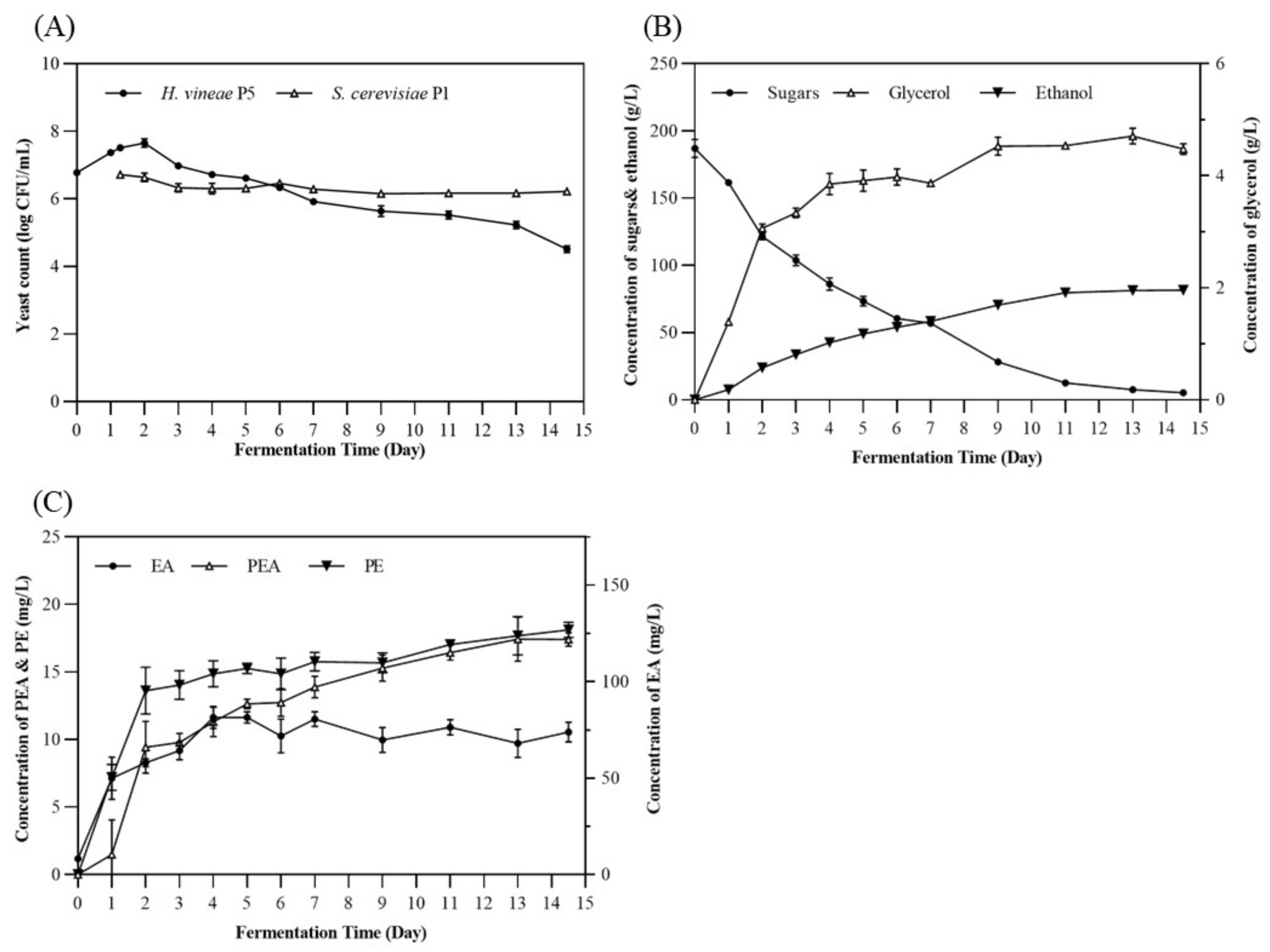
3.4. Changes in Chemical Composition and Yeast Flora during Fermentation
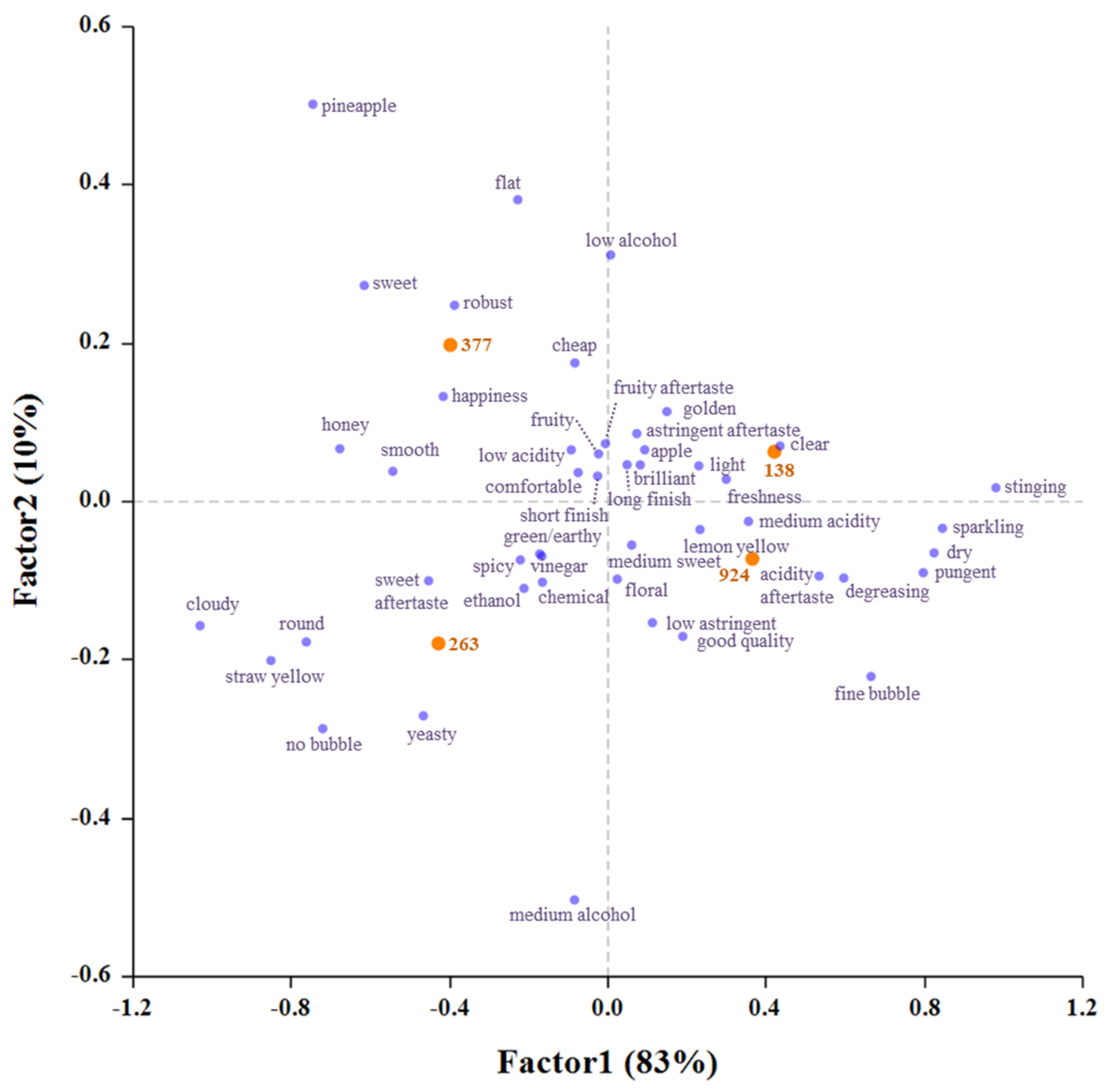
3.5. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ANOVA | Analysis of variance; |
| CATA | Check-all-that-apply; |
| CCM | Central carbon metabolism; |
| EA | Ethyl acetate; |
| GC–FID | Gas chromatography with flame-ionization detection; |
| GRAS | Generalized recognized as safe; |
| HPLC | High-performance liquid chromatography; |
| ITS | Internal transcribed spacer; |
| PE | 2-phenylethanol; |
| PEA | 2-phenylethyl acetate; |
| RSM | Response surface methodology; |
| WL | Wallerstein Laboratory; |
| YPD | Yeast extract peptone dextrose |
References
- Cousin, F.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.-M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lea, A.G.H.; Drilleau, J.-F. Cidermaking. In Fermented Beverage Production; Lea, A.G.H., Piggott, J.R., Eds.; Springer: Boston, MA, USA, 2003; pp. 59–87. ISBN 978-1-4615-0187-9. [Google Scholar]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef]
- Rainieri, S.; Pretorius, I.S. Selection and improvement of wine yeasts. Ann. Microbiol. 2000, 50, 15–32. [Google Scholar]
- González Flores, M.; Rodríguez, M.E.; Origone, A.C.; Oteiza, J.M.; Querol, A.; Lopes, C.A. Saccharomyces uvarum isolated from patagonian ciders shows excellent fermentative performance for low temperature cidermaking. Food Res. Int. 2019, 126, 108656. [Google Scholar] [CrossRef]
- Guichard, H.; Poupard, P.; Legoahec, L.; Millet, M.; Bauduin, R.; Le Quéré, J.-M. Brettanomyces anomalus, a double drawback for cider aroma. LWT 2019, 102, 214–222. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Portillo, M.D.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.-K.; Wang, J.; Chen, F.-S.; Zhang, X.-Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT 2019, 116, 108477. [Google Scholar] [CrossRef]
- Gschaedler, A.; Iñiguez-Muñoz, L.E.; Flores-Flores, N.Y.; Kirchmayr, M.; Arellano-Plaza, M. Use of non-Saccharomyces yeasts in cider fermentation: Importance of the nutrients addition to obtain an efficient fermentation. Int. J. Food Microbiol. 2021, 347, 109169. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [Green Version]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The ecology of insect–yeast relationships and its relevance to human industry. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef]
- Qian, X.; Yan, W.; Zhang, W.; Dong, W.; Ma, J.; Ochsenreither, K.; Jiang, M.; Xin, F. Current status and perspectives of 2-phenylethanol production through biological processes. Crit. Rev. Biotechnol. 2019, 39, 235–248. [Google Scholar] [CrossRef]
- Lai, Y.-T.; Cheng, K.-C.; Lai, C.-N.; Lai, Y.-J. Isolation and identification of aroma producing strain with esterification capacity from yellow water. PLoS ONE 2019, 14, e0211356. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of Aroma Compounds in Apple Cider Using Solvent-Assisted Flavor Evaporation and Headspace Solid-Phase Microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.B. (Eds.) Copyright. In The Yeasts, 5th ed.; Elsevier: London, UK, 2011; p. iv. ISBN 978-0-444-52149-1. [Google Scholar]
- Duarte-coelho, A.C.; Dumoulin, E.D.; Guerain, J.T. High-Performance Liquid Chromato-Graphic Determination of Sucrose, Glucose, Fructose in Complex Products of Distilleries. J. Liq. Chromatogr. 1985, 8, 59–73. [Google Scholar] [CrossRef]
- Liou, B.-K.; Jaw, Y.-M.; Chuang, G.C.-C.; Yau, N.N.J.; Zhuang, Z.-Y.; Wang, L.-F. Important Sensory, Association, and Postprandial Perception Attributes Influencing Young Taiwanese Consumers’ Acceptance for Taiwanese Specialty Teas. Foods 2020, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Sommer, S.; Sommer, S.J.; Cohen, S.D. Proposal of the head-heart-base technique as an innovative method to describe and evaluate the aroma profile of wine and cider. J. Wine Res. 2018, 29, 204–219. [Google Scholar] [CrossRef]
- Masneuf-Pomarede, I.; Bely, M.; Marullo, P.; Albertin, W. The Genetics of Non-conventional Wine Yeasts: Current Knowledge and Future Challenges. Front. Microbiol. 2016, 6, 1563. [Google Scholar] [CrossRef]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Aung, M.T.; Lee, P.-R.; Yu, B. Yeast and volatile evolution in cider co-fermentation with Saccharomyces cerevisiae and Williopsis saturnus. Ann. Microbiol. 2016, 66, 307–315. [Google Scholar] [CrossRef]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Giorello, F.; Valera, M.J.; Martin, V.; Parada, A.; Salzman, V.; Camesasca, L.; Fariña, L.; Boido, E.; Medina, K.; Dellacassa, E.; et al. Genomic and Transcriptomic Basis of Hanseniaspora vineae’s Impact on Flavor Diversity and Wine Quality. Appl. Environ. Microbiol. 2019, 85, e01959-18. [Google Scholar] [CrossRef] [Green Version]
- Ciani, M.; Comitini, F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Microbial carboxyl esterases: Classification, properties and application in biocatalysis. FEMS Microbiol. Rev. 2002, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.; Bauer, F.F.; Lambrechts, M.G.; Swiegers, J.H.; Cozzolino, D.; Pretorius, I.S. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 2006, 23, 641–659. [Google Scholar] [CrossRef] [Green Version]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, B.; Bauer, F.F.; Cardinali, G.; Setati, M.E. Ecological interactions are a primary driver of population dynamics in wine yeast microbiota during fermentation. Sci. Rep. 2020, 10, 4911. [Google Scholar] [CrossRef] [Green Version]
- Salvadó, Z.; Arroyo-López, F.N.; Barrio, E.; Querol, A.; Guillamón, J.M. Quantifying the individual effects of ethanol and temperature on the fitness advantage of Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 1155–1161. [Google Scholar] [CrossRef]
- Crépin, L.; Truong, N.M.; Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C.; Cullen, D. Management of Multiple Nitrogen Sources during Wine Fermentation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, e02617-16. [Google Scholar] [CrossRef] [Green Version]
- Rollero, S.; Mouret, J.-R.; Bloem, A.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S.; Camarasa, C. Quantitative (13) C-isotope labelling-based analysis to elucidate the influence of environmental parameters on the production of fermentative aromas during wine fermentation. Microb. Biotechnol. 2017, 10, 1649–1662. [Google Scholar] [CrossRef]
- Singh, A.; Kocher, G.S. Standardization of seed and peel infused Syzygium cumini -wine fermentation using response surface methodology. LWT 2020, 134, 109994. [Google Scholar] [CrossRef]
- Llorente, D.D.; Abrodo, P.A.; González-Álvarez, J.; de la Fuente, E.D.; Alonso, J.J.M.; Álvarez, M.D.G.; Gomis, D.B. A New Analytical Method to Volatile Compounds in Cider Apples: Application to Evaluate the Starch Index. Food Bioprocess Technol. 2013, 6, 2447–2454. [Google Scholar] [CrossRef]
- Gamero, A.; Ferreira, V.; Pretorius, I.S.; Querol, A. Wine, Beer and Cider: Unravelling the Aroma Profile. In Molecular Mechanisms in Yeast Carbon Metabolism; Springer: Berlin/Heidelberg, Germany, 2014; pp. 261–297, ISBN 978-3-642-55012-6 (Print) 978-3-642-55013-3 (Online). [Google Scholar]
- de Barros, H.E.A.; Natarelli, C.V.L.; de Carvalho Tavares, I.M.; de Oliveira, A.L.M.; Araújo, A.B.S.; Pereira, J.; Carvalho, E.E.N.; de Barros Vilas Boas, E.V.; Franco, M. Nutritional Clustering of Cookies Developed with Cocoa Shell, Soy, and Green Banana Flours Using Exploratory Methods. Food Bioprocess Technol. 2020, 13, 1566–1578. [Google Scholar] [CrossRef]
- Brennan, J.G.; Grandison, A.S.; Lewis, M.J. Separations in Food Processing. In Food Processing Handbook; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 429–511. ISBN 9783527307197. [Google Scholar]
- Ye, M.; Yue, T.; Yuan, Y. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [Green Version]

| Independent Variables | Dependent Variables (PEA) | |||||||
|---|---|---|---|---|---|---|---|---|
| Actual Value | Code Value | Predicted | Actual | |||||
| X1 | X2 (d) | X3 (°C) | X1 | X2 (d) | X3 (°C) | Ypre. (mg/L) | Yact. (mg/L) | |
| 1 | S2H0 | 15 | 20 | 1 | 1 | 0 | 15.71 | 15.62 |
| 2 | S2H0 | 7 | 20 | 1 | −1 | 0 | 13.39 | 13.29 |
| 3 | S0H0 | 15 | 20 | −1 | 1 | 0 | 11.63 | 11.74 |
| 4 | S0H0 | 7 | 20 | −1 | −1 | 0 | 12.37 | 12.46 |
| 5 | S2H0 | 11 | 25 | 1 | 0 | 1 | 13.77 | 13.76 |
| 6 | S2H0 | 11 | 15 | 1 | 0 | −1 | 11.96 | 12.16 |
| 7 | S0H0 | 11 | 25 | −1 | 0 | 1 | 8.98 | 8.78 |
| 8 | S0H0 | 11 | 15 | −1 | 0 | −1 | 11.64 | 11.65 |
| 9 | S1H0 | 15 | 25 | 0 | 1 | 1 | 12.61 | 12.70 |
| 10 | S1H0 | 15 | 15 | 0 | 1 | −1 | 15.91 | 15.80 |
| 11 | S1H0 | 7 | 25 | 0 | −1 | 1 | 14.69 | 14.80 |
| 12 | S1H0 | 7 | 15 | 0 | −1 | −1 | 12.24 | 12.14 |
| 13 | S1H0 | 11 | 20 | 0 | 0 | 0 | 16.67 | 17.00 |
| 14 | S1H0 | 11 | 20 | 0 | 0 | 0 | 16.67 | 16.41 |
| 15 | S1H0 | 11 | 20 | 0 | 0 | 0 | 16.67 | 16.59 |
| Source | Df | Adj SS | Adj MS | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 75.2956 | 8.3662 | 121.01 | 0.000 |
| Linear | 3 | 14.6221 | 4.8740 | 70.50 | 0.000 |
| X1 | 1 | 13.0027 | 13.0027 | 188.08 | 0.000 |
| X2 | 1 | 1.2574 | 1.2574 | 18.19 | 0.008 |
| X3 | 1 | 0.3620 | 0.3620 | 5.24 | 0.071 |
| Square | 3 | 45.0622 | 15.0207 | 217.27 | 0.000 |
| X12 | 1 | 29.6026 | 29.6026 | 428.19 | 0.000 |
| X22 | 1 | 1.1543 | 1.1543 | 16.70 | 0.009 |
| X32 | 1 | 18.6665 | 18.6665 | 270.00 | 0.000 |
| Interaction | 3 | 15.6113 | 5.2038 | 75.27 | 0.000 |
| X1 × X2 | 1 | 2.3339 | 2.3339 | 33.76 | 0.002 |
| X1 × X3 | 1 | 5.0026 | 5.0026 | 72.36 | 0.000 |
| X2 × X3 | 1 | 8.2748 | 8.2748 | 119.69 | 0.000 |
| Error | 5 | 0.3457 | 0.0691 | ||
| Lack of fit | 3 | 0.1602 | 0.0534 | 0.58 | 0.684 |
| Pure error | 2 | 0.1855 | 0.0927 | ||
| Total | 14 | 75.6413 |
| Sample Designation 1 | 138 2 | 263 | 377 | 924 |
|---|---|---|---|---|
| Appearance | 7.30 ± 0.88 a | 6.33 ± 1.62 b | 6.63 ± 1.31 b | 7.33 ± 0.92 a |
| Aroma | 6.73 ± 1.22 a | 5.95 ± 1.44 b | 6.35 ± 1.41 ab | 6.20 ± 1.36 ab |
| Flavor | 6.30 ± 1.34 a | 6.58 ± 1.39 a | 6.50 ± 1.60 a | 6.00 ± 1.26 a |
| Texture | 6.23 ± 0.83 a | 6.65 ± 1.27 a | 6.35 ± 1.66 a | 6.28 ± 1.06 a |
| Aftertaste | 6.00 ± 1.24 a | 6.48 ± 1.43 a | 6.00 ± 1.75 a | 6.10 ± 1.08 a |
| Overall liking | 6.38 ± 1.00 a | 6.48 ± 1.45 a | 6.30 ± 1.67 a | 6.33 ± 1.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.-Y.; Huang, P.-H.; Lai, Y.-T.; Lin, S.-P.; Liou, B.-K.; Lin, H.-W.; Hsieh, C.-W.; Cheng, K.-C. Screening and Identification of Yeasts from Fruits and Their Coculture for Cider Production. Fermentation 2022, 8, 1. https://doi.org/10.3390/fermentation8010001
Hou C-Y, Huang P-H, Lai Y-T, Lin S-P, Liou B-K, Lin H-W, Hsieh C-W, Cheng K-C. Screening and Identification of Yeasts from Fruits and Their Coculture for Cider Production. Fermentation. 2022; 8(1):1. https://doi.org/10.3390/fermentation8010001
Chicago/Turabian StyleHou, Chih-Yao, Pei-Hsiu Huang, Yen-Tso Lai, Shin-Ping Lin, Bo-Kang Liou, Hui-Wen Lin, Chang-Wei Hsieh, and Kuan-Chen Cheng. 2022. "Screening and Identification of Yeasts from Fruits and Their Coculture for Cider Production" Fermentation 8, no. 1: 1. https://doi.org/10.3390/fermentation8010001
APA StyleHou, C.-Y., Huang, P.-H., Lai, Y.-T., Lin, S.-P., Liou, B.-K., Lin, H.-W., Hsieh, C.-W., & Cheng, K.-C. (2022). Screening and Identification of Yeasts from Fruits and Their Coculture for Cider Production. Fermentation, 8(1), 1. https://doi.org/10.3390/fermentation8010001









