Abstract
Sparkling wine production using the traditional method involves a second fermentation of still wines in bottles, followed by prolonged aging on lees. The key factors affecting the organoleptic profiles of these wines are the grape varieties, the chemical and sensory attributes of the base wines elaborated, the yeast strains used for first and second fermentation, and the winery practices. While Chardonnay and Pinot noir are gold standard grape varieties in sparkling wine production, other valuable grape cultivars are used worldwide to elaborate highly reputable sparkling wines. Fundamental research on the chemical and sensory profiles of innovative sparkling wines produced by the traditional method, using non-conventional grape varieties and novel yeast strains for first and/or second fermentation, is accompanying their market diversification. In this review, we summarize relevant aspects of sparkling wine production using the traditional method and non-conventional grape varieties and yeast starters.
1. Introduction
The traditional method for sparkling wine production (i.e., méthode champenoise) consists of the fermentation of a base wine following the addition of a liqueur de tirage (i.e., wine, sucrose, ethanol-adapted yeast cells, nutrients, and a clarifying agent), in characteristic bottles [1] (Figure 1). The development of this second fermentation in sealed bottles greatly contributes to the sensory complexity of the resulting wine [2,3]. Among other factors, the in bottle fermentation method contributes to a refined development of foam and bubbles, which are key properties identifying champenoise sparkling wines [3,4].
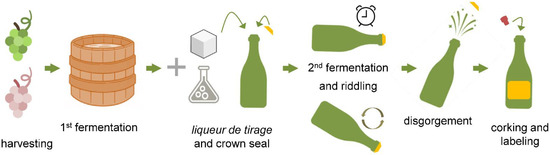
Figure 1.
Schematic representation of sparkling wine production using the traditional method. During the first fermentation, the grape juice is converted into a base wine, which is then subjected to a second fermentation following the addition of wine, sucrose, ethanol-adapted yeast cells, nutrients, and a clarifying agent, in characteristic bottles. After the second fermentation and aging, riddling is performed to allow accumulation of the lees at the bottle neck, facilitating their mechanical elimination. Disgorging (i.e., lees removal) is followed by the addition of a liqueur de expedition, bottle corking, and labelling.
Conventional grape varieties used in this industry are linked to prestigious sparkling wines produced in France (e.g., Chardonnay, Pinot noir, and Pinot meunier), Italy (e.g., Chardonnay, Pinot nero, Pinot bianco, and Lambrusco), and Spain (e.g., Macabeo, Xarel.lo, Parellada, Pinot noir, and Chardonnay) (Table 1). Each country has different officially authorized grape varieties for their production. These varieties are linked to various Protected Designations of Origin [3,4] (Table 1).

Table 1.
Conventional grape varieties used for sparkling wine production by the traditional method.
Accompanying the global expansion of sparkling wine production, emerging grape varieties for the elaboration of fine quality sparkling wines are being explored worldwide [3,4] (Table 2). Non-conventional grape varieties challenge the wine industry to recognize and market products with novel sensory profiles, and to anticipate potential consumer acceptance [2,5]. Adding complexity to the scenario of innovation in sparkling wine production, first and second fermentation strategies are also being explored, based on novel and/or indigenous yeast starters [2]. In this review, we summarize relevant studies of sparkling wine production, innovating both in grape varieties and in yeast starters, and using the traditional method.

Table 2.
Non-conventional grape varieties used for sparkling wine production by the traditional method.
2. Non-Conventional Grape Varieties for Sparkling Wine Production Using the Traditional Method
Sugar content (i.e., 16–21 °Brix), titratable acidity (i.e., >8 g/L tartaric acid) and pH (i.e., 2.9–3.2) of grape musts are significant chemical parameters when harvesting grapes destined for sparkling wine production [39,40]. This “ripened” stage is dramatically influenced by the grape variety, and by the soil and climate (i.e., terroir) [39]. Organoleptic analyses of grape musts and base wines produced with various non-conventional grape varieties (Table 2) have shown highly valuable chemical and sensory profiles for the production of white, rosé, and red sparkling wines (Table 3), comparable to those obtained by using the gold standard grape varieties (Table 1) [3,4,41]. In addition to market innovation, the use of alternative grape cultivars would enable, for example, development of this industry in geographic areas where climatic conditions do not allow more conventional grape cultivars to reach optimal grape maturity [15].

Table 3.
Chemical profiles of base wines obtained using non-conventional grape varieties in sparkling wine production by the traditional method.
2.1. White Sparkling Wines
White and/or red grape varieties can be used in the production of white sparkling wines [3,39]. Golden yellow sparkling wines are typically manufactured using white grapes (i.e., blanc de blanc) [3,39,40]. On the other hand, when red grape varieties are used, they are vinified as base white wines by eliminating or greatly reducing must maceration, to produce sparkling wines known as blanc de noirs [3,39,40]. In addition to traditional grape cultivars (Table 1), several non-conventional varieties are used for the production of white sparkling wine (Table 2 and Table 4). Interestingly, Muscat-related sparkling wines have a worldwide distribution [9,10,22], and the volatile profile of these wines has revealed a homogeneous signature for this grape family [9,10,22]. Major classes of volatile compounds in these sparkling wines are terpenes, a predominant class of compounds associated with their floral profiles, and higher alcohols and esters, which contribute to their fruity aromas [9,10,22]. In particular, Moscato Giallo sparkling wines from Brazil have shown high concentrations of 2-phenylethanol, ethyl octanoate, linalool, and α-terpineol [10] (Table 5). Similarly, Romanian Muscat Ottonel sparkling wines show high levels of ethyl octanoate and ethyl decanoate, as well as high levels of linalool, regardless of the yeast used for second fermentation [22] (Table 5). In Argentina, Torrontés Riojano (Listan Prieto × Moscatel de Alejandría), an aromatic cultivar with rich and variegated profiles comparable to that of Muscat in terms of intensity and bouquet [43], is also used for sparkling wine production [6]. The volatile fraction of Torrontés Riojano sparkling wines revealed a high concentration of volatile compounds associated with fruit aromas, as well as terpene compounds associated with the floral Muscat-like aromas [6] (Table 5). In fact, the final sensory properties of Muscat, Moscato Giallo, Muscat Ottonel, and Torrontés Riojano sparkling wines appears to depend on their unique terpenic profiles [5,7,22,44,45]. As shown in Table 5, even if differences in total terpene concentrations are evident between the various Muscat-type varietals, their total contribution is higher (above 0.5 mg/L) than in other varietals used for sparkling wine production, such as Chardonnay and Riesling [46,47]. In the last sparkling wines, the composition of volatile compounds show a relevant contribution of esters, alcohols, and acetates [46,47].

Table 4.
Chemical profiles of sparkling wines.

Table 5.
Aromatic compounds in Muscat-related and Chardonnay, Pinot noir, and Riesling sparkling wines.
Other cultivars, such as the white grapes Albarín, Fernão-Pires, Godello, Malvasía, Verdejo, and Viura, as well as the red grapes Bobal, País, and Baga, which are traditional varieties used to produce still wines, have also been employed for the production of high-quality white sparkling wines [12,13,19,25,29] (Table 3 and Table 4). Studies on the organoleptic profiles of these grape varieties, their various ripening stages, the influence of the alternative soils, as well as other factors, have been conducted for the production of Spanish (i.e., Albarín, Viura, Malvasía, Verdejo, and Godello), German (i.e., Sauvignon blanc), Chilean (i.e., País), and Portuguese (i.e., Fernão-Pires and Baga) sparkling wines [12,20,21,25,26,27,51]. The grape variety and/or soil have been shown to significantly influence the relative contribution of various volatile and phenolic compounds in these wines [12,21,25,51]. These parameters influenced their organoleptic profiles more than shorter or longer aging times on lees [12,27]. Although sparkling wines made with Verdejo, Viura, Malvasía, Albarín, and Godello presented excellent physicochemical parameters (Table 4), Albarín and Verdejo wines showed more color and olfactory intensity than those elaborated with the other three grape varieties [25]. Albarín also had the highest contents of catechins, proanthocyanidins, and, together with Viura, hydroxycinnamates, all of which contribute to their phenolic profile [25]. Differences in the abundance and glycosylation profiles of grape glycoproteins could influence foam production and foam quality in non-conventional Sauvignon blanc Sekt versus conventional Riesling Sekt [13]. Portuguese Fernão-Pires sparkling wines show higher contents of volatile compounds that contribute to their varietal character, including linalool, hotrienol, α-terpineol, geraniol, and nerol, than Baga sparkling wines [21]. Baga wines, however, showed a higher maximum foam height than Fernão-Pires [20]. A detailed sensory characterization of Chilean País sparkling wines highlighted strong floral nuances, dependent on ethyl isobutyrate, isoamyl acetate, ethyl hexanoate, and β-phenylethanol, which are high-impact aromatic compounds [12]. Taken together, these studies reveal how the character of non-conventional grape cultivars may influence the innovative arena of sparkling wine production.
Distinctive characteristics of white grape cultivars can also be enhanced, for example, by applying skin pre-fermentative maceration, a technique that is usually performed in red and rosé sparkling wine production [39]. This strategy has been used to manufacture Spanish Pedro Ximenez sparkling wines, increasing the extraction of grape skin compounds that influence their ester profiles [33]. In the study by Ruiz Moreno et al. (2017), wines elaborated with pre-fermentative maceration displayed higher contents of ethyl esters of branched acids and cinnamates, while the absence of maceration rendered higher levels of ethyl esters of fatty acids and higher alcohol acetates [33]. Thus, classic methods used for red wine production may constitute alternative strategies for the elaboration of white sparkling wines with improved organoleptic characteristics.
In warm and dry climates, like traditional Mediterranean winemaking regions, harvesting at optimal grape maturity may be associated with insufficient acidity for sparkling wine production [39,40]. Innovative base wines manufactured with Maresco and Grillo grape musts, however, show high total acidity even when fully ripened grapes are used [15,17]. Maresco grapes, a minor variety grown in the Apulia region of Italy, besides high acidity values, also showed floral, fruity, and fatty notes, as a result of the presence of linalool and phenyl acetate (floral descriptors), isoamyl acetate, ethyl hexanoate, ethyl octanoate, and ethyl decanoate (fruity descriptors), and octanoic acid (fatty descriptors) [17]. Sicilian Grillo grape musts, also from a dry Mediterranean region, have shown optimal concentrations of tartaric and malic acids, with the experimentally produced wines presenting remarkable values of total acidity and low pH values (Table 3) [15]. Similarly, in tropical semi-arid winemaking regions in Northeastern Brazil (i.e., São Francisco Valley), Syrah and Chenin Blanc are adapted varieties for the production of high quality sparkling wines (Table 4) [11]. These Syrah (blanc de noir) and Chenin Blanc (blanc de blanc) wines showed different varietal-related volatile profiles: Chenin Blanc was characterized by the presence of 2,3-butanediol, 3-ethoxypropan-1-ol, diethyl succinate, and ethyl decanoate, while Syrah by benzaldehyde, butyric acid, and some acetates [11]. Interestingly, due to high temperatures, solar radiation rates, and irrigation throughout the year, one vine can produce two harvests per year in the São Francisco Valley region [11].
In geographic regions where the agricultural conditions do not favor the proper development of most V. vinifera grapes, V. non-vinifera species and their hybrids are commonly cultivated [52]. In Brazil, non-vinifera grape varieties (e.g., Villenave -V. labrusca × Riesling renano-, Niagara, Goethe) and innovative V. vinifera varieties, such as Manzoni Bianco, mostly employed in the elaboration of grape juices and wines, express interesting characteristics of aroma and a good balance between acidity and sugar content, resulting in appealing alternatives for sparkling wines [7,8,9]. In this context, studies of changes in phenolic composition, browning index, and glutathione content during 18 months of biological aging sur lies, for sparkling wines produced with Villenave, Niagara, Manzoni, and Goethe cultivars, revealed highly suitable changes in the phenolic profiles during the aging period [7]. For example, (+)-catechin and (−)-epicatechin, flavanols with an important influence on the astringency and color of wines, show concentrations of 1.31–14.05 mg/L and 2.64–5.70 mg/L, respectively, in these non-conventional sparkling wines [7]. Catechin and epicatechin levels of 3.52–5.80 mg/L and 1.56–2.15 mg/L, respectively, were measured in sparkling wines made with Sauvignon blanc, Riesling Renano, Pinot Grigio, Pinot noir, and Chardonnay [7]. These studies show that non-vinifera grape varieties could enable sparkling wine production in latitudes where V. vinifera cultivars are more sensitive to different pests, environmental stresses and/or high annual rainfall [15].
2.2. Rosé Sparkling Wines
For the production of rosé sparkling wines, red grape pressing conditions and/or maceration strategies render base wines with varying colors, depending on the concentration of color-determining compounds extracted from the grape skins [3]. The impact of various non-conventional red grape varieties on the quality of the resulting rosé sparkling wines has been evaluated. Pioneer studies using the red varieties Trepat and Monastrell, for the manufacture of rosé sparkling wine in Spain, largely contributed to the current use of these grapes for Cava production [31] (Table 3 and Table 4). These original studies showed that rosé Trepat was more similar to the white Cava wines (using a conventional blend of Xarel.lo, Macabeo, and Parellada) than to the rosé wines manufactured with Monastrell [31]. Organoleptic characterization of these sparkling wines showed that Trepat and Monastrell had very good sensory attributes and even slightly better foam profiles than the white Cava [31]. Interestingly, it was also reported that the foamability and color intensity of the sparkling wines significantly increased when Trepat base wines were blended with white varieties (Macabeo, Xarel.lo, and Parellada) to manufacture Cava [32]. Thus, these qualities of Trepat grapes validated their use for elaborating either blanc de noirs sparkling wines or novel versions of rosé Cava, and highlighted the value of autochthonous grape varieties for sparkling wine production.
Other Spanish cultivars such as Prieto Picudo, Tempranillo, and Garnacha, traditionally used to produce still wines, have also been characterized for their potential for manufacturing high quality rosé sparkling wines using the traditional method [25,27,28,53] (Table 3 and Table 4). These wines are rich in typical ethyl esters and alcohol acetates that contribute to their fruity aroma [51] and preserve their varietal characteristics, even after a long aging time (i.e., thirty months) [27]. Furthermore, the sensory and analytical characteristics of five Garnacha Tinta rosé sparkling wines, where second fermentation was performed using five alternative yeast strains, showed that their organoleptic properties mostly depend on the grape variety itself rather than on the strain used for second fermentation [53]. Garnacha rosé has also shown high phenolic potential and hydroxycinnamic acid concentrations, while Prieto Picudo rosé sparkling wines had high color intensity and anthocyanin concentrations, with high olfactory intensity and freshness in sensory analyses [25]. Also, these sparkling wines, besides optimal chemical parameters (Table 4), presented good foaming characteristics, similar to high quality sparkling wines like Champagne and Cava, with low levels of biogenic amines, which are desirable attributes in sparkling wine production [25,27,51,53].
2.3. Red Sparkling Wines
Red sparkling or semi-sparkling wines represent a small fraction of the sparkling wine production worldwide, and are mostly characterized by a slight red color and a relatively poor complexity [34,35], because red grapes harvested at the proper time for sparkling wine production (i.e., <21 °Brix), have not yet achieved adequate phenolic maturity and, due to the relatively low final content of alcohol (i.e., 10.0–11.5% v/v; Table 3), the extraction of phenolic compounds and aroma precursors from the grape berries into the base wine is relatively low [35]. To face many of the challenges associated with red sparkling wine production, various agricultural and enological practices have been proposed, including alternative harvest dates, mixing of different grape varieties, pre-fermentative cold maceration, rack and return, carbonic maceration, and treatment of pre-mature grapes by applying pectolytic enzymes [23,34,35,36].
The impact of Tempranillo grape maturity on the alcohol concentrations, acidity, pH, and color intensity of red sparkling wine production and aging definitely depends on the ripening stage of grapes (Table 3 and Table 4). Levels of soluble polysaccharides and oligosaccharides in Tempranillo base wines also increased with grape maturity, suggesting that these compounds are more easily extracted during maceration-fermentation [35]. Following these results, Pérez-Magariño et al. (2019) applied pre-fermentative cold maceration of pre-mature Tempranillo grapes, using dry ice to favor the extraction of skin compounds into the must [34]. The resulting wines showed a volatile composition similar to those produced from mature grapes, and also had good values in foam and sensory descriptors [34]. The study, however, also showed that the differences in overall volatile composition of the wines were impacted more by grape maturity than by the use of enological techniques [34]. In fact, sparkling wines made with mature Tempranillo grapes, despite their high alcohol content, showed better volatile composition and foam characteristics than those obtained from pre-mature Tempranillo grapes [34]. The use of pectinolytic enzymes and/or carbonic maceration on premature Tempranillo grapes, intended to contribute to the extraction of polyphenols and varietal aromas, did not improve the chemical and sensory qualities of the sparkling wines compared with those manufactured using mature Tempranillo grapes [36]. Moreover, Tempranillo red sparkling wines made with mature grapes have shown high contents of polyphenols, ethyl esters, alcohol acetates, and total volatile acids, as well as foam stability [34,36], while wines made using unripe grapes have vegetal aroma notes [34,36]. These studies suggest that mature grapes and traditional winemaking practices are options to be preferred for elaborating red sparkling wines using the traditional method (Table 3 and Table 4).
3. Indigenous Yeast Starters for First and Second Fermentation of Sparkling Wines Using Non-Conventional Grape Varieties and the Traditional Method
Yeast starters used in both grape must fermentations and secondary fermentations of base wines are exposed to highly hostile physicochemical environments. Grape juices and musts destined for base wine production have high osmotic pressure (i.e., 16–21 °Brix), high titratable acidity (i.e., >8 g/L tartaric acid), low pH (i.e., 2.9–3.2), growing ethanol and glycerol amounts, presence of sulfites, and progressive consumption of nutrients [2]. On the other hand, yeast starters used for second fermentation must meet additional technological requisites to those needed for first fermentation [2]. Second fermentation of base wines in bottles requires yeast strains capable of growing under high ethanol contents (10–12% v/v), low pH (2.9–3.2), low temperature (10–15 °C), high CO2 pressure (up to 6 atmospheres), relatively high levels of SO2 (50–80 mg/L), and high total acidity (7–10 g/L; measured as tartaric acid) (Table 3) [2]. In addition, these second-fermentation yeast strains should be able to properly flocculate and autolysate during aging [1,3,54,55], allowing the wines to remain in contact with the lees (i.e., mostly yeast cell debris and clarifying agents), shaping their sensory complexity [4,56,57]. The aging process also contributes to other sparkling wine properties such as foam (i.e., maximum height and stability) and bubble (i.e., size and persistence) profiles [25,27,39,58]. Thus, because sparkling wine organoleptic properties largely correlate with the physiological and metabolic characteristics of the yeasts used for fermentation, a proper selection of starters is essential [14,54,59].
Although a wide variety of Saccharomyces cerevisiae strains are commercialized for first and second fermentation of grape musts and base wines, respectively, there is increased interest in indigenous S. cerevisiae and non-Saccharomyces yeasts to improve the sensory attributes of the resulting wines [15,54,59]. Producing both base and sparkling wines with native yeast starters can give typicality to the wines and may offer valuable technological alternatives to the application of commercial starters that can lead to wine flavor standardization [54,59]. Interesting technological screening methods have been proposed to identify, characterize, and select novel and indigenous yeast strains with valuable potential in the sparkling wine industry [2,6,14,54,57,59,60,61]. Enological and technological traits (i.e., fermenting power and vigor, SO2 tolerance, alcohol tolerance, flocculence, production of acetic acid, glycerol, H2S, and volatile compounds, autolytic capacity, and foaming properties), sensory analyses, and genotypic screenings are currently being used as selection strategies for these yeast strains [6,14,54,57,59,60] (Figure 2). As a result, various non-commercial S. cerevisiae strains for first and second fermentation have been proposed to provide regional character to sparkling wines as a driver of innovation/segmentation in this market [14,54,57,59,60].
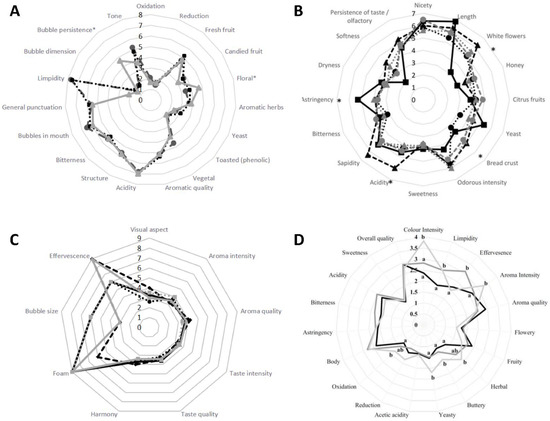
Figure 2.
Influence of yeast starters, used in secondary fermentation of base wines, on the sensory qualities of white, rosé, and red sparkling wines. (A) Mean ratings of the attributes of Torrontés Riojano white sparkling wines after 10 months of aging on lees, obtained using commercial yeasts EC1118 (gray dashes), bayanus C12 (black dots), and mutant S. cerevisiae strain IFI473I (gray line). (A) was prepared based on data from reference [6]. (*) Attributes with significant differences between treatments (p < 0.05). (B) Mean ratings of the attributes of Verdicchio white sparkling wines obtained using the following single or combined starters: S. cerevisiae DiSVA 527 (black line), T. delbrueckii DiSVA130 (black dashes), DiSVA 313 (black dots), S. cerevisiae DiSVA 527 plus T. delbrueckii DiSVA 130 (gray dashes), and S. cerevisiae DiSVA 527 plus T. delbrueckii DiSVA 313 (gray dots). The figure was licensed from reference [18]. (*) Attributes with significant differences between treatments (p < 0.05). (C) Mean ratings of the attributes of Garnacha tinta rosé sparkling wines obtained with the following yeast starters: S. cerevisiae IMIA-2010 (black line), IMIA-2011 (black dashes), IMIA-2012 (black dots), IMIA-2013 (gray dashes), and EC1118 (gray line). The figure was prepared based on data from reference [53]. (D) Mean ratings of the attributes of Tempranillo red sparkling wines obtained using the following yeast starters: S. cerevisiae 7VA (black line), Saccharomycodes ludwigii 979 (dark gray line), and Schizosaccharomyces pombe 938 (light gray line). Values in the same line with the same letter are not significantly different (p < 0.05). The figure was licensed from reference [24].
Producing sparkling wine with the use of alternative indigenous yeast strains renders wines with varying organoleptic profiles [14,15,60]. What is more, native S. cerevisiae strains have revealed similar [53,59] and even improved [54,62] physicochemical parameters when compared with commercial starters for second fermentation [53,54,59,62] (Figure 2). Studies by Di Gianvito et al. (2018) compared the second fermentation performance of the commercial strains FI (i.e., a mixture of strains IOC-18-2007 and FRC) and EC1118 with six indigenous Italian S. cerevisiae wine strains (i.e., F6789, F6030, F10599, F10477, F10471, and F7101), selected on the basis of their degree of flocculation and autolytic capacity [54]. Their results showed that the indigenous yeast strains had an enological performance comparable, in terms of fermentation rate and the maximum pressure reached, to the commercial strains [54]. This study also highlighted the phenotypic differences of some yeasts, in terms of their autolysis profiles, as well as the different levels of aromatic compounds released after six months of aging [54]. Sparkling wines obtained with strains F10471 and F10477 presented the highest amount of esters (e.g., 3-methylbut-1-yl ethanoate, ethyl ethanoate, ethyl octanoate, ethyl decanoate and ethyl dodecanoate), which can be attributed to the strong autolytic ability of these strains [54]. In similar studies, Martí-Raga et al. (2016) showed that Cava wines fermented with the indigenous yeast strain P29, isolated from the Penedes grape-growing region in Spain, reached a remarkable foam height and sensory profiles compared to sparkling wines elaborated using the commercial S. cerevisiae strains PDM and ARM [62].
In a detailed study of first and second fermentation of Apulian grape varieties (i.e., Nero di Troia and Bombino bianco) using native yeast strains for the production of white and rosé sparkling wines, Garofalo et al. (2018) reported a yeast strain-dependent profile of the release of volatile compounds [14]. Interestingly, they also showed that alternative native yeast strains influenced the final CO2 pressure values of white Bambino bianco sparkling wines, confirming the suitability of native S. cerevisiae strains for improving the quality of these wines [14]. Indigenous Italian yeast strains have also been tested for the production of Grillo base wines in dry Italian Mediterranean climates [15]. These native strains (i.e., CS182, GR1, MSE13 and MSE41) showed their ability to start fermentation at low pH and in the presence of high amounts of organic acids, contributing to some herbaceous and vegetal, floral, and exotic fruit descriptors that clearly distinguished each of the Grillo base wines produced [15]. Taken together, these results support the idea that indigenous S. cerevisiae yeasts could be exploited as starter cultures, differentiating between sparkling wines, and linking them with their region of production.
Besides the selection of native S. cerevisiae strains, Saccharomyces non-cerevisiae and non-Saccharomyces yeasts have also been proposed as alternatives for improving the enological features and flavor complexity of second fermentations of base wines [37,38,63,64]. Saccharomyces bayanus and Saccharomyces oviformis have been used both in free forms and immobilized within coated alginate beads as inoculums for the second fermentation of Turkish Emir and Drimit sparkling wines [37,38]. During the second fermentation of Emir and Drimit base wines, no significant differences were found in free amino acids and amino acids in peptides between the use of immobilized and free yeasts [37,38]. However, significant differences in these compounds were found due to the aging time and the yeast strains used [37,38]. Free amino acids were higher in the Emir sparkling wine made with S. oviformis than with S. bayanus [38], and in the Drimit sparkling wine fermented with in S. cerevisiae than with S. bayanus [37].
Among non-Saccharomyces species, Metschnikowia pulcherrima (Flavia® strain) and Torulaspora delbrueckii (Biodiva™ strain) have been tested, in sequential inoculations with S. cerevisiae, for their impact on the composition and quality of Macabeo base wine for sparkling wine production [64]. This study showed that base wines fermented with the addition of M. pulcherrima resulted in an increase in foam persistence and changes in the aromatic profile, characterized by smoky and flowery notes [64]. Sequential addition of T. delbrueckii increased glycerol concentration, reduced volatile acidity, and exerted a positive effect on foaming properties when compared with control base wines fermented with S. cerevisiae [64]. Further studies showed that the foaming properties of the sparkling wines obtained by sequential inoculation with these T. delbrueckii and S. cerevisiae strains resulted in sparkling wines with significantly higher maximum foam heights than conventional S. cerevisiae inoculation, probably due to the autolysis of T. delbrueckii cells in the base wine [65]. Another report used T. delbrueckii strains (i.e., Td130/313) to conduct second fermentations of Verdicchio base wine, both in mixed fermentations with S. cerevisiae and as pure inocula [18] (Figure 2B). The T. delbrueckii strains tested were able to complete the secondary fermentation and confirmed the previously reported behavior of T. delbrueckii in still wines, leading to a reduction in acetaldehyde and some higher alcohols, and increasing the production of ester compounds [18]. Thus, T. delbrueckii sparkling wines showed overall different aromatic compositions and sensory profiles to those of pure S. cerevisiae starters, with higher scores for positive aromatic descriptors [18] (Figure 2B).
Finally, physicochemical properties and sensory evaluations of sparkling wines made by second fermentation in bottle with Saccharomycodes ludwigii and Schizosaccharomyces pombe have been studied by Ivit et al. (2018) [24] (Figure 2D). These non-Saccharomyces yeasts properly completed the second fermentation of Airén white base wine and of Tempranillo red base wine, showing significant differences in acidity parameters, non-volatile compound levels, and sensory evaluations compared to control sparkling wines produced with S. cerevisiae [24] (Figure 2D). These studies demonstrated the potential of non-Saccharomyces yeasts to develop flavor complexity in sparkling wine production. The wide diversity of non-Saccharomyces species and strains constitute a great resource in the arena of sparkling wine innovation. Further studies are needed to define the potential of these non-Saccharomyces yeast starters [2].
4. Conclusions
Innovation and diversification in the manufacturing of high-quality sparkling wines is largely associated with the use of non-conventional grape varieties and yeast starters. A worldwide repertoire of prestigious grape cultivars, traditionally used in the production of high quality still wines, has demonstrated suitable enological properties for elaborating white, rosé, and red sparkling wines. These non-conventional grape varieties can positively contribute to the sensory diversification of sparkling wines, while showing comparable physicochemical profiles to Champagne and Cava. The diversity of these non-conventional grape varieties opens a window of opportunities for winemakers to innovate and satisfy consumer expectations. Novel and/or indigenous yeast starters for first and/or second fermentation can make interesting contributions. Native S. cerevisiae strains and/or S. non-cerevisiae and/or non-Saccharomyces yeasts offer new tools for innovation. The creative use of alternative technological operations in winemaking would also contribute to diversifying the sensory profiles of the growing number of non-conventional sparkling wines.
Author Contributions
M.L.R.E. and A.L.R. equally contributed to the conception, drafting, revising of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Católica de Córdoba grant SIV-2015. M.L.R.E. is supported by a postdoctoral fellowship from the Argentine National Research Council (CONICET) and A.L.R. is a Principal Investigator of CONICET (Argentina).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank J. Seballe for a critical reading of the manuscript and J. Heywood for English editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cebollero, E.; Rejas, M.T.; Gonzalez, R. Chapter 12 Autophagy in Wine Making, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2008; Volume 451. [Google Scholar]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter cultures for sparkling wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Buxaderas, S.; López-Tamames, E. Sparkling Wines: Features and Trends from Tradition; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 66, ISBN 9780123945976. [Google Scholar]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, N.; Ristic, R.; Culbert, J.A.; Pearce, K.; Wilkinson, K.L. Investigating australian consumers’ perceptions of and preferences for different styles of sparkling wine using the fine wine instrument. Foods 2021, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Raymond Eder, M.L.; Fariña, L.; Dellacassa, E.; Carrau, F.; Rosa, A.L. Chemical and sensory features of Torrontes Riojano sparkling wines produced by second fermentation in bottle using different Saccharomyces strains. Food Sci. Technol. Int. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sartor, S.; Burin, V.M.; Ferreira-Lima, N.E.; Caliari, V.; Bordignon-Luiz, M.T. Polyphenolic Profiling, Browning, and Glutathione Content of Sparkling Wines Produced with Nontraditional Grape Varieties: Indicator of Quality During the Biological Aging. J. Food Sci. 2019, 84, 3546–3554. [Google Scholar] [CrossRef]
- Caliari, V.; Burin, V.M.; Rosier, J.P.; BordignonLuiz, M.T. Aromatic profile of Brazilian sparkling wines produced with classical and innovative grape varieties. Food Res. Int. 2014, 62, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Nicolli, K.P.; Welke, J.E.; Closs, M.; Caramão, E.B.; Costa, G.; Manfroi, V.; Zini, C.A. Characterization of the volatile profile of Brazilian moscatel sparkling wines through solid phase microextraction and gas chromatography. J. Braz. Chem. Soc. 2015, 26, 1411–1430. [Google Scholar] [CrossRef]
- Caliari, V.; Panceri, C.P.; Rosier, J.P.; Bordignon-Luiz, M.T. Effect of the traditional, charmat and asti method production on the volatile composition of moscato giallo sparkling wines. LWT-Food Sci. Technol. 2015, 61, 393–400. [Google Scholar] [CrossRef]
- Nascimento, A.M.d.S.; de Souza, J.F.; Lima, M.D.S.; Pereira, G.E. Volatile profiles of sparkling wines produced by the traditional method from a semi-arid region. Beverages 2018, 4, 103. [Google Scholar] [CrossRef] [Green Version]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, Á. Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Pegg, C.L.; Phung, T.K.; Caboche, C.H.; Niamsuphap, S.; Bern, M.; Howell, K.; Schulz, B.L. Quantitative Data-Independent Acquisition Glycoproteomics of Sparkling Wine. Mol. Cell. Proteom. 2021, 20, 100020. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, A.; Francesca, N.; Mercurio, V.; Prestianni, R.; Settanni, L.; Spanò, G.; Naselli, V.; Moschetti, G. Use of grape racemes from Grillo cultivar to increase the acidity level of sparkling base wines produced with different Saccharomyces cerevisiae strains. Yeast 2020, 37, 475–486. [Google Scholar] [CrossRef]
- Montevecchi, G.; Masino, F.; Simone, G.V.; Cerretti, E.; Antonelli, A. Aromatic profile of white sweet semi-sparkling wine from Malvasia di candia aromatica grapes. S. Afr. J. Enol. Vitic. 2015, 36, 267–276. [Google Scholar] [CrossRef]
- Tufariello, M.; Pati, S.; D’Amico, L.; Bleve, G.; Losito, I.; Grieco, F. Quantitative issues related to the headspace-SPME-GC/MS analysis of volatile compounds in wines: The case of Maresco sparkling wine. Lwt 2019, 108, 268–276. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for secondary fermentation in sparkling wine production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.C.; Riponi, C.; Chinnici, F. Chitosan in sparkling wines produced by the traditional method: Influence of its presence during the secondary fermentation. Foods 2020, 9, 1174. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Reis, A.; Domingues, M.R.M.; Rocha, S.M.; Coimbra, M.A. Synergistic effect of high and low molecular weight molecules in the foamability and foam stability of sparkling wines. J. Agric. Food Chem. 2011, 59, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Coimbra, M.A.; Nogueira, J.M.F.; Rocha, S.M. Quantification approach for assessment of sparkling wine volatiles from different soils, ripening stages, and varieties by stir bar sorptive extraction with liquid desorption. Anal. Chim. Acta 2009, 635, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Cotea, V.V.; Focea, M.C.; Luchian, C.E.; Colibaba, L.C.; Scutarașu, E.C.; Marius, N.; Zamfir, C.I.; Popîrdă, A. Influence of different commercial yeasts on volatile fraction of sparkling wines. Foods 2021, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Coldea, T.E.; Mudura, E.; Fărcaș, A.; Marc, L. Valorisation of hybrid grape variety into processing of red sparkling wine. J. Agroaliment. Process. Technol. 2016, 22, 6–9. [Google Scholar]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J.A. Making natural sparkling wines with non-Saccharomyces yeasts. Eur. Food Res. Technol. 2018, 244, 925–935. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Ortega-Heras, M.; Pérez-Magariño, S. Sparkling wines produced from alternative varieties: Sensory attributes and evolution of phenolics during winemaking and aging. Am. J. Enol. Vitic. 2013, 64, 39–49. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Ortega-Heras, M.; Pérez-Magariño, S. Changes in polysaccharide composition during sparkling wine making and aging. J. Agric. Food Chem. 2013, 61, 12362–12373. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; Ortega-Heras, M.; Bueno-Herrera, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Grape variety, aging on lees and aging in bottle after disgorging influence on volatile composition and foamability of sparkling wines. LWT-Food Sci. Technol. 2015, 61, 47–55. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S. Role of major wine constituents in the foam properties of white and rosé sparkling wines. Food Chem. 2015, 174, 330–338. [Google Scholar] [CrossRef]
- García, M.J.; Aleixandre, J.L.; Álvarez, I.; Lizama, V. Foam aptitude of Bobal variety in white sparkling wine elaboration and study of volatile compounds. Eur. Food Res. Technol. 2009, 229, 133–139. [Google Scholar] [CrossRef]
- Liu, P.H.; Vrigneau, C.; Salmon, T.; Hoang, D.A.; Boulet, J.C.; Jégou, S.; Marchal, R. Influence of grape berry maturity on juice and base wine composition and foaming properties of sparkling wines from the champagne region. Molecules 2018, 23, 1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozo-Bayón, M.A.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Andujar-Ortiz, I.; Pueyo, E. Impact of using Trepat and Monastrell red grape varieties on the volatile and nitrogen composition during the manufacture of rosé Cava sparkling wines. LWT-Food Sci. Technol. 2010, 43, 1526–1532. [Google Scholar] [CrossRef] [Green Version]
- Girbau-Solà, T.; López-Barajas, M.; López-Tamames, E.; Buxaderas, S. Foam aptitude of Trepat and Monastrell red varieties in Cava elaboration. 2. Second fermentation and aging. J. Agric. Food Chem. 2002, 50, 5600–5604. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, M.J.; Muñoz-Redondo, J.M.; Cuevas, F.J.; Marrufo-Curtido, A.; León, J.M.; Ramírez, P.; Moreno-Rojas, J.M. The influence of pre-fermentative maceration and ageing factors on ester profile and marker determination of Pedro Ximenez sparkling wines. Food Chem. 2017, 230, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Magariño, S.; Bueno-Herrera, M.; López de la Cuesta, P.; González-Lázaro, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Volatile composition, foam characteristics and sensory properties of Tempranillo red sparkling wines elaborated using different techniques to obtain the base wines. Eur. Food Res. Technol. 2019, 245, 1047–1059. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Apolinar-Valiente, R.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S.; Williams, P.; Doco, T. Influence of Grape Maturity on Complex Carbohydrate Composition of Red Sparkling Wines. J. Agric. Food Chem. 2016, 64, 5020–5030. [Google Scholar] [CrossRef] [PubMed]
- González-Lázaro, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestaran, B.; Bueno-Herrera, M.; López de la Cuesta, P.; Pérez-Magariño, S. Evaluation of grape ripeness, carbonic maceration and pectolytic enzymes to improve the chemical and sensory quality of red sparkling wines. J. Sci. Food Agric. 2020, 100, 2618–2629. [Google Scholar] [CrossRef]
- Bozdogàn, A.; Canbaş, A. The effect of yeast strain, immobilisation, and ageing time on the amount of free amino acids and amino acids in peptides of sparkling wines obtained from cv. dimrit grapes. S. Afr. J. Enol. Vitic. 2012, 33, 257–263. [Google Scholar] [CrossRef]
- Bozdogan, A.; Canbas, A. Influence of yeast strain, immobilisation and ageing time on the changes of free amino acids and amino acids in peptides in bottle-fermented sparkling wines obtained from Vitis vinifera cv. Emir. Int. J. Food Sci. Technol. 2011, 46, 1113–1121. [Google Scholar] [CrossRef]
- Zoecklein, B. A Review of Methode Champagne Production; Virginia Coop. Ext.: Ettrick, VA, USA, 2002. [Google Scholar]
- Jones, J.E.; Kerslake, F.L.; Close, D.C.; Dambergs, R.G. Viticulture for sparkling wine production: A review. Am. J. Enol. Vitic. 2014, 65, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.D.; Welke, J.E.; Nicolli, K.P.; Zanus, M.; Caramão, E.B.; Manfroi, V.; Zini, C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015, 183, 291–304. [Google Scholar] [CrossRef]
- Ganss, S.; Kirsch, F.; Winterhalter, P.; Fischer, U.; Schmarr, H.G. Aroma changes due to second fermentation and glycosylated precursors in Chardonnay and Riesling sparkling wines. J. Agric. Food Chem. 2011, 59, 2524–2533. [Google Scholar] [CrossRef]
- Pérez, D.; Assof, M.; Bolcato, E.; Sari, S.; Fanzone, M. Combined effect of temperature and ammonium addition on fermentation profile and volatile aroma composition of Torrontés Riojano wines. LWT-Food Sci. Technol. 2018, 87, 488–497. [Google Scholar] [CrossRef]
- Culbert, J.A.; Ristic, R.; Ovington, L.A.; Saliba, A.J.; Wilkinson, K.L. Sensory profiles and consumer acceptance of different styles of Australian Moscato. Aust. J. Grape Wine Res. 2018, 24, 96–104. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Boidron, J.N.; Terrier, A. Aroma of Muscat Grape Varieties. J. Agric. Food Chem. 1975, 23, 1042–1047. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Simpson; Miller Aroma composition of Chardonnay wine. VITIS-J. Grapevine Res. 1984, 23, 143.
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef] [Green Version]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Song, M.; Xia, Y.; Tomasino, E. Investigation of a Quantitative Method for the Analysis of Chiral Monoterpenes in White Wine by HS-SPME-MDGC-MS of Different Wine Matrices. Molecules 2015, 20, 7359–7378. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Magariño, S.; Ortega-Heras, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Multivariate analysis for the differentiation of sparkling wines elaborated from autochthonous Spanish grape varieties: Volatile compounds, amino acids and biogenic amines. Eur. Food Res. Technol. 2013, 236, 827–841. [Google Scholar] [CrossRef]
- Raymond Eder, M.L.; Rosa, A.L. Yeast diversity in Vitis non-vinifera ecosystems. Rev. Argent. Microbiol. 2019, 51, 278–283. [Google Scholar] [CrossRef]
- Hidalgo, P.; Pueyo, E.; Pozo-Bayón, M.Á.; Martínez-Rodríguez, A.; Martín-Álvarez, P.; Polo, M.C. Sensory and analytical study of rosé sparkling wines manufactured by second fermentation in the bottle. J. Agric. Food Chem. 2004, 52, 6640–6645. [Google Scholar] [CrossRef]
- Di Gianvito, P.; Perpetuini, G.; Tittarelli, F.; Schirone, M.; Arfelli, G.; Piva, A.; Patrignani, F.; Lanciotti, R.; Olivastri, L.; Suzzi, G.; et al. Impact of Saccharomyces cerevisiae strains on traditional sparkling wines production. Food Res. Int. 2018, 109, 552–560. [Google Scholar] [CrossRef]
- Borrull, A.; Poblet, M.; Rozès, N. New insights into the capacity of commercial wine yeasts to grow on sparkling wine media. Factor screening for improving wine yeast selection. Food Microbiol. 2015, 48, 41–48. [Google Scholar] [CrossRef]
- Nunez, Y.P.; Carrascosa, A.V.; Gonzalez, R.; Polo, M.C.; Martínez-Rodríguez, A. Effect of accelerated autolysis of yeast on the composition and foaming properties of sparkling wines elaborated by a champenoise method. J. Agric. Food Chem. 2005, 53, 7232–7237. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Carrascosa, A.V.; Martín, V.; Moreno-Arribas, M.V.; Polo, M.C. Influence of the yeast strain on the changes of the amino acids, peptides and proteins during sparkling wine production by the traditional method. J. Ind. Microbiol. Biotechnol. 2002, 29, 314–322. [Google Scholar] [CrossRef]
- Kemp, B.; Condé, B.C.; Jégou, S.; Howell, K.S.; Vasserot, Y.; Marchal, R. Chemical compounds and mechanisms involved in the formation and stabilization of foam in sparkling wines. Crit. Rev. Food Sci. Nutr. 2019, 59, 2072–2094. [Google Scholar] [CrossRef]
- Vigentini, I.; Cardenas, S.B.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of native yeast strains for in-bottle fermentation to face the uniformity in sparkling wine production. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Carrascosa, A.V.; Barcenilla, J.M.; Angeles Pozo-Bayón, M.; Carmen Polo, M. Autolytic capacity and foam analysis as additional criteria for the selection of yeast strains for sparkling wine production. Food Microbiol. 2001, 18, 183–191. [Google Scholar] [CrossRef]
- Carrau, F.; Boido, E.; Ramey, D. Yeasts for Low Input Winemaking: Microbial Terroir and Flavor Differentiation, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 111. [Google Scholar]
- Marti-Raga, M.; Martín, V.; Gil, M.; Sancho, M.; Zamora, F.; Mas, A.; Beltran, G. Contribution of yeast and base wine supplementation to sparkling wine composition. J. Sci. Food Agric. 2016, 96, 4962–4972. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The impact of non-Saccharomyces yeast on traditional method sparkling wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Medina-Trujillo, L.; González-Royo, E.; Sieczkowski, N.; Heras, J.; Canals, J.M.; Zamora, F. Effect of sequential inoculation (Torulaspora delbrueckii/Saccharomyces cerevisiae) in the first fermentation on the foaming properties of sparkling wine. Eur. Food Res. Technol. 2017, 243, 681–688. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).