Abstract
Yakju, a traditional fermented beverage in Korea, is prepared using various raw materials and methods, and, hence, exhibits various characteristics. Low-temperature-fermented yakju can inhibit the growth of undesirable bacteria and is known for its unique flavor and refreshing taste. To increase the production of volatile aromatic compounds in yakju, strains with strong resistance to low temperatures and excellent production of volatile aromatic compounds were screened from indigenous fruits (grape, persimmon, plum, aronia, wild grape) and nuruk in Korea. One Saccharomyces cerevisiae and three non-Saccharomyces strains were finally screened, and yakju was fermented at 15 °C through mono/co-culture. The analysis of volatile aromatic compounds showed that S. cerevisiae W153 produced 1.5 times more isoamyl alcohol than the control strain and reduced the production of 2,3-butanediol by a third. Similarly, a single culture of Pichia kudriavzevii N373 also produced 237.7 mg/L of ethyl acetate, whereas Hanseniaspora vineae G818 produced ~11 times greater levels of 2-phenethyl acetate than the control. Alternatively, Wickerhamomyces anomalus A159 produced 95.88 mg/L of ethyl hexadecanoate. During principal component analysis, we also observed that the co-culture sample exhibited characteristics of both volatile aroma compounds of the single cultured sample of each strain. Our results suggest that yakju with unique properties can be prepared using various non-Saccharomyces strains.
1. Introduction
Yakju is a genre of traditional Korean fermented liquor fermented with rice as the primary ingredient. It is characterized by a clean and intrinsic flavor by removing solids through filtration at the end of fermentation. Yakju is fermented between 20 and 25 °C, and when the fermentation temperature is lowered, the pH is reduced to suppress harmful bacteria that spoil the liquor; organic acids and volatile aromatic compounds are also different from those produced by fermentation at room temperature [1]. Low-temperature fermentation prevents the loss of primary aromatic compounds and increases the synthesis of secondary aromatic compounds (ethyl and acetate esters) [2,3,4]. Furthermore, studies have reported that yeast in a low-temperature fermentation environment is influenced by several factors, such as protein transcription, cell membrane fluidity, RNA structure stability, and enzyme activity, as well as aroma component-related reactions [5,6]; therefore, when low-temperature fermentation is performed using a low-temperature-resistant strain, it is possible to prevent spoilage and create yakju with a unique profile [7].
The following microorganisms are involved in the fermentation of yakju: for saccharification of rice, fungi such as Aspergillus, Rhizopus, and Mucor produce amylase to decompose starch into small molecules of saccharides [8], and then Saccharomyces cerevisiae and other microorganisms work to produce ethanol and numerous organic substances [9,10]. Depending on the yeast that is involved in the fermentation, the result shows significant differences, and the overall preference is affected because of its influence on the senses [11,12].
It is difficult to use most non-Saccharomyces strains as ethanol-producing strains in liquor fermentation because of their high sensitivity to ethanol. Moreover, non-Saccharomyces yeast has low primary metabolic efficiency, which limits fermentation performance; however, it can affect the organoleptic properties of the final fermentation product due to secondary metabolism [13,14]. It is often used alone or through co-fermentation with S. cerevisiae for liquor production [11,13,15,16]. To date, non-Saccharomyces strains, such as Torulaspora spp. [15,17], Metschnikowia spp. [18,19], Pichia spp. [20], Hanseniaspora spp. [21,22,23], and Brettanomyces spp. have been investigated. Fermentation performed using controlled inoculum can improve the quality of the final product and can ensure better wine quality by utilizing multiple metabolic pathways of non-Saccharomyces yeast [24]. Some yeasts have killer activity against spoiled microorganisms, which can be a biological inhibitor to prevent contamination by external microorganisms during fermentation [25,26].
In this study, to improve the flavor of low-temperature-fermented yakju, strains with excellent low-temperature tolerance and volatile aromatic compound production ability were first selected among ~700 strains of yeast derived from native fruits and nuruk (a traditional Korean fermentation starter). Then, using the selected strain, yakju fermentation at 15 °C (single/co-culture) was performed, and the characteristics were analyzed.
2. Materials and Methods
2.1. Strains and Media
A total of 661 isolates were used in this experiment, which were isolated from indigenous fruits (persimmon, wild grape, grape, aronia, plums) and nuruk owned by the Microbial Biotechnology Laboratory, Department of Food Science and Biotechnology, Kyungpook National University (Daegu, Korea). All strains were subcultured twice at 30 °C by shaking (150 rpm) in sterilized yeast extract peptone dextrose (YPD) media (1% yeast extract, 2% peptone, and 2% glucose (w/v)). Then, a lysine medium (Oxoid ltd, Basingstoke, Hampshire, UK) was used to confirm the growth of non-Saccharomyces strains. All strains were stored at −70 °C in 10% glycerol until they were used for the experiments. S. cerevisiae W-3, an industrial wine yeast strain, was used as a control for all experiments.
2.2. Screening of Yakju Yeast
2.2.1. Low-Temperature Fermentation Activity
After inoculation in YPD broth medium, the strains were cultured at 15 °C for 48 h, after which the absorbance was measured at 600 nm using a spectrophotometer (UV-1601, Shimadzu Co., Kyoto, Japan) to select strains with excellent growth. Subsequently, a strain possessing excellent fermentation ability was first screened through its gas-producing ability and sniffing tests performed by culturing in a rice saccharification solution at 15 °C for 96 h. In the case of the sniffing test, it was classified into positive and negative according to the occurrence of an off-flavor.
2.2.2. Screening by Internal Transcribed Spacer (ITS) Region Sequencing
The identification of the first screened strain was performed through phylogenetic analysis based on the nucleotide sequence of the ITS I-5.8S rDNA-ITS II region. After DNA extraction using the method by Looke et al. [27], ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) primers were then used with a Tpersnal 48 PCR machine (Biometra Co., Göttingen, Germany) for amplification. Then, for PCR-RFLP, Hinf I, Hae III, and Hpa I (Enzynomics Co., Daejeon, Korea) restriction enzymes were reacted at 37 °C for 1 h, after which DNA fragments were identified through electrophoresis. DNA sequencing was performed using the sequencing service of Solgent (Daejeon, Korea), and the nucleotide sequence homology was checked using the Basic Local Alignment Search Tool of the National Center for Biotechnology Information. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 6 (Tamura, Stecher, Peterson, Filipski, and Kumar, 2013).
2.2.3. β-Glucosidase Enzyme Activity
A modified Rodríguez’s method was used for measuring β-glucosidase activity [28]. The first screened strain was incubated for 48 h in YPD broth, and 1 mL was collected and centrifuged (1536, GYROZEN Co., Daejeon, Korea) at 9425× g for 3 min. Next, 0.2 mL of the supernatant, 0.2 mL of 20 mM pNPG (ρ-nitrophenyl-β-d-glucopyranoside), and 0.4 mL of 0.1 M citrate phosphate buffer (pH 5.0) were mixed and reacted in a water bath at 40 °C for 30 min. Finally, 0.8 mL of 2 M sodium carbonate was added to terminate the reaction, and absorbance was measured at 405 nm. One unit (U) of enzyme activity was defined as the amount of enzyme that released 1 μmol pNPG per minute under the experimental conditions.
2.2.4. Biosynthesis Inhibitor Resistance Activity
Cerulenin agar (0.67% yeast nitrogen base, 0.5% 10 mM cerulenin, 2% glucose, and 2% agar) and FPA agar (0.67% yeast nitrogen base, 0.4% 1 M FPA (ρ-fluoro phenylalanine), 2% glucose, and 2% agar) were inoculated with a single colony of the first screened strain. Resistance was confirmed by colony formation after incubation for 48 h at 30 °C [29].
2.3. Analysis of Low-Temperature-Fermented Yakju
2.3.1. Brewing Yakju
Steamed rice 270 g (Shindongjin, Iksan Rice Processing Plant, Iksan, Jeollabuk-do, Korea), koji 50 g (Aspergillus luchuensis, sp 60, Cheokgokshik, Hwaseong, Gyeonggi-do, Korea), 480 mL water, and 2.5% of the total volume of pre-cultured yeast were mixed. It was mixed once a day while fermentation was proceeding at 15 °C. For co-fermented samples, pre-cultured S. cerevisiae and non-Saccharomyces were mixed in a ratio of 1:9 and inoculated simultaneously. Growth of S. cerevisiae and non-Saccharomyces in co-fermented samples was compared every 5 days using Lee’s method [30]. Fermentation was terminated when no change was observed in the content of reducing sugars as assessed using colorimetry. Finally, all samples were centrifuged at 3578× g for 10 min and stored at 4 °C for further experiments.
2.3.2. Fermentation Characteristics of Yakju
The pH value was measured using an automatic titration device (DL22 Food and Beverage Analyzer, Mettler-Toledo AG Analytical, Schwerzenbach, Switzerland). Soluble solid content (° Brix) was measured using a refractometer (ATAGO, Co., N-1a, Kyoto, Japan). The reducing sugar content was measured by colorimetric quantification using DNS (3,5-dinitrosalicylic acid, Sigma Chemical Co., St. Louis, MO, USA) reagent. After adding 1 mL of DNS reagent to 0.3 mL of a sample to induce a reaction at 95 °C for 5 min, 7 mL of distilled water was added to measure the absorbance at 550 nm. The reducing sugar content was converted from the glucose standard curve. The alcohol content was measured using a hydrometer based on the specific gravity of yakju distillates (expressed as % [v/v]) at 15 °C.
2.3.3. Volatile Aromatic Compound Analysis by Gas Chromatography
The volatile aromatic compounds were determined using a gas chromatography mass spectrometer (7890A GC-MS; Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector, and analysis methods were referred to previously described methods [31]. The separation was performed using a DB-WAX column (60 m × 250 μm × 0.25 mm; Waters, Milford, MA, USA). The detector was an Agilent 5975C Inert XL MSD with a Triple-Axis Detector. Helium was used as a carrier gas at a constant flow rate of 1 mL/min. The temperature of the chromatographic oven was initially held at 40 °C for 2 min, increased at 2 °C/min to 220 °C, increased continuously at 20 °C/min to 240 °C, and then maintained at 240 °C for 5 min. The volatile ester compounds were collected using a solid-phase microextraction fiber (50/30 μm DVB/CAR/PDMS; Supelco, Bellefonte, PA, USA). Extraction of the volatile ester compounds from yakju was performed in a headspace (HS) mode with magnetic stirring. Briefly, 5 mL of the sample was placed in an HS vial (20 mm, PTFE/silicone septum, magnetic cap), and 1.25 g of NaCl was added to increase the volatile ester compound concentration in the HS. Before extraction, the sample was shaken in a water bath at 35 °C for 20 min to achieve equilibrium. The volatile ester compounds were identified based on a comparison of their GC retention times and mass spectrometry with spectral data from the Wiley 9th Edition/Nist 2008 library program(Wiley 9th Edition/Nist 2008 library version 5.0; John Wiley & Sons Inc., Hoboken, NJ, USA).
2.4. Statistical Analysis
Data were expressed as the mean ± standard deviation (SD) of three experiments. The SAS program (Statistics Analysis System, SAS Institute Inc., Cary, NC, USA) was used to perform analysis of variance and Duncan’s multi-range testing. The threshold level for statistical significance was set at p < 0.05. Principal component analysis (PCA) was conducted using R studio’s factoextra (https://cran.r-project.org/web/packages/factoextra/index.html, accessed on 6 November 2021) and ggplot2 (https://cran.r-project.org/web/packages/ggplot2/index.html, accessed on 6 November 2021).
3. Results and Discussion
3.1. Screening of Yakju Strains
The results of the screening of strains are expressed in Tables S1 and S2. Through the first screening (Table S1), 79 strains with excellent low-temperature growth and sniffing test results were first selected. Based on the result of ITS region nucleotide sequencing, 22 S. cerevisiae and 57 non-Saccharomyces were identified. Among them, there were 40 Wickerhamomyces anomalus, 12 Hanseniaspora vineae, 3 Torulaspora delbrueckii, and 2 Pichia kudriavzevii strains. Then, the biosynthesis inhibitor resistance test and β-glucosidase enzyme activity experiment were conducted, and the results are shown in Supplementary Table S2 (Table S2). The β-glucosidase enzyme activity was higher than 1 U/mL in 11 strains, and in the case of P. kudriavzevii N373, 1.41 U/mL was the highest activity. In the case of resistance to FPA, most H. vineae strains showed high resistance (11/12), and P. kudriavzevii also demonstrated the same result in both strains (2/2). For cerulenin resistance, colonies were identified in W. anomalus (39/40), T. delbreuckii (3/3), and P. kudriavzevii (2/2).
As β-glucosidase hydrolyzes β-1,4 bonds of nonvolatile glycosidic compounds to release volatile aromatic compounds [32,33], strains with high β-glucosidase enzyme activity can produce a large amount of volatile aromatic compounds include 2-phenethyl alcohol. Cerulenin is an antibiotic that inhibits the biosynthesis of fatty acids. The higher the resistance to cerulenin, the more favorable it is to produce medium-chain fatty acid (MCFA) ethyl esters, especially ethyl caproate, a flavor representing traditional Korean liquor, which can be produced up to five times [34]. ρ-Fluoro-phenylalanine (FPA) is a biosynthesis inhibitor through feedback and exists in the form of fluoroalanine attached to phenylalanine. The stronger the resistance to this compound, the more it produces 2-phenylethanol and 2-phenylethyl acetate, which gives off a rose-like scent [29].
Through the two-step screening, one S. cerevisiae and three non-Saccharomyces strains were finally selected, and one isolate from each strain was selected for the diversification of fragrance components, as shown in Table 1.

Table 1.
List of final selected strains.
S. cerevisiae W153 exhibited the best cell growth at 15 °C, and P. kudriavzevii N373 was resistant to both cerulenin and FPA. H. vineae G818 and W. anomalus A159 strains exhibited resistance to FPA and cerulenin, respectively.
3.2. Characteristics of Yakju
The characteristics of yakju after fermentation are shown in Figure 1. In the case of the co-culture samples, the growth of each strain was confirmed by the viable cell count and is shown in Supplementary Figure S1 (Figure S1, the results of the single cultures are not shown). The co-culture with non-Saccharomyces strains showed the lowest pH value of 3.32–3.35 (Figure 1a). The soluble solid content was at the maximum (10.37 Brix°) in W. anomalus A159, and H. vineae G818 showed the highest reducing sugar content (Figure 1b,c). S. cerevisiae single/co-culture samples consumed all the reducing sugars, whereas the non-Saccharomyces group did not, with the expectation of a decrease or death occurring as the ethanol content increased during fermentation [35]. Because most non-Saccharomyces species had lower ethanol resistance and titers than those of S. cerevisiae [36], the final ethanol content was also lower than that of S. cerevisiae. Nevertheless, all fermented samples showed a high ethanol content of 8% or more, which was expected to be characteristic of low-temperature long-term fermentation and yakju [7,37].
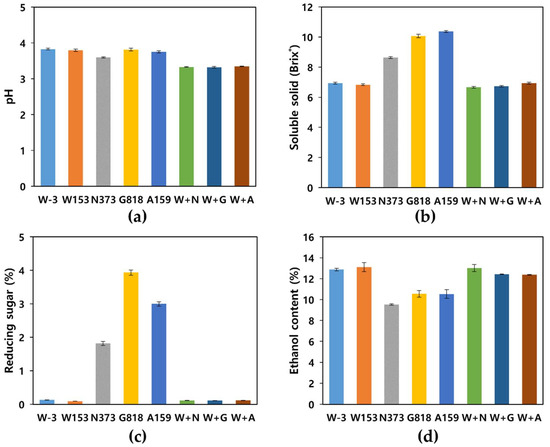
Figure 1.
Characteristics of yakju after fermentation: (a) pH; (b) Soluble solid (Brix°); (c) Reducing sugar (%); (d) Ethanol content (%). W + N, co-culture of W153 + N373; W + A, co-culture of W153 + A159; W + G, co-culture of W153 + G818.
In the case of the co-culture group (W153 + N373, W153 + G818, and W153 + A159), an amount of ethanol similar to that of the single culture of S. cerevisiae was produced (Figure 1d), which was consistent with the results of previous studies [36,38,39]. The S. cerevisiae W153 strain exhibited fermentation characteristics similar to those of the control.
3.3. Volatile Aromatic Compounds
Regarding the volatile aromatic compounds of yakju, six alcohols and fourteen esters were detected by headspace GC-MS, as shown in Table 2. Compared with the results obtained from the analysis of volatile aroma components of commercial yakju [40], a large concentration of higher alcohols and esters was produced. This high concentration was expected due to differences in fermentation processes (dilution) and conditions (temperature, fermentation time, etc.). In the single culture, S. cerevisiae (W-3 and W153) produced more total higher alcohol content (507–556 mg/L) than non-Saccharomyces strains (206–397 mg/L), whereas regarding esters, non-Saccharomyces strains produced two to three times higher ester content. In the case of co-culture, the characteristics of the strains used for fermentation appeared similar, and the content of total volatile aromatic compounds increased. Some components showed higher values than those of single fermentation (isoamyl alcohol, 1-propanol, 2-phenethyl alcohol, and ethyl decanoate).

Table 2.
Volatile aromatic compounds of yakju after fermentation.
Higher alcohols were quantitatively the most abundant group in yakju and contributed to the aroma and essential characteristics [40]. However, excessive production of higher alcohols has a negative impact on the aroma, and an appropriate concentration can have a positive impact, such as a fruity flavor [41]. Isoamyl alcohol is a major volatile compound among higher alcohols, which boosts the aroma and flavor [42]. S. cerevisiae W153 produced ~1.5 times more isoamyl alcohol than the control strain (S. cerevisiae W-3). One of the primary components of wine, 2,3-Butanediol, is produced from carbohydrates during the alcohol fermentation process and has a negative impact on liquor by causing bitter taste, vinegar odor, and yeast odor [43,44]. S. cerevisiae W153 could reduce the production of 2,3-butanediol by one-third. Ethyl acetate levels with higher quantities of 150 mg/L are a measure of non-Saccharomyces-dependent spoilage in the wine industry [45]. However, a large quantity of ethyl acetate is preferred for grain-based alcoholic beverages and spirits [46,47]. During this study, P. kudriavzevii N373 produced approximately nine times more ethyl acetate (237 mg/L) than the control.
MCFA ethyl esters (ethyl hexanoate, ethyl octanoate, ethyl nonanoate, and ethyl decanoate) are fatty acid ethyl esters composed of 6–12 carbon atoms and have a low threshold, which can cause a large sensory change even in a small amount [48]. Particularly, ethyl hexanoate (ethyl caproate) is an important fragrance ingredient in products such as premium sake and exhibits a pleasant, fruity apple-like flavor [49]. Two S. cerevisiae strains (W-3, W153) produced the highest amount of ethyl hexanoate (0.50–0.54 mg/L) [50], and co-culture increased the production compared to that with a non-Saccharomyces single culture. In the case of ethyl nonanoate, W. anomalus A159 showed the highest value of 11.54. The production of 2-phenylethyl acetate by yeast cells is responsible for a fruity, flowery flavor. H. vineae G818, FPA biosynthesis-resistant-positive strain, produced ~33.76 mg/L of 2-phenethyl acetate, which was ~11 times higher than that of the control and co-culture with S. cerevisiae W153 also demonstrated similar results (28.38 mg/L) [51]. Ethyl tetradecanoate (ethyl myristate) and ethyl hexadecanoate (ethyl palmitate), components of aged whiskey [52], were four times and nine times higher, respectively, in a single culture of W. aomalus A159.
3.4. Principal Component Analysis
Figure 2 shows the biplot results for the volatile fragrance components. Based on PC1, it could be divided into two groups: non-Saccharomyces single culture (B) and S. cerevisiae single/co-culture samples (A). Moreover, it was confirmed that PC2 showed similar values depending on the non-Saccharomyces strain involved in fermentation. In the case of P. kudriavzevii N373, a significant difference was observed between the single culture and co-culture (W + N, N373), but the single fermentation of A159 and G818 strains showed similar PC2 values to those of co-culture (W + A and W + G, respectively). Results of the loading plot (blue arrow) indicate that most aromatic compounds were contained in group A (13/20), and the aromatic compounds were strengthened through co-culture. Methionol, ethyl acetate, methyl salicylate, and ethyl tetradecanoate were specifically produced in large amounts in the single-culture non-Saccharomyces group.
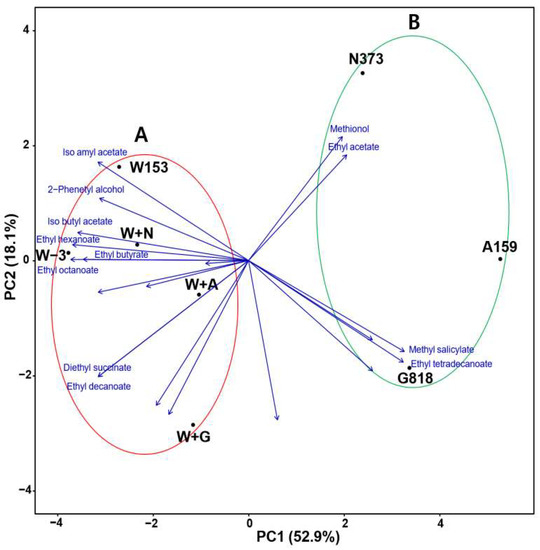
Figure 2.
Biplot of variable loadings (represented by vectors) and scores (marked by points) from the principal component analysis (PCA) of volatile fragrance. W + N, co-culture of W153 + N373; W + A, co-culture of W153 + A159; W + G, co-culture of W153 + G818.
4. Conclusions
Low-temperature fermentation prevents bacteria-dependent spoilage and preserves flavor; therefore, this method produces a different aroma component profile from that observed with room temperature fermentation. To strengthen the flavor components of yakju, a traditional Korean alcoholic beverage, strains possessing excellent low-temperature fermentation abilities, and which could produce numerous volatile aromatic compounds were selected from indigenous fruits and nuruk. Its fermentation characteristics were then compared after the preparation of low-temperature yakju. In this study, yakju was prepared using selected strains, which were mono or co-cultured with Saccharomyces cerevisiae and fermented at 15 °C. Due to the analysis of volatile aroma components produced, characteristic esters and higher alcohols were detected in each strain. Therefore, it was confirmed that the co-culture samples had characteristics of both strains. Based on these studies, it was, however, expected that yakju, having various aroma component profiles, would be produced using several indigenous yeasts. In addition to S. cerevisiae, a traditional yeast used in yakju brewing, studies have shown that yakju, having a unique aromatic profile, was produced using non-Saccharomyces yeast. However, specific correlation studies on the metabolism of the volatile fragrance component products in each strain and profile change assessments according to raw materials are required. Additional studies on changes in the fermentation profile according to the inoculation time and ratio of mixed samples are also required. Through these studies, we propose that it will be possible to contribute to the localization of yakju-based yeast and the manufacture of yakju using various volatile aroma components.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fermentation7040260/s1, Table S1: Low-temperature-tolerant and fermentation activity of isolated strains; Table S2: Biosynthesis inhibitor resistance and enzyme activity of first screened strains; Figure S1: Viable cell number of co-culture strain used in yakju fermentation.
Author Contributions
Conceptualization, J.-B.P., J.-S.C., H.-W.P., S.-B.L. and H.-D.P.; methodology, J.-B.P. and J.-S.C.; software, J.-B.P.; validation, J.-S.C., H.-W.P. and H.-D.P.; formal analysis, J.-B.P.; investigation, J.-B.P. and J.-S.C.; resources, J.-B.P., J.-S.C. and H.-W.P.; data curation, J.-B.P. and J.-S.C.; writing—original draft preparation, J.-B.P.; writing—review and editing, S.-B.L. and H.-D.P.; visualization, J.-B.P.; supervision, H.-D.P.; project administration, H.-D.P.; funding acquisition, H.-D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Rural Development Administration, Republic of Korea (Research grant PJ014550022021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, K.; Tuszyński, T. The effect of temperature on fermentation and beer volatiles at an industrial scale. J. Inst. Brew. 2018, 124, 230–235. [Google Scholar] [CrossRef]
- Torija, M.J.; Rozes, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef]
- Gamero, A.; Tronchoni, J.; Querol, A.; Belloch, C. Production of aroma compounds by cryotolerant Saccharomyces species and hybrids at low and moderate fermentation temperatures. J. Appl. Microbiol. 2013, 114, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.; Randez-Gil, F.; Prieto, J.A. Cold response in Saccharomyces cerevisiae: New functions for old mechanisms. FEMS Microbiol. Rev. 2007, 31, 327–341. [Google Scholar] [CrossRef]
- Schade, B.; Jansen, G.; Whiteway, M.; Entian, K.D.; Thomas, D.Y. Cold adaptation in budding yeast. Mol. Biol. Cell 2004, 15, 5492–5502. [Google Scholar] [CrossRef]
- Seo, D.-J.; Yeo, S.-H.; Mun, J.-Y.; Baek, S.Y. Effects of low temperature-adapted Saccharomyces cerevisiae Y297 strain and fermentation temperature on the quality characteristics of Yakju. Korean J. Food Preserv. 2016, 23, 666–672. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-R.; Kim, J.-H.; Bae, D.-H.; Ahn, B.-H. Characterization of Yakju brewed from glutinous rice and wild-type yeast strains isolated from nuruks. J. Microbiol. Biotechnol. 2010, 20, 1702–1710. [Google Scholar] [PubMed]
- Kim, H.-R.; Kwon, Y.-H.; Jo, S.-J.; Kim, J.-H.; Ahn, B.-H. Characterization and volatile flavor components in glutinous rice wines prepared with different yeasts of nuruks. Korean J. Food Sci. Technol. 2009, 41, 296–301. [Google Scholar]
- Rhee, S.J.; Lee, C.-Y.J.; Kim, K.K.; Lee, C.-H. Comparison of the traditional (Samhaeju) and industrial (Chongju) rice wine brewing in Korea. Food Sci. Biotechnol. 2003, 12, 242–247. [Google Scholar]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef]
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suárez-Lepe, J.A.; Han, S.; Benito, S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Larroque, M.; Carrau, F.; Fariña, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Fusco, V.; Böhnlein, C.; Kabisch, J.; Logrieco, A.F.; Habermann, D.; Cho, G.-S.; Benomar, N.; Abriouel, H.; Schmidt-Heydt, M. The life and times of yeasts in traditional food fermentations. Crit. Rev. Food Sci. Nutr. 2020, 60, 3103–3132. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Bañuelos, M.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT-Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; De Schutter, D.P.; Daenen, L.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Application of non-Saccharomyces yeasts isolated from kombucha in the production of alcohol-free beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Chasseriaud, L.; Coulon, J.; Marullo, P.; Albertin, W.; Bely, M. New oenological practice to promote non-Saccharomyces species of interest: Saturating grape juice with carbon dioxide. Appl. Microbiol. Biotechnol. 2018, 102, 3779–3791. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Portillo, M.d.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Tao, Y.-S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Patrignani, F.; Lanciotti, R.; Perpetuini, G.; Schirone, M.; Di Gianvito, P.; Pizzoni, D.; Arfelli, G.; Suzzi, G. Aroma profile of montepulciano d’abruzzo wine fermented by single and co-culture starters of autochthonous Saccharomyces and non-Saccharomyces yeasts. Front. Microbiol. 2016, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Ciani, M.; Fatichenti, F. Killer toxin of Kluyveromyces phaffii DBVPG 6076 as a biopreservative agent to control apiculate wine yeasts. Appl. Environ. Microbiol. 2001, 67, 3058–3063. [Google Scholar] [CrossRef]
- Comitini, F.; Ingeniis De, J.; Pepe, L.; Mannazzu, I.; Ciani, M. Pichia anomala and Kluyveromyces wickerhamii killer toxins as new tools against Dekkera/Brettanomyces spoilage yeasts. FEMS Microbiol. Lett. 2004, 238, 235–240. [Google Scholar] [CrossRef]
- Lõoke, M.; Kristjuhan, K.; Kristjuhan, A. Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques 2011, 50, 325–328. [Google Scholar] [CrossRef]
- Rodríguez, M.; Lopes, C.; Van Broock, M.; Valles, S.; Ramon, D.; Caballero, A. Screening and typing of Patagonian wine yeasts for glycosidase activities. J. Appl. Microbiol. 2004, 96, 84–95. [Google Scholar] [CrossRef]
- Akita, O.; Ida, T.; Obata, T.; Hara, S. Mutants of Saccharomyces cerevisiae producing a large quantity of β-phenethyl alcohol and β-phenethyl acetate. J. Ferment. Bioeng. 1990, 69, 125–128. [Google Scholar] [CrossRef]
- Lee, S.B.; Banda, C.; Park, H.D. Effect of inoculation strategy of non-Saccharomyces yeasts on fermentation characteristics and volatile higher alcohols and esters in Campbell Early wines. Aust. J. Grape Wine Res. 2019, 25, 384–395. [Google Scholar] [CrossRef]
- Lee, S.-B.; Park, H.-D. Isolation and investigation of potential non-Saccharomyces yeasts to improve the volatile terpene compounds in Korean Muscat Bailey A wine. Microorganisms 2020, 8, 1552. [Google Scholar] [CrossRef] [PubMed]
- Sarry, J.-E.; Günata, Z. Plant and microbial glycoside hydrolases: Volatile release from glycosidic aroma precursors. Food Chem. 2004, 87, 509–521. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.L.; Ullah, N.; Tao, Y.S. Aroma glycosides in grapes and wine. J. food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef]
- Ho, C.J.; Yeo, S.H.; Park, J.-H.; Choi, H.S.; Gang, J.-E.; Kim, S.I.; Jeong, S.T.; Kim, S.R. Isolation of aromatic yeasts (non-Saccharomyces cerevisiae) from Korean traditional nuruks and identification of fermentation characteristics. Agric. Sci. 2013, 4, 136. [Google Scholar]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Henschke, P.; Chambers, P.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, J.-H.; Bai, D.-H.; Ahn, B. Feasibility of brewing Makgeolli using Pichia anomala Y197-13, a non-Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2012, 22, 1749–1757. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Zhang, B.-Q.; Shen, J.-Y.; Duan, C.-Q.; Yan, G.-L. Use of indigenous Hanseniaspora vineae and Metschnikowia pulcherrima co-fermentation with Saccharomyces cerevisiae to improve the aroma diversity of Vidal Blanc icewine. Front. Microbiol. 2018, 9, 2303. [Google Scholar] [CrossRef]
- Heo, J.; Kwak, H.S.; Kim, M.; Kim, J.-H.; Baek, H.H.; Shin, H.; Lee, Y.-s.; Lee, S.; Kim, S.S. Major sensory attributes and volatile compounds of Korean rice liquor (yakju) affecting overall acceptance by young consumers. Foods 2020, 9, 722. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef]
- Ehsani, M.; Fernández, M.R.; Biosca, J.A.; Julien, A.; Dequin, S. Engineering of 2, 3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Palla, G.; Caligiani, A.; Brandolini, V.; Maietti, A.; Salzano, G. Evaluation of stereoisomers of 2, 3-butanediol and acetoin to differentiate Saccharomyces cerevisiae and Kloeckera apiculata wine strains. Biotechnol. Lett. 2000, 22, 1947–1951. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Boscolo, M.; Bezerra, C.W.; Cardoso, D.R.; Lima Neto, B.S.; Franco, D.W. Identification and dosage by HRGC of minor alcohols and esters in Brazilian sugar-cane spirit. J. Braz. Chem. Soc. 2000, 11, 86–90. [Google Scholar] [CrossRef][Green Version]
- Fu, Z.; Sun, B.; Li, X.; Fan, G.; Teng, C.; Alaa, A.; Jia, Y. Isolation and characterization of a high ethyl acetate-producing yeast from Laobaigan Daqu and its fermentation conditions for producing high-quality Baijiu. Biotechnol. Biotechnol. Equip. 2018, 32, 1218–1227. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tsuchiya, F.; Isogai, A. Relationship between medium-chain fatty acid contents and organoleptic properties of Japanese sake. J. Agric. Food Chem. 2014, 62, 8478–8485. [Google Scholar] [CrossRef]
- Carrascosa, A.V.; Muñoz, R.; González, R. Molecular Wine Microbiology; Elsevier: Cham, Switzerland, 2011. [Google Scholar]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.A.; Elkins, J.T. Comparison of unaged and barrel aged whiskies from the same Mash Bill using gas chromatography/mass spectrometry. J. Brew. Distill. 2019, 8, 1–6. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).