Abstract
This study aimed at examining the effects of rumen inoculum of steers receiving different combinations of ionophore and probiotics in their diets on in vitro gas production of corn silage. The fitting of gas production was performed with five mathematical models and its kinetics was evaluated. Four crossbred steers (403.0 ± 75.5 kg body weight) with ruminal cannula were assigned to a 4 × 4 Latin square design. The additives used were Monensin sodium (Rumensin® 100, 3 g/day), Bacillus toyonensis (Micro-Cell Platinum® 109, 1 g/day) and Saccharomyces cerevisiae boulardii (ProTernative®20, 0.5 g/day). Additives were arranged into the following treatments, supplied daily into total mixed diet: (1) Monensin; (2) Monensin + B. toyonensis; (3) Monensin + S. boulardii; and (4) B. toyonensis + S. boulardii. The gas production data were fitted into the models of Gompertz, Groot, Ørskov, Brody, Richards, and Dual-pool Logistic. A perfect agreement between observed and predicted values in curves of accumulated in vitro gas production was observed in the Groot and Richards models, with higher coefficient of determination (R2 = 0.770 and 0.771, respectively), concordance correlation coefficient (CCC = 0.871 and 0.870, respectively), and root mean square error of prediction (RMSEP = 1.14 and 1.15, respectively). Evaluating the feed additives throughout the Groot model, the B. toyonensis + S. boulardii treatment presented higher VF (12.08 mL/100 mg of DM; p = 0.0022) than Monensin and Monensin + S. boulardii (9.16 and 9.22 mL/100 mg of DM, respectively). In addition, the fractional rate of gas production (k) was higher (p = 0.0193) in B. toyonensis + S. boulardii than in Monensin, not presenting a statistical difference (p > 0.05) from the other two treatments. Additionally, with the time of beginning to gas production, the lag time (λ), was greater (p < 0.001) with Monensin and Monensin + B. toyonensis than with Monensin + S. boulardii and B. toyonensis + S. boulardii. The combination of Monensin and probiotics (B. toyonensis + S. boulardii) resulted in better kinetics of degradation of corn silage, being that the Groot and Richards models had the best fit for estimates of the in vitro gas production data of corn silage tested with different feed additive combinations.
1. Introduction
Over the years, several mathematical models have been developed to describe biological phenomena. For instance, Brody [1] proposed a model to describe the growth after an inflection point or inhibition act on growth, commonly related to human growth after birth. However, to study growth on plants, an adaptation of an existing model used for animal growth was necessary [2], resulting in Richard’s model [3]. Despite being old, the model developed by Benjamin Gompertz in 1825 to study the laws of mortality and natality in humans [4] was considered one of the most used models to describe growth of animals, embryos, plants, tumors and populations of organisms [5].
From these proposed models, it was necessary to expand and elucidate new analysis techniques, through the development of new specific models, such as the models that explain the kinetics of in vitro gas production in research with ruminants [6,7,8]. This variety of mathematical models is still used to fit in vitro gas production of cattle feeds, additives or diverse conditions due to that its complexity of biological factors fit perfectly in one single model for posterior statistical analysis of the studied treatments. However, the Dual-pool Logistic model [8] has been widely used to fit the in vitro gas production of feedstuffs and diets for ruminants [9,10,11,12,13]. Nevertheless, it should be noted that a single model should not be used for all types of feed; rather, it is essential that different models be adjusted for each nutritional situation [14].
One common research line for cattle is the feed additives. Monensin sodium, or just monensin, is an ionophore widely used as a nutritional feed additive for improvement on feed efficiency, cattle performance, rumen modulation and methane emission reduction [15,16,17]. However, in 2006 the Europe Union prohibited its use as a feed additive for cattle, instigating researchers to test alternative feed additives in order to sustain a high animal production demand [18].
Probiotics are natural feed additives, related to improved health, increased feed intake and nutrient digestibility [19,20,21,22]. Saccharomyces cerevisiae boulardii, a fungus kingdom member, has been related to increasing in vitro rumen degradability of forages and associated with the ability of yeast to stimulate growth and activity of fiber degradation bacteria [23,24]. In addition, the inclusion of S. cerevisiae in high concentrate or high fiber substrate diet and different process of the probiotic (inactivated yeast extract or live yeast cell) presented a diet-dependent effect of yeasts, been more evident in high fiber substrate [25].
Bacillus toyonensis, a bacterial kingdom member, has not been much studied in ruminants, especially in cattle, even though it has been released by the European Food Safety Authority since 2012 and reevaluated in 2014 [26]. Sheep who received Bovine herpesvirus type-5 vaccine and were supplemented with B. toyonensis had an improvement at vaccine immune response [27].
Due to probiotics being related to improvements in high fiber diets, and the corn silage being considered the most common food used as roughage by dairy cows and feedlot cattle [23,28,29], we studied the effects of different combinations of ionophore and probiotics in steer diets on the kinetics of in vitro gas production of corn silage. This study aimed to evaluate the kinetics of in vitro gas production and fit mathematical models of corn silage, using rumen liquid of steers fed different combinations of ionophore and probiotics.
2. Material and Methods
The study was carried out at College of Veterinary Medicine and Animal Science of the Federal University of Mato Grosso do Sul, Campo Grande, MS, Brazil (20°26′50′′ S, 54°50′21′′ W, altitude 417 m). In this study, all procedures involving the animals were in accordance with the Brazil’s National Council for the Control of Animal Experimentation [30] guidelines under the institutional Ethics Committee on Animal Use case of Federal University of Mat Grosso do Sul (protocol 1186/2021).
2.1. Animals, Experimental Design and Treatments
The rumen inoculum was obtained from four crossbred steers (Bos taurus indicus x Bos taurus taurus) with average initial body weight of 403 ± 75.5 kg, provided with ruminal cannula and housed individually in covered stalls (3 m × 4.5 m) with concrete floors, concrete feed bunkers and ad libitum waterer. They were assigned to a 4 × 4 Latin square design, consisting of four twenty-one day periods and four treatments.
Once a day at 09 h00 min, steers received a total mix ration containing 300 g/kg corn silage and 700 g/kg concentrate (Table 1) plus the additives, allowing 10% of orts. The feed additives used were Monensin sodium (Rumensin® 100, Eli Lilly do Brasil Ltda, São Paulo, SP, Brazil; 3 g/day), Bacillus toyonensis (Micro-Cell Platinum® 109, Lallemand Brazil; 1 g/day) and Saccharomyces cerevisiae boulardii (ProTernative®20, Lallemand Brazil; 0.5 g/day). Additives were arranged into the following treatments, supplied daily into total mixed diet: (1) Monensin; (2) Monensin + B. toyonensis; (3) Monensin + S. boulardii; and (4) B. toyonensis + S. boulardii supplied in the total mixed diet.

Table 1.
Composition of experimental diet offered to steers without the feed additives.
Steers were randomly distributed on treatments every period, in a way that all animals received all treatments by the end of trial. Treatments were: (1) Monensin; (2) Monensin + B. toyonensis; (3) Monensin + S. boulardii; and (4) B. toyonensis + S. boulardii.
2.2. Chemical Analysis of Silage
Sample of corn silage was dried in forced-air oven at 55 °C for 72 h, then grounded in a Wiley mill with a 1 mm screen and stored in plastic bags. The sample was analyzed for DM (method 930.15), Ash (method 942.05) and CP (method 955.04) according to the methodologies of AOAC [31]; EE was analyzed with ANKOMXT15 Extractor (ANKOM Technology, Macedon, NY, USA) according the manufacture’s instruction; while aNDF and ADF were analyzed with Tecnal TE-149 fiber analyzer (Tecnal, Piracicaba, SP, Brazil) according to the methodology of Van Soest et al. [32] using thermostable α-amylase. The non-fibrous carbohydrate (NFC) was calculated according Sniffen et al. [33]: NFC = 1000 − (CP + EE + Ash + NDF)
2.3. In Vitro Gas Production Data
In each period, approximately 500 mL of rumen liquid was obtained from steers by collecting throughout rumen cannula, filtered through layers of gauze and placed into individual pre-warmed thermal bottles to maintain at 39 °C. The rumen liquid of each animal in each period was used as inoculum in the in vitro gas production analysis.
The ANKOM RF Gas Production System (ANKOM Technology, Macedon, NY, USA) containing 12 modules was used to measure in vitro gas production. For each rumen liquid obtained from the four steers, in each period was prepared 2 bottles with silage samples and 1 blank, just with buffer and inoculum. Sample of corn silage was dried at 55 °C for 48 h and grinded in a Wiley mill with a 1 mm screen. Samples (500 mg) were accurately weighed into 250 mL serum bottle, and heated with 100 mL of phosphate bicarbonate buffer [34], then 25 mL of rumen liquid was added as inoculum; after being prepared they were placed in an incubator (MA093 Dubnoff metabolic bath model with agitation, Marconi Ltd.a, Piracicaba, SP, Brazil) and connected to the fully automatic system of ANKOM RF Gas Production System, and data were recorded every 5 min for 48 h. The pressure data in terms of volume were converted for cumulative gas production and corrected for blanks.
2.4. Models and Curve-Fitting
In vitro gas data were fitted into five mathematical models (Table 2). The sigmoidal models Gompertz and Dual-pool Logistic equations are described by Schofield et al. [8]. Groot et al. [6] and Richards [3] describe sigmoidal model’s equations. The exponential models Ørskov, Brody and Richards are described by Ørskov and McDonald [7] and Brody [1], respectively.

Table 2.
Nonlinear models considered in this study to describe the in vitro gas production of corn silage combined with different feed additives.
2.5. Statistical Analysis
The parameters of models Gompertz, Groot, Ørskov, Brody and Dual-pool Logistic were estimated by the method of Gauss Newton modified by the NLIN procedure of SAS (SAS University Edition, Sas Institute Inc., Cary, NC, USA). The maximum number of interactions used was 100 (one hundred). Due the difficulty of adjustment of Richards models by the Gauss Newton, because of the non-convergence of the interactive process, the Marquardt algorism was used at interaction of adjustment, and 300 (three hundred) interactions were used as the maximum number.
The coefficient of determination (R2) and the F test for the identity of parameters (β0 = 0 e β1 = 1) of regression of the observed data were used on the predicted values; the concordance correlation coefficient (CCC); the root mean square error of prediction (RMSEP); and the decomposition of mean square error of prediction (MSEP) in mean error (ME), systematic bias (SB) and the random error (RE) were used as criteria to evaluate the models. Comparison between models on their accuracy were carried out by the analysis of mean square error of prediction (MSEP) and the precision utilized the delta of Akaike information criterion (AIC) [35].
The statistical calculations of evaluation and comparison of models were made by the program Model Evaluation System version 3.2.2. Once the model was chosen that best described the medium curve of in vitro gas production of treatments, the parameters of selected model were submitted to a variance analysis by the PROC GLM procedure and means were compared by the Tukey test on SAS program (SAS University Edition, Sas Institute Inc., Cary, NC, USA). A significance level of 5% in all statistical analyses was adopted.
The in vitro gas production parameters were analyzed by a one-way ANOVA using the General Linear Models procedure of the SAS statistical package (SAS University Edition, Sas Institute Inc., Cary, NC, USA) in a Latin square design. The means among the treatments were compared by the Tukey test at α = 0.05 probability using the following statistical model:
where: μ = general mean; Ai = animal effect i, with i ranging from 1 to 4; Pj = period effect j, with j ranging from 1 to 4; Tk = treatment effect k, with k ranging from 1 to 4; eijk = random error associated with each observation.
Yijk =µ + Ai + Pj +Tk + eijk
3. Results
The chemical composition of corn silage incubated on in vitro gas production presented: Dry matter (DM) 310.27 g kg−1 on natural matter; Ash 62.39 g kg−1 at DM; Crude protein 97.70 g kg−1 at DM; Ethereal extract 12.56 g kg−1 at DM; neutral detergent fiber assayed with α-amylase 606.75 g kg−1 at DM; acid detergent fiber 428.19 g kg−1 at DM; non-fibrous carbohydrate 220.60 g kg−1 at DM.
The evaluation of models fitting the criteria presented means close to the observed data (Table 3), although standard deviations were lower when fitted into models. The Groot and Richards models presented the higher coefficient of determination (R2) indicating better fit of these equations to the in vitro data. In addition, the concordance correlation coefficient (CCC) presented the same pattern as R2, with the Groot and Richards models closer to ideal coefficient than other models, reflecting precision and accuracy.

Table 3.
Evaluation of models fitting to estimate the in vitro gas production of corn silage combined with feed additives.
When observing the root mean square error (RMSEP), the absolute measure for fit of models, the lower value was presented by the Groot model, followed by the Richards and Dual-pool Logistic models (Table 3). After the decomposition of mean square error of prediction (MSEP), it was possible to address the error of prediction, and the models Groot, Ørskov, Brody and Richards presented one hundred percent (100%) random error (RE), while Gompertz and Dual-pool Logistic models, in addition to the RE, also presented mean error (ME) and systematic bias (SB).
The comparison between models, for accuracy and precision, showed that the models of Groot and Richards did not differ from each other and were more precise (p < 0.05) and accurate (p < 0.05) than other models.
Perfect agreement between observed and predicted curves of accumulated in vitro gas production was observed in Groot and Richards models (Figure 1). Predictions from models Brody, Gompertz and Ørskov predicted a gas production starting around 2 mL of gas/100 mg DM, mismatching the intercept and curve shape with observed data (Figure 1). The Dual-pool Logistic model almost fit the data, although the intercept was also overestimated and a slight inflection point predicted, but not observed (Figure 1).
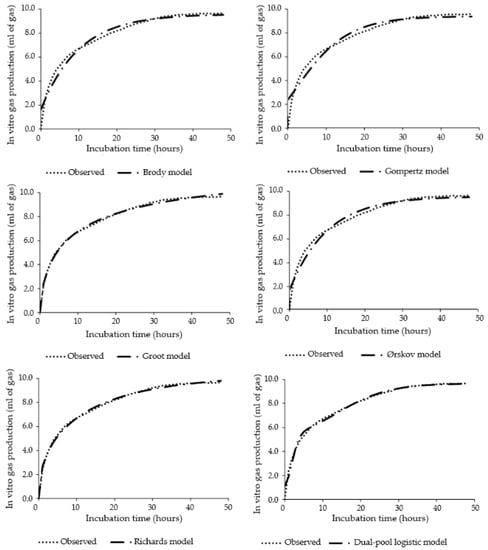
Figure 1.
Curves of accumulated in vitro gas production of corn silage with probiotics and ionophore combination fitted in different models.
Exploring in vitro gas production data throughout the Groot model for evaluation of feed additives, the estimated parameters presented statistical differences between treatments, as presented in Table 4. The B. toyonensis + S. boulardii treatment presented higher VF (12.08 mL/100 mg of DM; p = 0.0022) than Monensin and Monensin + S. boulardii (9.16 and 9.22 mL/100 mg of DM, respectively). In addition, the fractional rate of gas production (k) was higher (p = 00193) in B. toyonensis + S. boulardii than in Monensin, not presenting statistical difference (p > 0.05) from the other two treatments. Additionally, the time from the beginning to gas production and the lag time (λ) was greater (p < 0.001) with Monensin and Monensin + B. toyonensis than with Monensin + S. boulardii and B. toyonensis + S. boulardii.

Table 4.
Estimation of in vitro gas production parameters of corn silage with different feed additive combinations obtained by the Groot model.
When exploring the data using the Dual-pool Logistic model, which is the most used to this kind of evaluation (Table 5), all coefficients of determination adjusted for degrees of freedom, (R2), had values higher than 0.95, evincing that the obtained data from gas production fitted correctly on the Dual-pool Logistic equation. When comparing the gas production parameter of treatments, the volume of gas derived from the rapid degradation fraction (V1F) and the lag time (λ) of rumen microorganisms starting the degradation of incubated matter presented higher values (p = 0.024) on B. toyonensis + S. boulardii than Monensin treatment (Table 5). However, the parameters’ degradation rate (k1 and k2), and V2F did not present statistical difference between treatments. Despite that the maximum potential of gas production (VF) did not differ between treatments (p = 0.76), demonstrated in Table 5 and Figure 2, numerically the Monensin + B. toyonensis treatment had higher gas production (12.29 mL) and Monensin + S. boulardii had the lowest gas production (11.15 mL).

Table 5.
Estimation of in vitro gas production parameters of corn silage with different feed additive combinations obtained by the Dual-pool Logistic model.
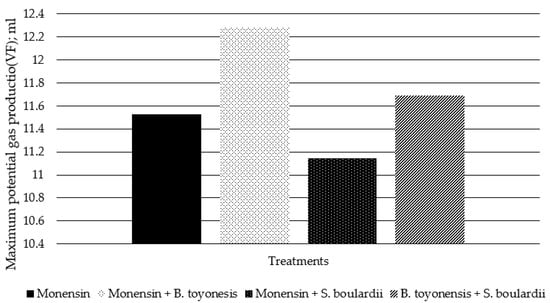
Figure 2.
Maximum potential of in vitro gas production (VF) from corn silage performed with inoculum of steers receiving different nutritional additives estimated by the Dual- pool Logistic model.
4. Discussion
According to NRCS [36], to be classified as roughage the feed must have crude protein lower than 18%, therefore corn silage is considered a roughage. The NRC [37] brings a slightly different classification, where silage is a class of feed apart from forages and roughage due to the ensiling process.
The chemical composition of corn silage can be affected by a variety of elements, such as the soil fertility, weather conditions and genetics of seeds, that may interfere in final feed composition. The DM (310.27 g kg−1 at NM), OM (937.61 g kg−1 at DM) and EE (12.56 g kg−1 at DM ) obtained in this work were lower than that presented in a meta-analysis of corn silage cultivated in Brazil (DM 325.20 g kg−1 at NM; OM 949.80 g kg−1 at DM; EE 28.40 g kg−1 at DM), although CP (97.70 g kg−1 at DM), NDF (606.75 g kg−1 at DM) and ADF (428.19 g kg−1 at DM) contents were slightly higher than the meta-analysis (CP 75.60 g kg−1 at DM; NDF 548.2 g kg−1 at DM; ADF 299.30 g kg−1 at DM) [29]. This pattern was also observed in the NRC (2021) and the North America typical corn silage database, where DM was slightly lower, but Ash, CP, NDF and ADF were above the mean values (DM 354 g kg−1 at NM; Ash 38 g kg−1 at DM; CP 77 g kg−1 at DM; NDF 409 g kg−1 at DM; ADF243 g kg−1 at DM).
The CP having higher than mean values (NRC, 2021; Zardin et al., 2017) may be due to the nitrogen fertilization level applied to the crop, as enlightened by Nematpour et al. [38] when studying different irrigation regimes and nitrogen fertilizer levels on different silage crops, and found that higher doses of nitrogen fertilizer result in higher crude protein in silages.
Another factor that changes a plant’s composition is their maturity. The interference of maturity on the chemical composition of corn silage may be the reason for the slight difference from the analyzed sample compared to that described in the literature. It has been demonstrated that when comparing immature corn silage to mature corn silage data, is it possible to observe that the immature silage presents a high humidity, resulting in low DM and higher CP, NDF, ADF and Ash [37,39,40]. The stage of maturity when harvesting together with the level of nitrogen fertilization used on the crop could be the reason that leads to differences in chemical composition of the corn silage used in this study.
Regarding the mathematical models, non-sigmoidal shapes assume that gas production rates decrease continuously, while sigmoidal shapes work first with an increased gas production rate, reaching a maximum rate and then decreasing [41], and differences are expected between their estimation due to how data are interpreted. Because of the wide variety of elements submitted to an in vitro gas production test, and conditions made in tests, its kinetics and gas production are not equal.
Mjoun [42] evaluated the fit of several feeds for ruminants using eight models (Exponential, Logistic, Dual-pool Logistic, France, Gompertz, Dual-pool Gompertz, Groot, and McDonalds–Ørskov) and obtained the best fit of corn silage with the Dual-pool Logistic model. In comparison to Mjoun [42], the chosen models to be evaluated in this study only included the Dual-pool Logistic and Gompertz, which were not the best fits to our data. The evaluated criterions by the Dual-pool Logistic model were good (R2 = 0.700; CCC = 0.865; RMSEP = 1.16), but the Groot and Richards model were better (R2 = 0.770 and 0.771, CCC = 0.871 and 0.870, RMSEP = 1.14 and 1.15, respectively). Mello et al. [43] also found a better fitting of in vitro gas production of corn silage with the Dual-pool Logistic model after comparing to the models of Brody, Von Bertalanffy, Gompertz, France, Logistic and Modified Logistic.
However, despite the good fit, the Richards model showed convergence problems in the iterative process, requiring the use of the Marquardt algorithm and an increase in the number of iterations, possibly because this model needs to estimate an additional parameter. According to Gurgel et al. [14], some researchers have also reported convergence difficulties using the Richards model. In addition, a model with three parameters—as is the case with the Groot model—will exhibit more degrees of freedom in the estimates, which can be important when a curve displays a smaller amount of information or data [14]. It is also important that all parameters have biological significance. In this respect, the Groot and Dual-pool models have advantages over the other functions in that they do not assume a constant fractional rate of fermentation [6].
As demonstrated by Mjoun [42], each type of feed had a kinetic of gas production, matching with different models, so it is possible that Monensin and the two probiotics (S. boulardii and B. toyonensis) diluted into the rumen liquid used as inoculum in this study may have interfered with the kinetics and gas production of the corn silage. This interference by feed additives at in vitro gas production was observed by Elghandour et al. [44] evaluating three cultures of Saccharomyces cerevisiae, when the addition of two of the cultures was responsible for an increase on VF parameter (control = 359.5 mL g−1 of DM vs. S. cerevisiae = 409.6 and 428.8 mL g−1 of DM).
When comparing in vitro gas production, high attention to detail is necessary, because of the natural diversity of corn silage, rumen fluid and the interference of treatments submitted to the in vitro test. Macome et al. [45] evaluated the influence of corn silage maturity in gas production with the inoculum of Holstein cows adapted to a diet ratio of 80:20 of roughage:concentrate, and they found a corn silage DM = 318 g kg−1 at NM and VF = 101.38 mL g−1 of DM estimated by the Groot model [6], with the corn harvest with 32% of DM, similar to the findings of this study, where DM = 310.27 g kg−1 at NM and VF estimated by Groot model was 91.6 to 120.8 mL g−1 of DM. However, Zhang et al. [46], using inoculum from dairy cows receiving a diet ratio around 55:45 of roughage:concentrate, obtained a high VF (VF = 137.6 mL g−1 of DM), estimated by the France model [47], when compared to our study.
Faria et al. [48], using corn silage treated or not with Monensin sodium before ensiling observed that Monensin reduced the accumulated gas production at 48 h of incubation from 56.59 mL g−1 of DM (just corn silage) to 52.99 mL g−1 of DM (corn silage with Monensin). In addition, the VF of 96 h of incubation estimated by the France model went from 62.72 mL g−1 of DM (just corn silage) to 56.58 mL g−1 of DM (corn silage with Monensin). This was also supported by Shen et al. [49], who found a lower gas production with the addition of Monensin (181.9 vs. 131.0 mL of gas, control vs. Monensin) in a total mix ration with a 50:50 ratio (roughage:concentrate) used as an in vitro substrate. This effect of Monensin was similar to the obtained data in this study fitted by the Groot model. Monensin treatments presented lower VF than treatment only with probiotics (B. toyonensis + S. boulardii), but when fitted to the Dual-pool model, no significant difference was observed at VF parameter (p = 0.762). This result explicitly highlights the need to use models that fit as perfectly as possible to in vitro gas production data, otherwise treatment differences may be absent.
The supply of probiotics and Monensin via diet and subsequently sampling rumen liquid for use as inoculum on in vitro test has successfully resulted in differences in gas production of a high fiber feed, the corn silage. After submission of gas production data to models, B. toyonensis + S. boulardii presented higher VF at than Monensin treatment by the Groot model, and higher VF1 by Dual-pool Logistic model. The addition of S. cerevisiae in high fibrous feeds (corn stover, oat straw, sugarcane bagasse and sorghum straw) were also responsible for a high VF compared to control (0 mg of S. cerevisiae) [23]. Corroborating these results, an increase in VF by the addition of S. cerevisiae yeast live cells or cells extract was also observed by Rodriguez et al. [50], when compared to the control treatment (0 mg of S. cerevisiae) using a total mixed ration (50:50, concentrate:roughage ratio).
Due to the small number of studies with Bacillus toyonensis, mainly with the use as a feed additive to cattle, a comparison of results is difficult. Nonetheless, regarding the effect of B. toyonensis observed in this study when comparing the treatments Monensin + B. toyonensis vs. Monensin + S. boulardii, a difference between them was only observed at λ estimated by the Groot model, with a higher lag time of Monensin + B. toyonensis (1.11 h) than Monensin + S. boulardii (0.66 h).
It can be inferred that the in vitro evaluation of feed for ruminants is extremely important for animal nutrition, since feed directly influences the productive performance of animals, and this will have a direct impact on the economic performance of the livestock activity. Thus, in this study, the adjustment of the best models for evaluating silage (Groot and Dual-pool Logistic models) means that there was an understanding of how the kinetics of degradation in vitro occurs. This knowledge elucidates the mechanisms in which the ruminants transform this roughage into an animal product. In addition, a comparison was made of additives for steers, and the study showed that the combinations of Monensin with probiotics is the best way to include these additives to obtain better results in the use of forage feed included in the diet of confined steers.
5. Conclusions
The combination of Monensin and probiotics (B. toyonensis + S. boulardii) can improve the kinetics of degradation of corn silage, being that the Groot and Richards models had the best fit for the in vitro gas production data of corn silage tested with different feed additive combinations.
Further research using concentrates with the combination of Monensin and probiotics will provide a better insight into the kinetics of degradation on ruminant nutrition. In addition, the validation using animals in vivo will be interesting to validate these results. Understanding these relationships might contribute to the development of novel nutritional strategies based on modifications of the concentrate:roughage ratio in ruminant diets. The future directions for improving the research are to include different foods and diets in the evaluation of in vitro degradation kinetics using Monensin and probiotic combinations for beef and dairy cattle.
Author Contributions
C.d.S.Z. conducted experiment, laboratorial analysis, collection of samples and wrote the manuscript. L.C.V.Í. and C.C.B.F.Í. conceived and designed research, and wrote the manuscript. A.L.C.G. and G.d.S.D. conducted statistical analysis. G.T.d.S. and A.M.D. conceived and designed research. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brody, S. Bioenergetics and Growth; with Special Reference to the Efficiency Complex in Domestic Animals; Reinhold: Oxford, UK, 1945. [Google Scholar]
- Von Bertalanffy, L. Quantitative Laws in Metabolism and Growth. Q. Rev. Biol. 1957, 32, 217–231. [Google Scholar] [CrossRef]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–301. [Google Scholar] [CrossRef]
- Laird, A.K. Dynamics of relative growth. Growth 1965, 29, 249–263. [Google Scholar]
- Savageau, M.A. Growth equations: A general equation and a survey of special cases. Math. Biosci. 1980, 48, 267–278. [Google Scholar] [CrossRef]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Orskov, E.R.; Mcdonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef]
- Olivo, P.M.; Santos, G.T.; Ítavo, L.C.V.; Silva Junior, R.C.; Leal, E.S.; Prado, R.M. Assessing the nutritional value of agroindustrial co-products and feed through chemical composition, in vitro digestibility, and gas production technique. Acta Sci. Anim. Sci. 2017, 39, 289–295. [Google Scholar] [CrossRef]
- Diaz, T.G.; Branco, A.F.; Ítavo, L.C.V.; Santos, G.T.; Carvalho, S.T.; Teodoro, A.L.; Oliveira, R.L. In vitro gas production kinetics and digestibility in ruminant diets with diferente levels of cashew nut shell liquid. Semin. Cienc. Agrar. 2018, 39, 1669–1682. [Google Scholar] [CrossRef]
- Souza, A.D.V.; Ítavo, L.C.V.; Favaro, S.P.; Ítavo, C.C.B.F.; Petit, H.V.; Dias, A.M.; Morais, M.G.; Reis, F.A.; Roscoe, R. Thermal decomposition, chemical composition, in vitro digestibility and gas production and in situ degradability of oilseed residues from the biofuel industry. Anim. Sci. J. 2018, 89, 79–87. [Google Scholar] [CrossRef]
- Leal, E.S.; Ítavo, L.C.V.; Valle, C.B.; Ítavo, C.C.B.F.; Dias, A.M.; Difante, G.S.; Barbosa-Ferreira, M.; Nonato, L.M.; Melo, G.K.A.; Gurgel, A.L.C. AInfluence of protodioscin content on digestibility and in vitro degradation kinetics in Urochloa brizantha cultivars. Crop Pasture Sci. 2020, 71, 278–284. [Google Scholar] [CrossRef]
- Santana, J.C.S.; Morais, J.A.S.; Difante, G.S.; Ítavo, L.C.V.; Gurgel, A.L.C.; Oliveira, V.S.; Rodrigues, M.J.S.T. In vitro digestion characteristics of various combinations of elephant grass hay, Gliricidia hay or silage, soybean meal and corn meal in rations for sheep. Trop. Grassl. 2020, 8, 147–152. [Google Scholar] [CrossRef]
- Gurgel, A.L.C.; Morais, J.A.S.; Santana, J.C.S.; Difante, G.S.; Emerenciano Neto, J.V.; Ítavo, L.C.V.; Ítavo, C.C.B.F.; Oliveira, V.S.; Rodrigues, M.J.S.T. Mathematical models to adjust the parameters of in vitro cumulative gas production of diets containing preserved Gliricidia. Ciência Rural 2021, 51, e20200993. [Google Scholar] [CrossRef]
- Weiss, C.P.; Beck, P.A.; Gadberry, M.S.; Richeson, J.T.; Wilson, B.K.; Robinson, C.A.; Zhao, J.; Hess, T.; Hubbell, D. Effects of intake of monensin during the stocker phase and subsequent finishing phase on performance and carcass characteristics of finishing beef steers. Appl. Anim. Sci. 2020, 36, 668–676. [Google Scholar] [CrossRef]
- Tseu, R.J.; Perna Junior, F.; Carvalho, R.F.; Sene, G.A.; Tropaldi, C.B.; Peres, A.H.; Rodrigues, P.H.M. Effect of tannins and monensin on feeding behaviour, feed intake, digestive parameters and microbial efficiency of nellore cows. Ital. J. Anim. Sci. 2020, 19, 262–273. [Google Scholar] [CrossRef]
- Thompson, L.R.; Beck, M.R.; Gunter, S.A.; Williams, G.D.; Place, S.E.; Reuter, R.R. An energy and monensin supplement reduces methane emission intensity of stocker cattle grazing winter wheat. Appl. Anim. Sci. 2019, 35, 433–440. [Google Scholar] [CrossRef]
- İnal, F.; Gürbüz, E.; Coşkun, B.; Alataş, M.S.; Çitil, Ö.B.; Polat, E.S.; Şeker, E.; Özcan, C. The Effects of Live Yeast Culture (Saccharomyces cerevisiae) on Rumen Fermentation and Nutrient Degradability in Yearling Lambs. Kafkas Üniversitesi Vet. Fakültesi Derg. 2010, 16, 799–804. [Google Scholar] [CrossRef]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Young, T.R.; Ribeiro, F.R.B.; Sanchez, N.C.B.; Carroll, J.A.; Jennings, M.A.; Cribbs, J.T.; Rathmann, R.J.; Corley, J.R.; Johnson, B.J. Yeast cell wall supplementation alters the performance and health of beef heifers during the receiving period. Prof. Anim. Sci. 2017, 33, 166–175. [Google Scholar] [CrossRef]
- Diaz, T.G.; Branco, A.F.; Jacovaci, F.A.; Jobim, C.C.; Bolson, D.C.; Daniel, J.L.P. Inclusion of live yeast and mannan-oligosaccharides in high grain-based diets for sheep: Ruminal parameters, inflammatory response and rumen morphology. PLoS ONE 2018, 13, e0193313. [Google Scholar] [CrossRef]
- Diaz, T.G.; Branco, A.F.; Jacovaci, F.A.; Jobim, C.C.; Daniel, J.L.P.; Bueno, A.V.I.; Ribeiro, M.G. Use of live yeast and mannan-oligosaccharides in grain-based diets for cattle: Ruminal parameters, nutrient digestibility, and inflammatory response. PLoS ONE 2018, 13, e0207127. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Chagoyán, J.C.V.; Salem, A.Z.M.; Kholif, A.E.; Castañeda, J.S.M.; Camacho, L.M.; Cerrillo-Soto, M.A. Effects of Saccharomyces cerevisiae at direct addition or pre-incubation on in vitro gas production kinetics and degradability of four fibrous feeds. Ital. J. Anim. Sci. 2014, 13, 295–301. [Google Scholar] [CrossRef]
- Wambui, C.; Abdulrazak, S. Effect of yeast supplementation on in vitro ruminal degradability of selected browse species from Kenya. J. Food Agric. Environ. 2010, 8, 553–557. [Google Scholar]
- Opsi, F.; Fortina, R.; Tassone, S.; Bodas, R.; López, S. Effects of inactivated and live cells of Saccharomyces cerevisiae on in vitro ruminal fermentation of diets with different forage: Concentrate ratio. J. Agric. Sci. 2012, 150, 271–283. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on the safety and efficacy of Toyocerin® (Bacillus toyonensis) as a feed additive for chickens for fattening, weaned piglets, pigs for fattening, sows for reproduction, cattle for fattening and calves for rearing and for rabbits for fat. EFSA J. 2014, 12, 17. [Google Scholar] [CrossRef]
- Roos, T.B.; de Moraes, C.M.; Sturbelle, R.T.; Dummer, L.A.; Fischer, G.; Leite, F.P.L. Probiotics Bacillus toyonensis and Saccharomyces boulardii improve the vaccine immune response to Bovine herpesvirus type 5 in sheep. Res. Vet. Sci. 2018, 117, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Zardin, P.B.; Velho, J.P.; Jobim, C.C.; Alessio, D.R.M.; Haygert-Velho, I.M.P.; da Conceição, G.M.; Almeid, P.S.G. Chemical composition of corn silage produced by scientific studies in Brazil—A meta-analysis. Semin. Ciências Agrárias 2017, 38, 503–511. [Google Scholar] [CrossRef]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special Topics—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options 1. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Silva, A.C.; Diaz, B.L.; Rivera, E.A.B.; Granjeiro, J.M.; Braga, L.M.G.M.; Frajblat, M.; Stephano, M.A. Guia Brasileiro de Produção, Manutenção ou Utilização de Animais em Atividades de Ensino ou Pesquisa: Fascículo 1: Introdução Geral; Ministérios da Ciência, Tecnologia e Inovação, Ed.; Concelho Nacional de Controle de Experimentação Animal: Brasilia, Brazil, 2016; ISBN 9788588063310. [Google Scholar]
- AOAC Official Methods of Analysis of AOAC International. Official Methods of Analysis of AOAC; AOAC International: Rockville, MD, USA, 2005; ISBN 0935584544. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci 1991, 10, 3583–3597. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Marten, G.C.; Barnes, R.F. Prediction of energy digestibility of forages with in vitro rumen fermentation and fungal enzyme systems. Stand. Anal. Methodol. Feed. 1979, 4, 61–71. [Google Scholar]
- Tedeschi, L.O. Assessment of the adequacy of mathematical models. Agric. Syst. 2006, 89, 225–247. [Google Scholar] [CrossRef]
- NRCS. Animal Diets and Feed Management; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2012; pp. 1–14.
- National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-06997-7. [Google Scholar]
- Nematpour, A.; Eshghizadeh, H.R.; Zahedi, M. Comparing the Corn, Millet and Sorghum as Silage Crops Under Different Irrigation Regime and Nitrogen Fertilizer Levels. Int. J. Plant Prod. 2021, 15, 351–361. [Google Scholar] [CrossRef]
- Bryant, H.T.; Blaser, R.E.; Hammes, R.C.; Huber, J.T. Evaluation of Corn Silage Harvested at Two Stages of Maturity 1. Agron. J. 1966, 58, 253–255. [Google Scholar] [CrossRef]
- Colenbrander, V.F.; Martin, G. Relationships Between Stage of Maturity of the Corn Plant at Time of Harvest for Corn Silage and Chemical Composition1,2. J. Dairy Sci. 1965, 54, 533–536. [Google Scholar] [CrossRef]
- Wang, M.; Tang, S.X.; Tan, Z.L. Modeling in vitro gas production kinetics: Derivation of Logistic-Exponential (LE) equations and comparison of models. Anim. Feed Sci. Technol. 2011, 165, 137–150. [Google Scholar] [CrossRef]
- Mjoun, K. 72 Evaluation of Different Gas Production Models When Applied to Ruminants Feeds. J. Anim. Sci. 2018, 96, 37–38. [Google Scholar] [CrossRef]
- Mello, R.; Magalhães, A.L.R.; Breda, F.C.; Regazzi, A.J. Modelos para ajuste da produção de gases em silagens de girassol e milho. Pesqui. Agropecuária Bras. 2008, 43, 261–269. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Vázquez, J.C.; Salem, A.Z.M.; Kholif, A.E.; Cipriano, M.M.; Camacho, L.M.; Márquez, O. In vitro gas and methane production of two mixed rations influenced by three different cultures of Saccharomyces cerevisiae. J. Appl. Anim. Res. 2017, 45, 389–395. [Google Scholar] [CrossRef]
- Macome, F.M.; Pellikaan, W.F.; Hendriks, W.H.; Dijkstra, J.; Hatew, B.; Schonewille, J.T.; Cone, J.W. In vitro gas and methane production of silages from whole-plant corn harvested at 4 different stages of maturity and a comparison with in vivo methane production. J. Dairy Sci. 2017, 100, 8895–8905. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Wang, X.; Yu, Z.; Na, R. Ensiling alfalfa with whole crop corn improves the silage quality and in vitro digestibility of the silage mixtures. Grassl. Sci. 2017, 63, 211–217. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef]
- Faria, B.N.; Reis, R.B.; Maurício, R.M.; Lana, A.M.Q.; Soares, S.R.V.; Saturnino, H.M.; Coelho, S.G. Efeitos da adição de propilenoglicol ou monensina à silagem de milho sobre a cinética de degradação dos carboidratos e produção cumulativa de gases in vitro. Arq. Bras. De Med. Veterinária E Zootec. 2008, 60, 896–903. [Google Scholar] [CrossRef][Green Version]
- Shen, J.; Liu, Z.; Yu, Z.; Zhu, W. Monensin and nisin affect rumen fermentation and microbiota differently in vitro. Front. Microbiol. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.P.; Mariezcurrena, M.D.; Mariezcurrena, M.A.; Lagunas, B.C.; Elghandour, M.M.M.Y.; Kholif, A.M.; Kholif, A.E.; Almaraz, E.M.; Salem, A.Z.M. Influence of live cells or cells extract of Saccharomyces cerevisiae on in vitro gas production of a total mixed ration. Ital. J. Anim. Sci. 2015, 14, 590–595. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).