1. Introduction
Sheep are thought to be the first domesticated livestock species and thus integral to animal husbandry [
1]. China has experienced a significant economic growth in recent decades, and the growing consumption of mutton is leading to increased lamb production and sheep meat import. China is now the largest importer of sheep and goat meat in the world, with a volume forecast to reach 1.38 million metric tons in 2021 [
2]. As the structure of meat consumption changes, numbers of domestic animal husbandry enterprises have seized this development opportunity, and at the same time due to the strategy of rural vitalization, the scale and technology of animal husbandry have been continuously updated. Hu sheep are widely farmed in southern China because of their high reproductive performance (Multiple fetuses) [
3]. However, the main constraint for further improvement of lamb products is the lack of adequate and high-quality feed stuff [
4,
5]. Large amounts of forage are consumed by cattle and sheep, and the global trade of fodder has been limited due to the pandemic of COVID-19. Sino–US economic- and trade frictions have continued in the past few years, which have worsened the shortage of quality forages in domestic. Consequently, considerable interest has been given to the use of paper mulberry [
6,
7], a woody grass which could enjoy a broad array of uses for meeting the challenge of food–fuel–feed competition [
8], available substitution for enhancing lamb performance.
Paper mulberry, with its relatively high content of protein, has been used as a substituted feedstuff of protein [
7], especially with the current shortage of quality forage and soybean meal. Although the abundant rainfall in southern China can provide a large amount of biomass for paper mulberry, the rainfall makes it difficult to store feed after harvest. Consequently, ensiling is a way to store fresh plants which avoids excessive energy consumption (used for drying in rainy southern China) and improves the palatability of feed with lactic acid fermentation under anaerobic conditions [
4,
9,
10].
Paper mulberry contains higher condensed tannin [
11], which may positively affect the meat quality of livestock products by altering the rumen’s fermentation mode [
8,
12,
13] and the biohydrogenation of unsaturated fatty acids. Moreover, paper mulberry can improve the milk quality of dairy cattle and the meat quality of sheep [
14,
15], change the rumen microorganism [
16], and increase the content of polyunsaturated fatty acids in milk [
17,
18]. However, few studies have focused on the relationship between rumen microorganisms and meat quality [
19].
Therefore, this study explored the relationship between a diet of paper mulberry silage with lamb meat quality and rumen fermentation. We hypothesized that paper mulberry silage could help to improve the quality of lamb meat. We closely examined the effects of paper mulberry silage on the meat quality of Hu sheep, including correlations between rumen bacteria flora and fatty acid composition which have previously received little attention.
2. Materials and Methods
2.1. Diets, Animals, and Experimental Design
The experiment was conducted in accordance with the Chinese Guidelines for Animal Welfare and Experimental Protocol, and approved by the Animal Care and Use Committee of China Agricultural University (ID:AW22601202-5-1).
Paper mulberry (
B. papyrifera) were harvested in the third cutting and ensiled by Rongcheng Gouyang Modern Agriculture Co., Ltd. which is located in Rongchang, Chongqing, China (29°51′ N and 105°49′ E). The location has a subtropical monsoon climate. This study was also conducted on the farm of Rongcheng Gouyang Modern Agriculture Co., Ltd., and lasted for 56 d, with the first week for a preliminary trial period followed by 7 weeks of paper mulberry silage (PMS) treatment, beginning on 3 December 2020 and finishing on 28 January 2021. Forty weaned lambs (approximately 45-days old) were randomly divided into four treatments individually: control group, 20% (PMS20), 30% (PMS30), and 40% (PMS40). Four diets were formulated according to the values recommended by the Feeding Standard of Meat-Producing Sheep of Chinese agricultural industry standards (NY/T 816-2004) and produced as a total mixed ration (
Table 1). The nutrient and chemical composition of PMS diets were shown in
Table 1. The environment was comfortable and equipped with ventilation and moisture-proof measures. All lambs were fed twice a day (08:00 and 18:00), allowed to feed ad libitum, and given free clean water throughout the entire experimental period.
2.2. Sample Collection, Carcass Characteristics and Meat Quality Measurement
Body weight was recorded individually on the first day and at the end of the feeding experiment, and during the feeding period, feed offered and refused was quantified to determine dry matter intake (DMI) daily. Before slaughter, lambs were prevented from consuming feed and drinking for 12 h All lambs were recorded after all blood samples (for serum biochemical analysis) were collected and hot carcasses were weighted. We use an electronic platform scale with an accuracy of 10 g. The whole rumen contents of each Hu lamb was mixed immediately, and then squeezed through four layers of cheesecloth to separate liquid fractions and pH of rumen was recorded (PHS-3C with three-point calibration). Then they were collected into three 15 mL sterilized tubes and put into liquid nitrogen. All rumen microbe samples were stored at −80 °C before the volatile fatty acid and 16S rRNA gene analysis. Forty-five minutes after slaughter, longissimus dorsi (LD) muscle of lambs were collected from carcass for meat color, pH, drip loss, cooking loss, and shear force data measurement.
Measurements of muscle pH were made after postmortem using a pH meter (pH-STAR, MATTHAUS, Berlin, Germany). The meat color of LD was measured at 45 min postmortem by a mean of three random readings made with a portable chromameter (CR-400, Konica Minolta, Tokyo, Japan), which was calibrated with a white tile according to the manufacturer’s manual. The light was Illuminant D-65, the inclination Angle is 10, and the aperture radius is 10 mm. The drip loss percentage was measured as per the method previously described [
20]. Cooking loss was determined by Cardoso’s method [
21]. Briefly, muscle samples were heated in 80 °C water until the central temperature reached 75 °C, after which the samples were immediately removed from the steamer, and reweighed after cooling [
21]. Shear force was evaluated after muscle cooking loss were calculated. Three cylindrical cores (12.7 mm) were removed from cooled muscle samples to the fiber direction [
22]. Each sample was sheared using a muscle shear force measuring analyzer (C-LM3B, TENOVO, Beijing, China).
2.3. Fermentation Characteristics and Volatile Fatty Acid Profile Measurement
Rumen fluid thawed at 4 °C, then the supernatant was centrifuged at 3000 rpm·min−1 and the content of ammoniacal nitrogen (AN) was determined by the microplate testing system (SpectraMax iD5, Molecular Devices, Pleasanton, CA, USA). Thereafter, 25% (wt/vol) metaphosphoric acid was added in the supernatant samples and preserved at −4 °C for the measurement of ruminal volatile fatty acid (VFAs). The concentrations of VFAs were determined by gas chromatography (Shimadzu, Kyoto, Japan). The Coomassie Brilliant Blue G-250 assay was employed to measure the microbial protein content in the rumen liquid.
2.4. Serum Biochemical Analysis
Blood was sampled from a jugular vein of each animal using 10 mL serum tubes at 08:00 before slaughtering. The blood was centrifuged at room temperature (10 min, 2810 g), and the upper serum was obtained and stored at −20 °C [
23]. Samples were aliquoted for measurement via an automatic biochemical analyzer (CLS880, Zechengbio, Guangzhou, China), which included the measurement of growth hormone (GH), insulin (INS), glucose (GLU), total cholesterol (TC), triglyceride (TG), high-density lipoproteins (HDL), low-density lipoproteins (LDL), non-esterified fatty acid (NEFA), and leptin (LEP).
2.5. Analysis of the Amino Acid Composition
Samples of 50–100 mg were extracted with 0.5 mL 0.1 M hydrochloric acid for 1 h with gentle agitation on a shaker at room temperature. Each sample was filtered through a 0.22 μm pore membrane filter. Then 10 μL of the sample was taken into a UHPLC vial and added 70 μL Borate buffer and 20 μL AccQ Tag reagent. The reaction mixture was kept at room temperature for 1 min, heated at 55 ºC for 10 min and 1 μL was injected after cooling. The sample extracts were analyzed using an UPLC– Orbitrap-MS system (UPLC, Vanquish; MS, QE). The analytical conditions were as follows, UPLC: column, Waters BEH C18 (50 × 2.1 mm, 1.8 μm); column temperature, 55 °C; flow rate, 0.5 mL/min; injection volume, 1μL; solvent system, water (0.1% Formic acid): acetonitrile (0.1% Formic acid); gradient program, 95:5 v/v at 0 min, 90:10 v/v at 5.5 min, 75:25 v/v at 7.5 min, 40:60 v/v at 8 min, 95:5 v/v at 8.5 min, and 95:5 v/v at 13 min. HRMS data were recorded on a Q Exactive hybrid Q–Orbitrap mass spectrometer equipped with a heated ESI source (Thermo Fisher Scientific, Waltham, MA, USA) using the SIM MS acquisition methods. The ESI source parameters were set as follows: spray voltage, 3 kV; sheath gas pressure, 40 arb; aux gas pressure, 10 arb; sweep gas pressure, 0 arb; capillary temperature, 320 °C; and aux gas heater temperature, 350 °C. Data were acquired on the Q-Exactive using Xcalibur 4.1 (Thermo Scientific, Waltham, MA, USA), and processed using TraceFinder™4.1 Clinical (Thermo Scientific, Waltham, MA, USA). Quantified data were output into excel format.
2.6. Analysis of the Fatty Acid Composition
Samples of LD were collected to measure fatty acid composition. The fatty acid composition in the meat was determined using gas chromatography-mass spectrometric (GC-MS) according to the methods described in previous studies. Thirty-seven fatty acid methyl esters were used as mixed standard samples for quantitative analysis [
22]. Lipids were extracted from the meat samples using the trichloromethane and dichloromethane, successively. Then, free fatty acid mixture was esterified with 2 mL methylation reagent. After shaking for 30 s, the mixture was reacted in 80 °C water for 2 h. Fatty acid methyl esters were analyzed using an Agilent 6890 gas chromatographer equipped with a flame ionization detector (Agilent Technologies, Santa Clara, CA, USA). A CP-Sil 88 fused silica open tubular capillary column (100 m × 0.25 mm × 0.25 µm) was used. The initial oven temperature was set at 100 °C for 5 min and increased to 240 °C at 4 °C/min, kept at 240 °C for 5 min, then increased to 350 °C at 15 °C/min. The carrier gas was helium at a flow rate of 1 mL/min. The mass spectrometric system is the quadrupole mass spectrometric detection system (Agilent 5977; Agilent Technologies, Santa Rosa, CA, USA) with electron bombardment ion source (EI) and Mass hunter workstation. The fatty acid was detected by the electron bombardment ion source (EI) fully SCAN mode. The optimized conditions of mass spectrometry analysis were as follows: inlet temperature 260 °C, quadrupole temperature 150 °C; Scanning mode is full SCAN mode, mass scanning range (
m/
z): 30–550. The identification of individual fatty acid methyl esters was accomplished by the retention times of an authentic standard. The concentration of individual fatty acids was quantified according to the peak area and expressed as a percentage of total fatty acids.
2.7. DNA Extraction, 16S rRNA Gene Amplification, and High-Throughput Sequencing
DNA of the rumen liquid samples was extracted using the bead-eating and phenol-chloroform extraction method. The quality and concentration of the extracted DNA were measured on a NanoDrop2000 spectrophotometer. Then, polymerase chain reaction (PCR) was used to amplify the V3-V4 region of the bacterial 16S rRNA gene using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), and specific primers with Barcode were synthesized according to the specified sequencing region. The PCR condition was set as follows: initial denaturation at 95 °C for 2 min, 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 30 s, and extension at 72 °C for 5 min. Each treatment was conducted in triplicate and the mixture was purified to perform sequencing. PCR products were verified on agarose gel (2%, w/v), and the expected bands were extracted separately and purified via the Axy-PrepDNA Gel Extraction Kit. The concentrations of the purified DNA amplicons were quantified using Quantifluor™-ST Blue Fluorescence Quantitative System. Amplicons from different samples were mixed in equal ratio and sequenced via the 2 × 300 paired-end kit on an Illumina MiSeq platform. Any sequences with mismatches and ambiguous reads in the primers were removed for quality-control purposes.
2.8. Statistical Analysis
All data obtained in the present study were analyzed using One-Way Analysis of Variance (ANOVA) to determine different dietary treatments, and Duncan’s multiple range test was conducted to post hoc analyses via IBM SPSS 24.0 software (IBM Co., Armonk, NY, USA). Statistically significant difference was set at p < 0.05. Spearman correlation analysis was used to relate the abundance of the top 50 bacterial genera with the content of free amino acids and fatty acids using R (version 3.3.1). Only correlations with p < 0.05 for the linear model were considered as significant.
4. Discussion
In the present study, PMS30 and PMS40 contained higher DMI and ADG than others, which was similar to the results in goats, sheep, and beef cattle fed with paper mulberry or paper mulberry silage [
14,
15,
24]. This might be due to the replacement of low quality corn stover with high quality silage in the diets for Hu lambs, and it was also consistent with the result reported by Tao that PMS could increase the DMI in beef cattle [
24]. DMI in ruminants have been associated with neutral detergent fiber (NDF) content in diets [
25,
26,
27]. A negative correlation between NDF content and DMI in dairy cow diets has also been found [
28]. Miller et al. also reported that cows fed with higher NDF diet consumed less DM than cows fed with lower NDF diet [
29]. In general, this observation could be explained by the lower NDF content of PMS30 and PMS40 group compared with Control.
Serum biochemical indices reflect the health status and nutrients utilization of livestock [
24]. Si and Tao reported that PMS had no effects on the serum metabolites of cow and beef cattle [
18,
24], and similar results were found in the present study, which suggested that lambs were in healthy status with no adverse effects on their performance. Therefore, in this study, PMS had no negative effects on the growth and metabolism of Hu lambs.
AN is primarily used by microorganisms as raw material for producing MCP, an important source of high quality protein for ruminants [
30], as well as the end product of nitrogenous compound in mixed ration [
31,
32]. In this study, AN was at a reasonable concentration level [
33]. It was not conducive to the growth of rumen microorganisms with neither too high nor too low concentration of AN in rumen. Ruminants principally rely on the small intestine to absorb protein, which primarily comes from MCP and rumen bypass protein. PMS40 had a higher MCP than other groups. This observation is consistent with the previous study that MCP synthesis was increased in goat fed with 45% paper mulberry of diet [
14]. Therefore, higher content of PMS was speculated to synthesize more MCP in rumen. TFA concentration was affected by the forage offered [
34]. It was found that PMS would lead to a lower TVFA in rumen before slaughter. This may be due to the high digestibility of PMS, which can pass through rumen faster [
11]. In all treatments, the rumen TVFA content decreased after 12 h of fasting.
The meat quality of Hu lamb was not affected by PMS in the present study, which is consistent with the previous study in Hu rams after being fed with paper mulberry [
15]. However, the previous study showed that paper mulberry could improve the meat quality of goats compared to maize silage [
14]. Based on this, the response of meat quality to the PMS might mainly be affected by animal species.
We further studied the effects of PMS on fatty and amino acids in the LD muscle of lambs. Because of the active substances such as polyphenols, herbs and shrubs are generally used to improve the flavor of meat compared to crops [
19]. In the present study, we found that PMS40 feeding did not change the concentration of free amino acid except for Met and Tyr. It is noteworthy that flavor amino acid (glycine, alanine, serine, threonine, glutamic acid, and aspartic acid), which play an important role in determining the acceptability and directly affects meat taste, also showed no significant differences among the treatments in the present study. Although polyphenols and tannin, when possibly being considered rich in paper mulberry, were considered that could lead to a high content of rumen-protected protein, and the previous study also reported that PMS might not affect amino acid metabolism [
24]. It might be related to the type, erratic and dose-dependent responses of tannins in the paper mulberry silage diet [
8].
Fatty acid profiles of LD muscle also play a key role in meat quality [
35], and in particular, n-3 fatty acids are considered as functional FA to prevent non-alcoholic fatty liver disease (NAFLD) and Obesity. In the present study, PMS40 diet led to an increase of C24:1 and C20:5n-3(EPA). To the best to our knowledge, ruminal bio-hydrogenation (BH) promotes the accumulation of saturated FA in ruminant meat. Therefore, reducing rumen BH has become an important way to improve meat quality. Evidence supports the effect of tannin’s regulation of rumen lipid metabolism on meat polyunsaturated fatty acids (PUFA) [
8]. Based on this, paper mulberry may have the potential to reduce rumen BH and improve meat quality because of the high concentration of condensed tannin [
11]. It seems that the reasons for these different observations are not clear yet, and the underlying lipids metabolism mechanism of rumen needs to be clarified in the future study.
Forage-based or grain-based diet, and even starch type and tannin content of feed could affect rumen fermentation and meat quality [
13,
36,
37]. Therefore, we proposed that ruminal bacteria may indirectly influence the fatty acids in the muscle through the interaction between rumen bacteria and the host. At the phylum level, PMS30 and PMS40 had a lower spirochete abundance than others, which may lead to healthier body condition than the control group [
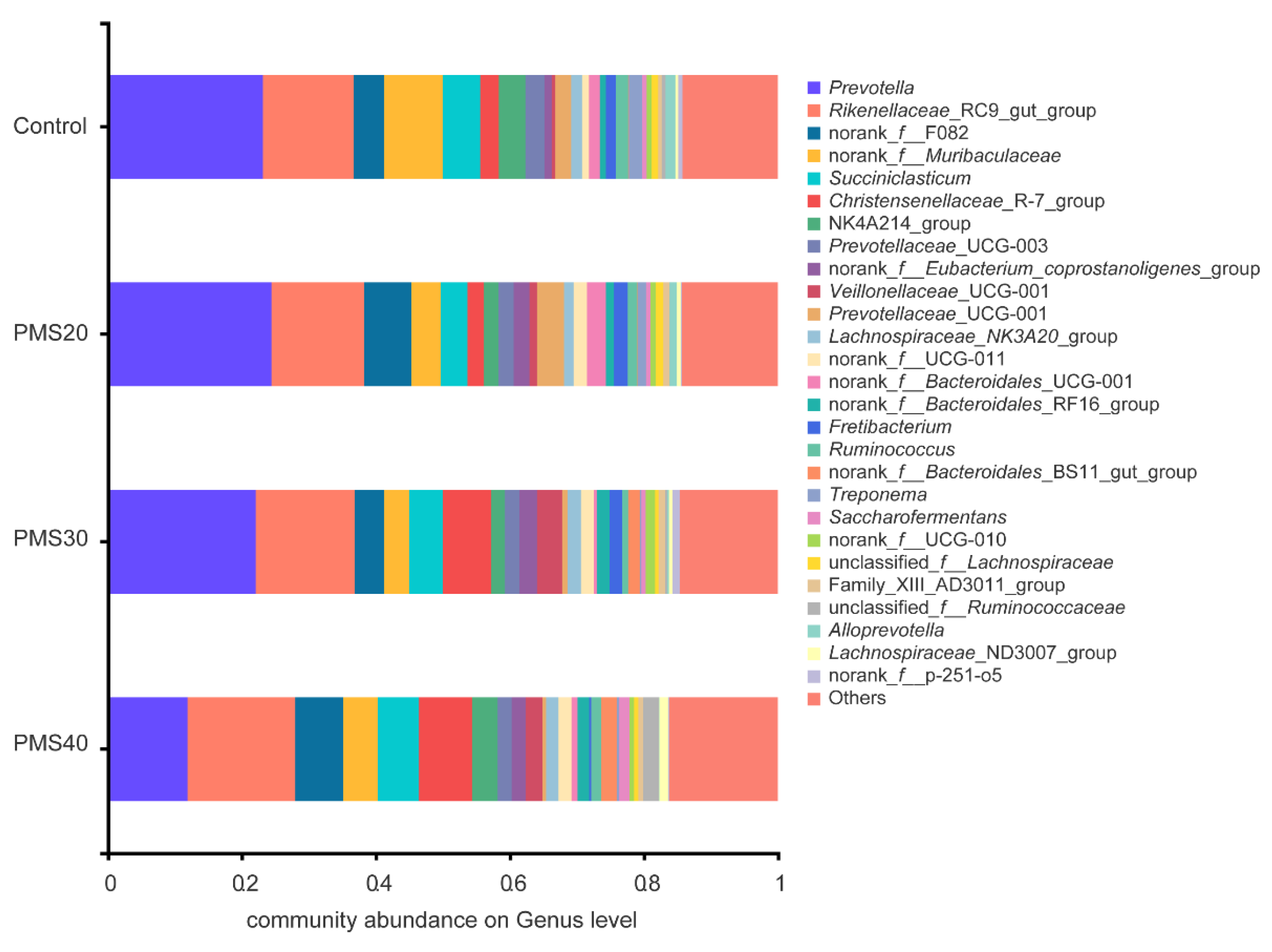
38]. PMS30 and PMS40 could result in a significant impact on rumen bacterial community. Although
Christensenellaceae _R-7_group and
norank_f_Eubacterium_coprostanoligenes_group were significantly higher in PMS30 and PMS40 than control group (
Supplementary Figure S2), very few studies have focused on the metabolism of
Eubacterium_coprostanoligenes. It had also been found in the rumen of high-yield dairy cows after being fed with
Perilla frutescens leaves reported by Sun [
39]. In the present study, it was indicated that
Christensenellaceae_R-7_group and
norank_f_Eubacterium_coprostanoligenes_group may have the largest contribution to the improvement of LD fatty acid distribution and growth performance via PMS diet. Then,
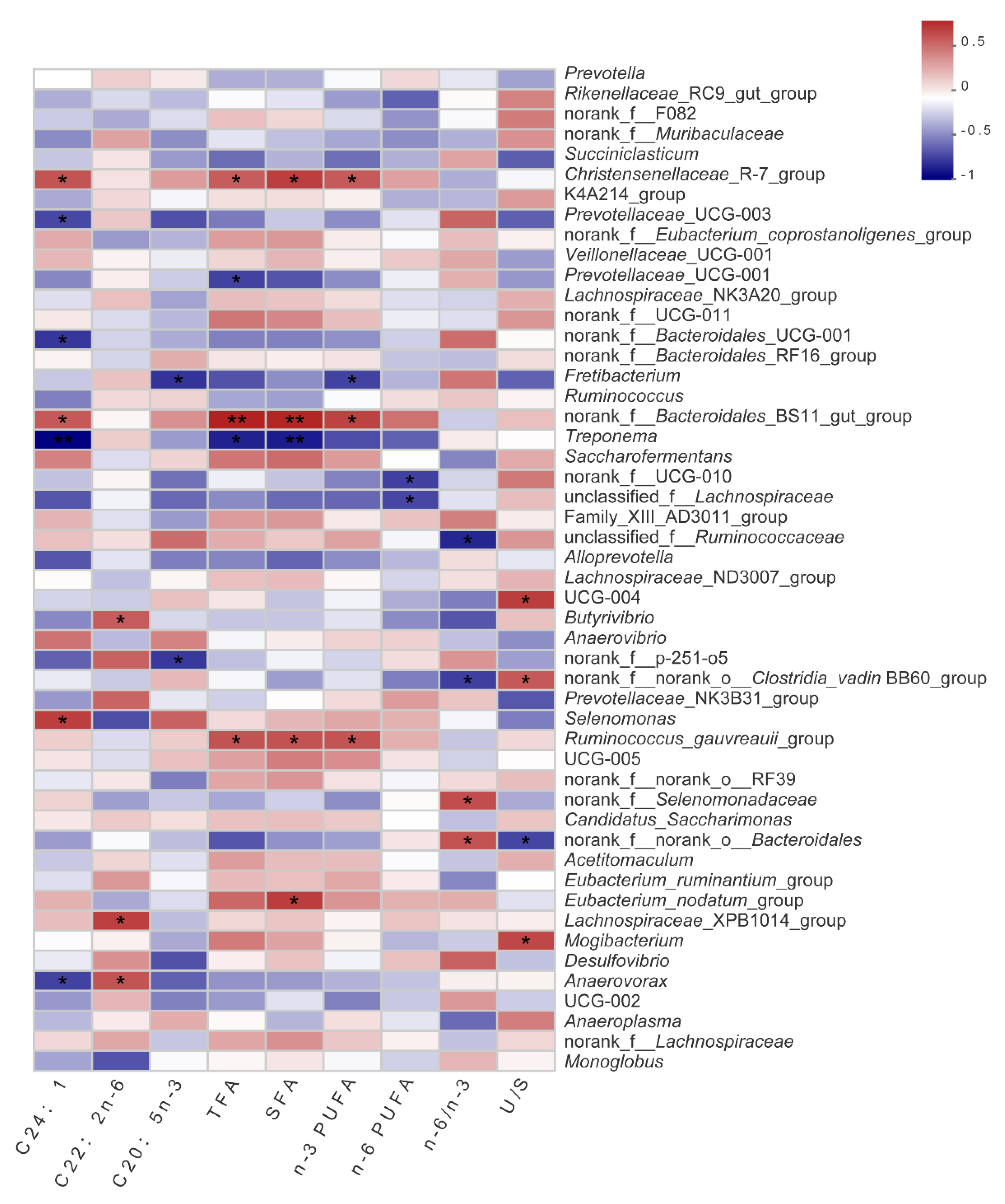
Christensenellaceae is mostly affected by the host genetic which may be beneficial to the body health [
40], and besides, we also found that
Christensenellaceae_R-7_group,
norank_f_Bacteroidales_BS11_gut_group and
Ruminococcus_gauvreauii_group were positively correlated with TFA, SFA, and n-3 PUFA. Besides, BS11 are cosmopolitan host-associated bacteria prevalent in ruminant gastrointestinal tracts, and it is also reported that they may play an important role in the degradation of recalcitrant carbohydrates and provide beneficial metabolites to host metabolism [
41]. In addition, Solden had identified BS11 that become dominant in rumen when the host consumes a high woody biomass diet [
42], which was verified in our research because paper mulberry is a typical woody plant. The
Ruminococcus spp. has been considered as a crucial cellulolytic bacteria generally detected in rumen [
43].
Ruminococcus_gauvreauii_group showed no significant difference between treatments, but a positive correlation with fatty acid was found, especially n-3PUFA (
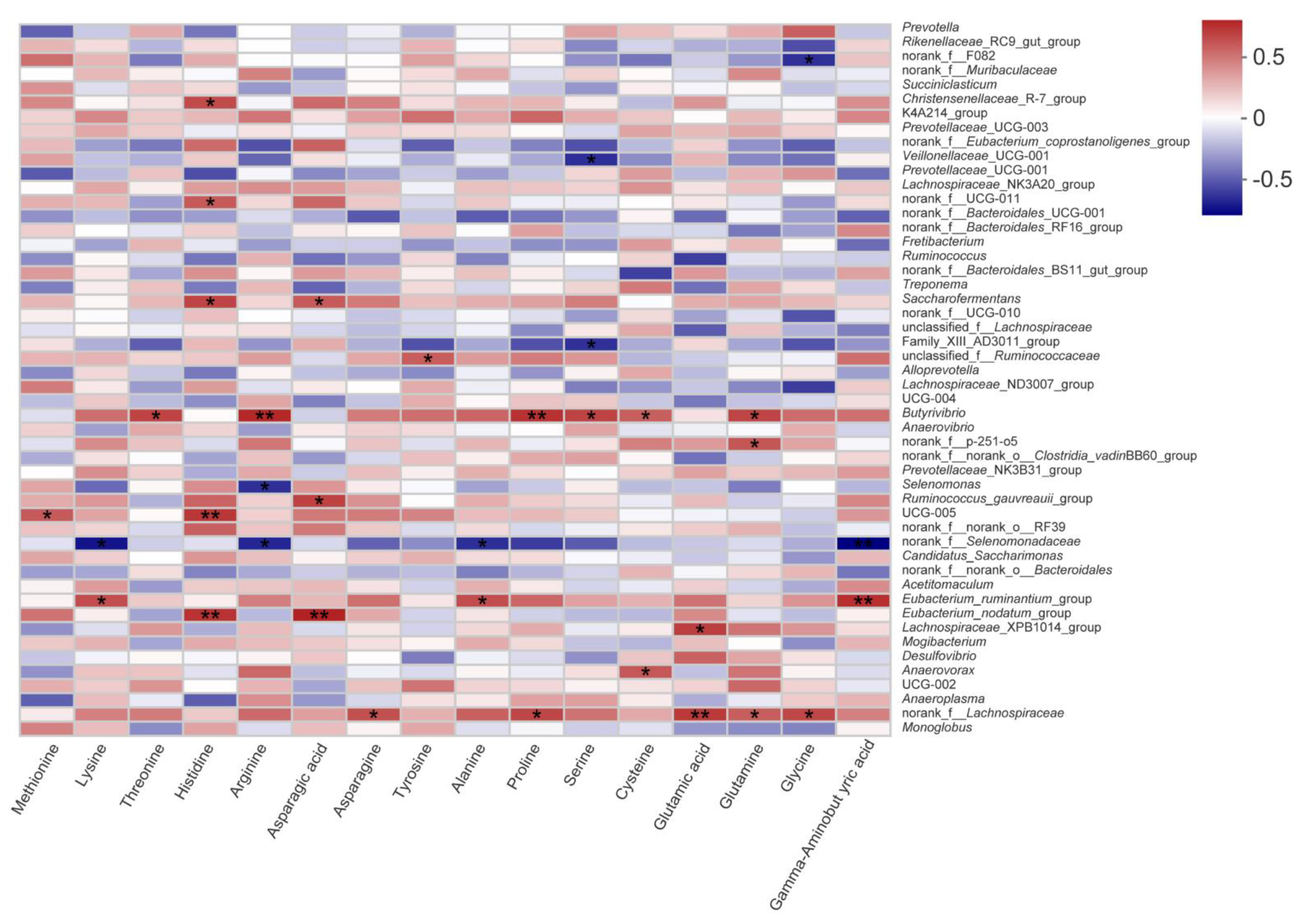
Figure 2). Rumen fermentation and metabolism are not only affected by dietary protein, structural carbohydrates, and starch, but also related to active metabolites such as flavonoids, tannins, and saponins, which can regulate the activities of microorganisms. Taking into account the correlation among paper mulberry silage, rumen bacterial community and fatty acids, we prefer that PMS improve fatty acids distribution in LD by changing rumen microbe composition and metabolic pathways. Unlike fatty acids, the changes in amino acids did not seem to be affected by rumen bacterial community in this study, although
Butyrivibrio,
Eubacterium spp. and
norank_f_Lachnospiraceae had a positive correlation with some amino acids. To the best of our knowledge, amino acids are mainly absorbed in the small intestine, so protein passing through rumen has become an important research direction for amino acid metabolism [
44]. Tannins are widely reported to reduce rumen BH, protect rumen protein degradation and reduce methane production [
8,
45,
46]. However, paper mulberry rich in tannins did not generate the expected results in amino acids of LD, probably because silage reduced the tannin content. Increasing research has been conducted to study not only which bacterial community are changing in rumen but also the functional metabolites shifting. Concomitantly, further study is still necessary to explore the relationship between rumen microorganism composition, rumen metabolites, and amino acid metabolism via metagenomics and metabolome.