Selection of Bacteriocinogenic Bacillus spp. from Traditional Fermented Korean Food Products with Additional Beneficial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Antibacterial Strains and Examination of Their Inhibitory Activity
2.2. Differentiation and Identification of Selected Isolates
2.3. Evaluation on Proteinaceous Nature and Stability of Produced Bacteriocins
2.4. Production of Antimicrobials and Change of pH during Bacterial Growth
2.5. The Effect of CFS on Growth and Survival of the Target Strains
2.6. Adhesion Properties
2.7. Spectrum of Activity
2.8. Presence of Genes Encoding for Antimicrobials
2.9. Safety Assessments
2.9.1. Virulence Genes
2.9.2. Biogenic Amines, Gelatinase, and Hemolytic Activity
2.9.3. Antibiotics
2.10. Detection of Beneficial Genes
2.11. Proteolytic Activity
2.12. Production of Lactic Acid
2.13. Hydrophobicity
3. Results
3.1. Isolation, Differentiation and Identification of Bacillus spp.
3.2. Evaluation of the Antimicrobial Activity
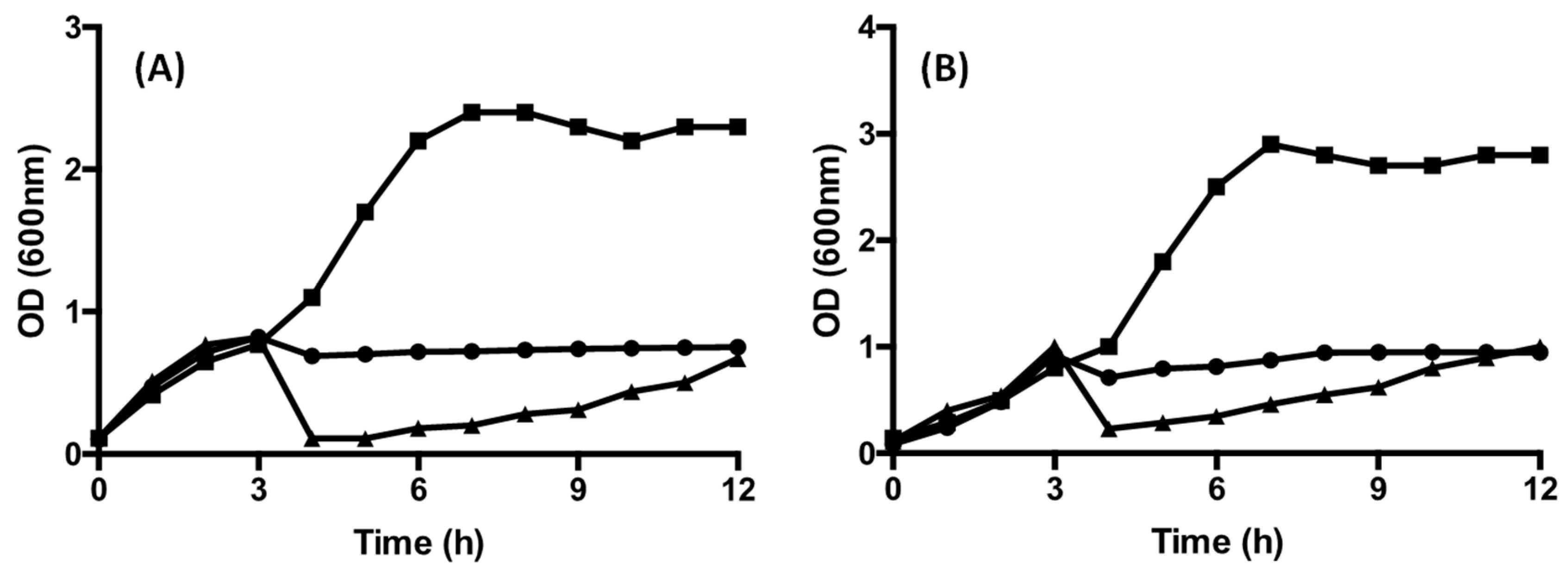
3.3. Bacterial Growth, Acidification (Changes in pH) and Production of Bacteriocin (AU/mL)
3.4. The Effect of CFS on Growth and Survival of the Target Strains
3.5. Adhesion Properties
3.6. Spectrum of Activity
3.7. Presence of Genes for Antimicrobials
3.8. Safety Features
3.8.1. Evaluation for Presence of Virulence Genes
3.8.2. Production of Biogenic Amines, Gelatinase, and Hemolytic Activity
3.8.3. Resistance/Susceptance to the Antibiotics
3.9. Beneficial Properties; Screening for Some Beneficial Genes
3.10. Proteolytic Activity
3.11. Production of Lactic Acid
3.12. Hydrophobicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, W.L. Roles of Bacillus endospores in the environment. Cell Mol. Life Sci. 2002, 59, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Claus, D.; Berkeley, R.C.W. Genus Bacillus. In Bergey’s Manual of Systematic Bacteriology; Sneath, P.H.A., Mair, N.S., Sharpe, M.E., Holt, J.G., Eds.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1986; Volume 2, pp. 1105–1139. [Google Scholar]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Oguntoyinbo, F.A.; Sanni, A.I.; Franz, C.M.; Holzapfel, W.H. In vitro fermentation studies for selection and evaluation of Bacillus strains as starter cultures for the production of okpehe, a traditional African fermented condiment. Int. J. Food Microbiol. 2007, 113, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Rooney, A.P.; Tsukakoshi, Y.; Nakagawa, R.; Hasegawa, H.; Kimura, K. Phylogenetic analysis of Bacillus subtilis strains applicable to natto (fermented soybean) production. Appl. Environ. Microbiol. 2011, 77, 6463–6469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chettri, R.; Tamang, J.P. Bacillus species isolated from Tungrymbai and Bekang, naturally fermented soybean foods of India. Int. J. Food Microbiol. 2015, 197, 72–76. [Google Scholar] [CrossRef]
- Compaoré, C.S.; Nielsen, D.S.; Ouoba, L.I.I.; Berner, T.S.; Nielsen, K.F.; Sawadogo, H.; Diawara, B.; Ouédraogo, G.A.; Jakobsen, M.; Thorsen, L. Co-production of surfactin and a novel bacteriocin by Bacillus subtilis subsp. subtilis H4 isolated from Bikalga, an African alkaline hibiscus sabdari, a seed fermented condiment. Int. J. Food Microbiol. 2013, 162, 297–307. [Google Scholar] [CrossRef]
- Todorov, S.D.; Ivanova, I.V.; Popov, I.; Weeks, R.; Chikindas, M.L. Bacillus spore-forming probiotics: Benefits with concerns? Crit. Revi. Microbiol 2021, in press. [Google Scholar] [CrossRef]
- Santos, R.A.; Oliva-Teles, A.; Pousão-Ferreira, P.; Jerusik, R.; Saavedra, M.J.; Enes, P.; Serra, C.R. Isolation and characterization of fish-gut Bacillus spp. as source of natural antimicrobial compounds to fight aquaculture bacterial diseases. Marine Biotechnol. 2021, 23, 276–293. [Google Scholar] [CrossRef]
- Abriouel, H.; Franz, C.M.; Ben Omar, N.; Galvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef] [Green Version]
- Lodemann, U.; Lorenz, B.M.; Weyrauch, K.D.; Martens, H. Effects of Bacillus cereus var. toyoi as probiotic feed supplement on intestinal transport and barrier function in piglets. Arch. Anim. Nutr. 2008, 62, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, A.L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haydushka, I.A.; Markova, N.; Kirina, V.; Atanassova, M. Recurrent Sepsis due to Bacillus licheniformis. J. Glob. Inf. Dis. 2012, 4, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Lagos, R. Bacteriocins. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 277–279. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Bacteriocins Produced by Lactic Acid Bacteria. A review article. APCBEE Procedia 2012, 2, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Begley, M.; Cotter, P.D.; Hill, C.; Ross, R.P. Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl. Environ. Microb. 2009, 75, 5451–5460. [Google Scholar] [CrossRef] [Green Version]
- De Vos, P.; Garrity, G.M.; Jones, D.; Kreig, N.R.; Ludwig, W.; Rainey, F.A.; Schleifel, K.-H.; Whitman, W.B. Bergey’s manual of systematic bacteriology. In The Firmicutes, 2nd ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 3. [Google Scholar]
- Dos Santos, K.M.O.; de Matos, C.R.; Salles, H.O.; Franco, B.D.G.M.; Arellano, K.; Holzapfel, W.H.; Todorov, S.D. Exploring beneficial/virulence properties of two dairy-related strains of Streptococcus infantarius subsp. infantarius. Probiotics Antimicrob. Proteins 2020, 12, 1524–1541. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M.T. Characterization of bacteriocins produced by lactic acid bacteria isolated from spoiled black olives. J. Basic Microbiol. 2005, 45, 312–322. [Google Scholar] [CrossRef]
- De Moraes, G.M.D.; de Abreu, L.R.; do Egito, A.S.; Salles, H.O.; da Silva, L.M.F.; Nero, L.A.; Todorov, S.D.; dos Santos, K.M.O. Functional properties of Lactobacillus mucosae strains isolated from Brazilian goat milk. Probiotics Antimicrob. Proteins 2016, 9, 235–245. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M.T. Screening for bacteriocin-producing lactic acid bacteria from boza, a traditional cereal beverage from Bulgaria. Comparison of the bacteriocins. Proc. Biochem. 2006, 41, 11–19. [Google Scholar] [CrossRef]
- Todorov, S.D.; Stojanovski, S.; Iliev, I.; Moncheva, P.; Nero, L.A.; Ivanova, I.V. Technology and safety assessment for lactic acid bacteria isolated from traditional Bulgarian fermented meat product “Lukanka”. Braz. J. Microbiol. 2017, 48, 576–586. [Google Scholar] [CrossRef]
- Chopra, L.; Singh, G.; Choudhary, V.; Sahoo, D.K. Sonorensin: An antimicrobial peptide, belonging to the heterocycloantharicin subfamily of bacteriocins, from a new marine isolate, Bacillus sonorensis MT93. Appl. Environ. Microbiol. 2014, 80, 2981–2990. [Google Scholar] [CrossRef] [Green Version]
- Guinebretière, M.H.; Broussolle, V.; Nguyen-The, C. Enterotoxigenic profile of food-poisoning and foodborne Bacillus cereus strains. J. Clin. Microbiol. 2002, 40, 3053–3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valledor, S.J.D.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Exploring beneficial properties of the bacteriocinogenic Enterococcus faecium ST10Bz strain isolated from boza, a Bulgarian cereal-based beverage. Microorganisms 2020, 8, 1474. [Google Scholar] [CrossRef] [PubMed]
- Irorita Fugaban, J.I.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Bacteriocinogenic Bacillus spp. isolated from Korean fermented cabbage (Kimchi)—Beneficial or hazardous? Fermentation 2021, 7, 56. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Gradient diffusion antibiotic susceptibility testing of potentially probiotic Lactobacilli. J. Food. Prot. 2001, 64, 2007–2014. [Google Scholar] [CrossRef]
- Doyle, R.J.; Rosenberg, M. Measurement of microbial adhesion to hydrophobic substrates. Meth. Enzymol. 1995, 253, 542–550. [Google Scholar]
- Savadogo, A.; Tapi, A.; Chollet, M.; Wathelet, B.; Traoré, A.S.; Jacques, P. Identification of surfactin producing strains in Soumbala and Bikalga fermented condiments using Polymerase Chain Reaction and Matrix Assisted Laser Desorption/Ionization-Mass Spectrometry methods. Int. J. Food Microbiol. 2011, 151, 299–306. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Hasenack, B.; Nout, M.J. Diversity and functionality of Bacillus and related genera isolated from spontaneously fermented soybeans (Indian Kinema) and locust beans (African Soumbala). Int. J. Food Microbiol. 2002, 77, 175–186. [Google Scholar] [CrossRef]
- Jung, S.; Woo, C.; Fugaban, J.I.I.; Bucheli, J.E.V.; Holzapfel, W.H.; Todorov, S.D. Bacteriocinogenic potential of Bacillus amyloliquefaciens isolated from Kimchi, a traditional Korean fermented cabbage. Probiotics Antimicrob. Proteins 2021, 13, 1195–1212. [Google Scholar] [CrossRef]
- Chikindas, M.L.; Weeks, R.M.; Drider, D.; Chistyakov, V.A.; Dicks, L.M.T. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef]
- Latorre, J.D.; Hernandez-Velasco, X.; Wolfenden, R.E.; Vicente, J.L.; Wolfender, A.D.; Menconi, A.; Bielke, L.R.; Hargis, B.M.; Tellez, G. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 2016, 3, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.M.; Lee, C.S.; Yoo, C.K.; Seo, W.S. Purification and characterization of fibrinolytic enzyme excreted by Bacillus subtilis K-54 isolated from Chung Guk Jang. Korean J. Appl. Microbiol. Biotechnol. 1998, 26, 507–515. [Google Scholar]
- Park, H.J.; Oh, H.H.; Jeong, D.Y.; Kim, Y.S. Probiotic characteristics of Bacillus tequilensis JBC17126 isolated from Korean traditional soybean paste. J. Kor. Soc. Food Sci. Nutr. 2019, 48, 710–717. [Google Scholar] [CrossRef]
- Yang, E.J.; Chang, H.C. Characterization of bacteriocin-like substances produced by Bacillus subtilis MJP1. Kor. J. Microbiol. Biotechnol. 2007, 35, 339–346. [Google Scholar]
- Jack, R.W.; Tagg, J.R.; Ray, B. Bacteriocins of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 1995, 59, 171–200. [Google Scholar] [CrossRef]
- Kuniyoshi, T.M.; Mendonça, C.M.N.; Vieira, V.B.; Robl, D.; Franco, B.D.G.M.; Todorov, S.D.; Tomé, E.; O’Connor, P.M.; Converti, A.; Araujo, W.L.; et al. Pediocin PA-1 production by Pediococcus pentosaceus ET34 using non-detoxified hemicellulose hydrolysate obtained from hydrothermal pretreatment of sugarcane bagasse. Bioresour. Technol. 2021, 338, 125565. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Salum, K.; Lee, H.A.; Kim, J.H. Properties of Bac W42, a bacteriocin produced by Bacillus subtilis W42 isolated from cheonggukjang. J. Microbiol. Biotechnol. 2012, 22, 1092–1100. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An antibiotic agent pyrrolo[1,2-a]pyrazine-1,4-dione,hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef] [Green Version]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, 039. [Google Scholar] [CrossRef]
- Hammami, R.; Fernandez, B.; Lacroix, C.; Fliss, I. Anti-infective properties of bacteriocins: An update. Cell. Mol. Life Sci. 2013, 70, 2947–2967. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, E.V.; Todorov, S.D.; Sesma, F.; Franco, B.D.G.M. Application of bacteriocinogenic Enterococcus mundtii CRL35 and Enterococcus faecium ST88Ch in the control of Listeria monocytogenesin fresh Minas cheese. Food Microbiol. 2012, 32, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-spectrum antibacterial agents. Medchemcomm 2018, 9, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Barboza-Corona, J.E.; de la Fuente-Salcido, N.; Alva-Murillo, N.; Ochoa-Zarzosa, A.; Lopez-Meza, J.E. Activity of bacteriocins synthesized by Bacillus thuringiensis against Staphylococcus aureus isolates associated to bovine mastitis. Vet. Microbiol. 2009, 138, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Inès, M.; Dhouha, G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 2015, 71, 100–112. [Google Scholar] [CrossRef]
- Huang, T.; Geng, H.; Miyyapuram, V.R.; Sit, C.S.; Vederas, J.C.; Nakano, M.M. Isolation of a variant of subtilosin A with hemolytic activity. J. Bacteriol. 2009, 191, 5690–5696. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.Z.V.; Nitschke, M. Evaluation of rhamnolipid and surfactin to reduce the adhesion and remove biofilms of individual and mixed cultures of food pathogenic bacteria. Food Control 2012, 25, 441–447. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, P. Disinfectant-like activity of lipopeptide biosurfactant produced by Bacillus tequilensis strain SDS21. Coll. Surf. B Biointerfaces 2020, 185, 110514. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef]
- Luis-Villaseñor, I.E.; Macías-Rodríguez, M.E.; Gómez-Gil, B.; Ascencio-Valle, F.; Campa-Córdova, Á.I. Beneficial effects of four Bacillus strains on the larval cultivation of Litopenaeus vannamei. Aquaculture 2011, 321, 136–144. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Hema, S.; Deepika, R.; Ravindran, A.D. Purification of antilisterial peptide (Subtilosin A) from novel Bacillus tequilensis FR9 and demonstrate their pathogen invasion protection ability using human carcinoma cell line. Front Microbiol. 2016, 7, 1910. [Google Scholar] [CrossRef] [Green Version]
- Hisano, T.; Abe, S.; Wakashiro, M.; Kimura, A.; Murata, K. Isolation and properties of a collagenase with caseinolytic activity from a Pseudomonas sp. J. Ferm. Bioeng. 1989, 68, 399–403. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalder, T.; Top, E. Plasmid transfer in biofilms: A perspective on limitations and opportunities. NPJ Biofilms Microbiomes 2016, 2, 16022. [Google Scholar] [CrossRef] [Green Version]
- Teuber, M. Spread of antibiotic resistance with food-borne pathogens. Cell. Mol. Life Sci. 1999, 56, 755–763. [Google Scholar] [CrossRef]
- Salyers, A.A.; Gupta, A.; Wang, Y. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 2004, 12, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A. What Is Wrong with Enterococcal Probiotics? Probiotics Antimicrob. Proteins 2020, 12, 1–4. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, M.E.; Yarrow, J.F.; McCoy, S.C.; Borst, S.E. Growth hormone isoform responses to GABA ingestion at rest and after exercise. Med. Sci. Sports Exerc. 2008, 40, 104–110. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Suwanmanon, K.; Hsieh, P.C. Effect of gamma-aminobutyric acid and nattokinase-enriched fermented beans on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. J. Food Drug. Anal. 2014, 22, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Huang, J.; Sun, L.; Xu, F.; Zhang, W.; Zhan, J. An efficient process for co-production of gamma-aminobutyric acid and probiotic Bacillus subtilis cells. Food Sci. Biotechnol. 2019, 28, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Vergnolle, N. Protease inhibition as new therapeutic strategy for GI diseases. Gut 2016, 65, 1215–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, R.R. Bacterial and fungal proteolytic enzymes: Production, catalysis and potential applications. Appl. Biochem. Biotechnol. 2017, 183, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Worsztynowicz, P.; Olejnik-Schmidt, A.; Białas, W.; Grajek, W. Identification and partial characterization of proteolytic activity of Enterococcus faecalis relevant to their application in the dairy industry. Acta Biochim. Pol. 2019, 66, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sarabhai, S.; Tamilselvan, T.; Prabhasankar, P. Role of enzymes for improvement in gluten-free foxtail millet bread: It’s effect on quality, textural, rheological and pasting properties. LWT Food Sci. Technol. 2020, 137, 110365. [Google Scholar] [CrossRef]
- Dos Santos, M.M.T.R.; de Aguiar, P.F.; Silva, J.B.A.; de Mello, P.P.M.; Sérvulo, E.F.C. Brewery wastes reuse for protease production by lactic acid bacteria fermentation. Food Technol. Biotechnol. 2017, 55, 218–224. [Google Scholar]
- Haard, N.F. A review of proteotlytic enzymes from marine organisms and their application in the food industry. J. Aquat. Food Prod. Technol. 1992, 1, 17–35. [Google Scholar] [CrossRef]
- Pokora, M.; Eckert, E.; Zambrowicz, A.; Bobak, Ł.; Szołtysik, M.; Dazbrowska, A.; Chrzanowska, J.; Polanowski, A.; Trziszka, T. Biological and functional properties of proteolytic enzyme-modified egg protein by-products. Food Sci. Nutr. 2013, 1, 184–195. [Google Scholar] [CrossRef]
- Park, H.; Lee, M.; Ji, Y.; Todorov, S.D.; Holzapfel, W.H. Safety evaluation and in vivo strain-specific functionality of Bacillus strains isolated from Korean traditional fermented foods. Probiotics Antimicrob. Proteins 2021, 13, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Montel, M.C.; Masson, F.; Talon, R. Bacterial role in flavour development. Meat Sci. 1998, 49 (Suppl. 1), S111–S123. [Google Scholar] [CrossRef]
- El Mecherfi, K.; Lupi, R.; Cherkaoui, M.; Albuquerque, M.A.C.; Todorov, S.D.; Tranquet, O.; Klingebiel, C.; Denery-Papini, S.; Onno, B.; Franco, B.D.G.M.; et al. Fermentation of gluten by Lactococcus lactis LLGKC18 reduces its antigenicity and allergenicity. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef] [PubMed]
- Biscola, V.; Tulini, F.L.; Choiset, Y.; Rabesona, H.; Ivanova, I.; Chobert, J.-M.; Todorov, S.D.; Haertle, T.; Franco, B.D.G.M. Proteolitic activity of Enterococcus faecium VB63F for reduction of allergenicity of bovine milk proteins. J. Dairy Sci. 2016, 99, 5144–5154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijeyeweera, R.L.; Kleinberg, I. Acid-base pH curves in vitro with mixtures of pure cultures of human oral microorganisms. Arch. Oral Biol. 1989, 34, 55–64. [Google Scholar] [CrossRef]
- Mack, D.R. D(−)-lactic acid-producing probiotics, D(−)-lactic acidosis and infants. Can. J. Gastroenterol. 2004, 18, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Todorov, S.D.; Furtado, D.N.; Saad, S.M.I.; Tome, E.; Franco, B.D.G.M. Potential beneficial properties of bacteriocin-producing lactic acid bacteria isolated from smoked salmon. J. Appl. Microbiol. 2011, 110, 971–986. [Google Scholar] [CrossRef] [Green Version]
- Todorov, S.D.; Botes, M.; Guigas, C.; Schillinger, U.; Wiid, I.; Wachsman, M.B.; Holzapfel, W.H.; Dicks, L.M.T. Boza, a natural source of probiotic lactic acid bacteria. J. Appl. Microbiol. 2008, 104, 465–477. [Google Scholar] [CrossRef]
- De Wouters, T.; Jans, C.; Niederberger, T.; Fischer, P.; Rühs, P.A. Adhesion potential of intestinal microbes predicted by physicochemical characterization methods. PLoS ONE 2015, 10, e0136437. [Google Scholar] [CrossRef]
- Schär-Zammaretti, P.; Ubbink, J. The cell wall of lactic acid bacteria: Surface constituents and macromolecular conformations. Biophys. J. 2003, 85, 4076–4092. [Google Scholar] [CrossRef] [Green Version]
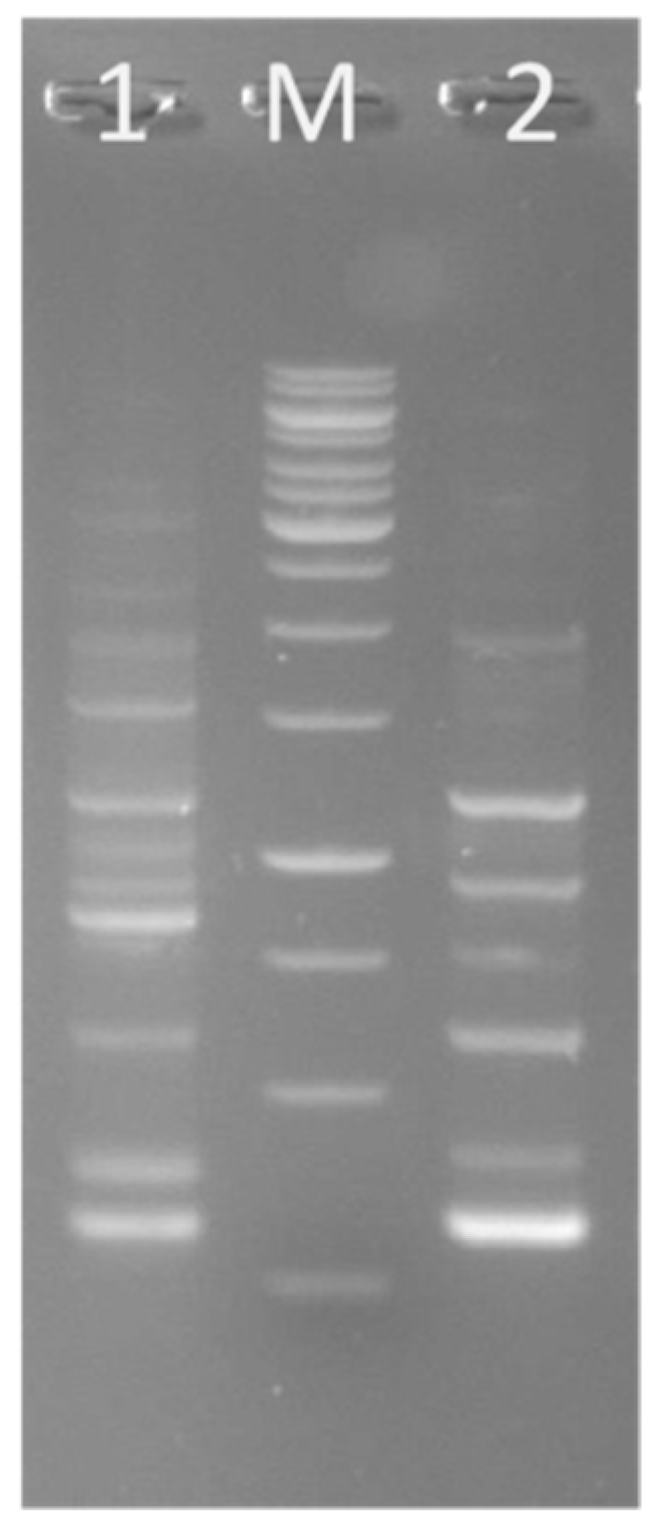

| Test Organisms | Environmental Factor | Effect of Factors on | |
|---|---|---|---|
| B. tequilensis ST816CD | B. subtilis ST830CD | ||
| Lactobacillus rhamnosus 911 | 50 # | 50 | |
| Lactobacillus brevis 384 | 0 | 0 | |
| Lactobacillus plantarum 187 | 0 | 0 | |
| Lactobacillus plantarum 211 | 50 | 50 | |
| Lactobacillus fermentum 792 | 0 | 0 | |
| Lactobacillus salivarius 851 | 0 | 50 | |
| Pediococcus acidilactici 867 | 0 | 50 | |
| Streptococcus sanguinis KACC 11301 | 75 | 75 | |
| Streptococcus mutans KACC 16833 | 0 | 50 | |
| Streptococcus mitis KACC 16832 | 0 | 0 | |
| Escherichia coli ATCC 25922 | 50 | 75 | |
| Listeria monocytogenes ATCC 15313 | 75 | 94 | |
| Staphylococcus simulans KACC 13241 | 75 | 94 | |
| Staphylococcus auricularis KACC 13252 | 50 | 50 | |
| Temperature, °C | |||
| Listeria monocytogenes ATCC 15313 | 25 | 50 | 0 |
| 30 | 50 | 0 | |
| 37 | 50 | 50 | |
| 40 | 0 | 50 | |
| Staphylococcus simulans KACC 13241 | 25 | 50 | 0 |
| 30 | 50 | 50 | |
| 37 | 50 | 75 | |
| 40 | 0 | 50 | |
| pH | |||
| Listeria monocytogenes ATCC 15313 | 4.0 | 50 | 50 |
| 6.0 | 50 | 50 | |
| 8.0 | 50 | 50 | |
| Staphylococcus simulans KACC 13241 | 4.0 | 50 | 0 |
| 6.0 | 50 | 0 | |
| 8.0 | 0 | 0 | |
| Additives | |||
| Listeria monocytogenes ATCC 15313 | skim milk * | 50 | 75 |
| enzyme salts ** | 75 | 75 | |
| oral hygienic powder *** | 50 | 75 | |
| Staphylococcus simulans KACC 13241 | skim milk * | 75 | 50 |
| enzyme salts ** | 75 | 50 | |
| oral hygienic powder *** | 75 | 0 | |
| Inhibition by CFS from | ||
|---|---|---|
| Test Microorganisms | B. tequilensis ST816CD | B. subtilis ST830CD |
| Lactobacillus brevis HEM384 | − | − |
| Lactobacillus fermentum HEM792 | − | − |
| Lactobacillus plantarum HEM187 | − | − |
| Lactobacillus plantarum HEM211 | + | − |
| Lactobacillus plantarun ST8Sh | − | − |
| Lactobacillus rhamnosus HEM911 | + | + |
| Lactobacillus salivarius HEM851 | − | + |
| Listeria monocytogenes ATCC 15313 | − | + |
| Pediococcus acidilactici HEM867 | − | + |
| Pediococcus acidilactici ST3522BG | − | − |
| Pediococcus pentosaceus ST3633BG | − | − |
| Staphylococcus arlettae KACC 13254 | + | + |
| Staphylococcus auricularis KACC 13252 | + | + |
| Staphylococcus capitis subsp. capitis KACC 13242 | − | − |
| Staphylococcus carnosus subsp. carnosus KACC 13250 | + | + |
| Staphylococcus cohnii subsp. cohnii KACC 13237 | − | − |
| Staphylococcus delphini KACC 13258 | + | − |
| Staphylococcus epidermidis KACC 13234 | − | − |
| Staphylococcus lentus KACC 13245 | + | − |
| Staphylococcus simulans KACC 13241 | + | + |
| Staphylococcus warneri KACC 13240 | − | − |
| Streptococcus mitis KACC 16832 | − | − |
| Streptococcus mutans KACC 16833 | − | − |
| Streptococcus sanguinis KACC 11301 | + | + |
| Escherichia coli ATCC 25922 | − | − |
| Gene | Primers | References | Presence in | ||
|---|---|---|---|---|---|
| B. tequilensis ST816CD | B. subtilis ST830CD | ||||
| Antimicrobial Genes | nisQ (nisin) | F: ATGAGTACAAAAGATTTCAACTT R: TTATTTGCTTACGTGAACGC | [23] | − | − |
| bli (lichenicidin) | F: GGAAATGATTCTTTCATGG R: TTAGTTACAGCTTGGCATG | [24] | − | − | |
| sbo (subtiloson) | F: GGTTGTGCAACATGCTCGAT R: CTCAGGAAGCTGGTGAACTC | + | + | ||
| thu (thurincin) | F: GTAGGTCAAATGGAAACAC R: TTAACTTGCAGTACTAGCTC | − | − | ||
| coa (coagulin) | F: GGTGGTAAATACTACGGTAATGGGGT R: GTGTCTAAATTACTGGTTGATTCGT | − | − | ||
| ped (pediocin PA-1) | F: CAAGATCGTTAACCAGTTT R: CCGTTGTTCCCATAGTCTAA | − | − | ||
| srfa (surfactin) | F: TCGGGACAGGAAGACATCAT R: CCTCTCAAACGGATAATCCTGA | + | + | ||
| ituc (iturin) | F: GGCTGCTGCAGATGCTTTAT R: TCGCAGATAATCGCAGTGAG | + | − | ||
| Virulence genes | hblA (hemolysin BL) | F: AAGCAATGGAATACAATGGG R: AGAATCTAAATCATGCCACTGC | [25] | − | − |
| hblB (hemolysin BL) | F: AAGCAATGGAATACAATGGG R: AATATGTCCCAGTACACCCG | − | − | ||
| hblC (hemolysin BL) | F: GATACYAATGTGGCAACTGC R: TTGAGACTGCTCGYTAGTTG | − | − | ||
| nheA (nonhemolytic enterotoxin) | F: GTGAGGATCACAATCACCGC R: ACGAATGTAATTTGAGTCGTCGC | [25] | − | − | |
| nheB (nonhemolytic enterotoxin) | F: TTTAGTGGATCTGTACGC R: TTAATGTTCGTTAATCCTGC | − | − | ||
| nheC (nonhemolytic enterotoxin) | F: TGGATTCCAAGATGTAACG R: ATTACGACTTCTGCTTGTGC | − | − | ||
| Vancomycin resistance genes | vanA | F: GTAGGCTGCGATATTCAAAGC R: CGATTCAATTGCGTAGTCCAA | [26] | − | − |
| vanB | F: GTAGGCTGCGATATTCAAAGC R: GCCGACAATCAAATCATCCTC | − | − | ||
| vanC | F: ATCCAAGCTATTGACCCGCT R: TGTGGCAGGATCGTTTTCAT | − | − | ||
| vanD | F: TGTGGGATGCGATATTCAA R: TGCAGCCAAGTATCCGGTAA | − | − | ||
| vanE | F: TGTGGTATCGGAGCTGCAG R: GTCGATTCTCGCTAATCC | − | − | ||
| vanG | F: GAAGATGGTACTTTGCAGGGCA R: AGCCGCTTCTTGTATCCGTTTT | − | − | ||
| Beneficial Genes | mapA (adhesion) | F: TGGATTCTGCTTGAGGTAAG R: GACTAGTAATAACGCGACCG | [19] | − | − |
| mub (adhesion) | F: GTAGTTACTCAGTGACGATCAATG R: TAATTGTAAAGGTATAATCGGAGG | − | − | ||
| eftu (adhesion) | F: TTCTGGTCGTATCGATCGTG R: CCACGTAATAACGCACCAAC | − | − | ||
| ef2380 (adhesion) | F: GCGGTCGACGACATCTATGAAAACAAT R: TCCGCGCCGCCTTAAACTTTCTCCTT | − | − | ||
| ef2662 (adhesion) | F: GGCGTCGACCACTTAAACTGATAGAGAGGAAT R: CGCGCCGCAATTAATTATTAACTAGTTTCC | − | − | ||
| ef1249 (adhesion) | F: GCGGTCGACAAACGAGGGATTTATTATG R: CTGGCGGCCGCGTTTAATACAATTAGGAAGCAGA | − | − | ||
| prg (adhesion) | F: GCCGTCGACTCGAGGAGAATGATACATGAAT R: CCTGCGGCCGCGTCCTTCTTTTCGTCTTCAA | − | − | ||
| folPE (folate production) | F: GAGATAGTCTTAACGACATCACGATT R: GCAGTCTATCAATTATTGGAAGCTTT | [26] | − | − | |
| folKQ (folate production) | F: CACTAGTGTCTATTGACTCAAATATTTT R: CGTTTTTATGGCTATCACGGGGCT | − | − | ||
| pabB (folate production) | F: CCTCAATTCATACAACCCTCTCACA R: CAGACAAATCTTCACTCACGCCATAA | − | − | ||
| pabC (folate production) | F: CGGACAAGCATAATGAATACTCGGAAT R: GGATTGATAACCGCTTCTATTGCCGA | − | − | ||
| gad (GABA production) | F: CCTCGAGAAGCCGATCGCTTAGTTCG R: TCATATTGACCGGTATAAGTGATGCCC | [26] | + | − | |
| B. tequilensis ST816CD | B. subtilis ST830CD | |
|---|---|---|
| Hemolytic activity (α, β, γ) | γ | α |
| Gelatinase | + | − |
| Biogenic amines: histidine to histamine ornithine to putrescine lysine to cadaverine tyrosine to tyramine | + + + + | + + + + |
| Antibiotics | Diameter Inhibition Zone (mm) | |
| ampicillin 10 μg per disc | 25 | 40 |
| ciprofloxacin 10 μg per disc | 45 | 45 |
| clindamycin 10 μg per disc | 27 | 55 |
| erythromycin 10 μg per disc | 29 | 40 |
| gentamycin 10 μg per disc | 15 | 30 |
| penicillin G 1 IU per disc | 0 | 35 |
| streptomycin 10 μg per disc | 15 | 25 |
| tobramycin 10 μg per disc | 15 | 20 |
| vancomycin 30 μg per disc | 22 | 30 |
| Bacteriocins Formed by | ||
|---|---|---|
| Bacillus tequilensis ST816CD | Bacillus subtilis ST830CD | |
| Effect of enzymes: proteinase K α-chymotrypsin α-amylase | +/− +/− + | +/− +/− + |
| Effect of temperature (°C): 4, 25, 30, 37, 60, 80, 100 For 1 h For 2 h | + + | + + |
| Effect of pH (for 2 h): 2.0 4.0, 6.0, 8.0 10.0 | + + − | − + + |
| Effect of chemicals/medium components (1%; for 1 h): NaCl, Tween 80, SDS, skim milk | + | + |
| Enzyme | B. tequilensis ST816CD | B. subtilis ST830CD |
|---|---|---|
| alkaline phosphatase | 3 | 3 |
| esterase (C 4) | 4 | 4 |
| esterase Lipase (C 8) | 2 | 2 |
| leucine arylamidase | 2 | 2 |
| valine arylamidase | 3 | 2 |
| cystine arylamidase | 2 | 1 |
| trypsin | 2 | 1 |
| acid phosphatase | 2 | 2 |
| naphthol-AS-BI-phosphohydrolase | 3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, G.H.; Fugaban, J.I.I.; Dioso, C.M.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Selection of Bacteriocinogenic Bacillus spp. from Traditional Fermented Korean Food Products with Additional Beneficial Properties. Fermentation 2021, 7, 271. https://doi.org/10.3390/fermentation7040271
Choi GH, Fugaban JII, Dioso CM, Vazquez Bucheli JE, Holzapfel WH, Todorov SD. Selection of Bacteriocinogenic Bacillus spp. from Traditional Fermented Korean Food Products with Additional Beneficial Properties. Fermentation. 2021; 7(4):271. https://doi.org/10.3390/fermentation7040271
Chicago/Turabian StyleChoi, Gee Hyeun, Joanna Ivy Irorita Fugaban, Clarizza May Dioso, Jorge Enrique Vazquez Bucheli, Wilhelm Heinrich Holzapfel, and Svetoslav Dimitrov Todorov. 2021. "Selection of Bacteriocinogenic Bacillus spp. from Traditional Fermented Korean Food Products with Additional Beneficial Properties" Fermentation 7, no. 4: 271. https://doi.org/10.3390/fermentation7040271
APA StyleChoi, G. H., Fugaban, J. I. I., Dioso, C. M., Vazquez Bucheli, J. E., Holzapfel, W. H., & Todorov, S. D. (2021). Selection of Bacteriocinogenic Bacillus spp. from Traditional Fermented Korean Food Products with Additional Beneficial Properties. Fermentation, 7(4), 271. https://doi.org/10.3390/fermentation7040271







