Abstract
The glucan-rich fraction, hemicellulosic compounds-rich fraction, and a mixture of both fractions obtained from organosolv pretreatment of oil palm empty fruit bunch (OPEFB) were used as substrates to produce volatile fatty acids (VFAs) in acidogenic fermentation. In this study, the effects of medium adjustment (carbon to nitrogen ratio and trace elements supplementation) and methanogenesis inhibition (through the addition of 2-bromoethanesulfonate or by heat shock) to enhance VFAs yield were investigated. The highest VFA yield was 0.50 ± 0.00 g VFAs/g volatile solid (VS), which was obtained when methanogens were inhibited by heat shock and cultivated in a mixture of glucan-rich and hemicellulosic compounds-rich fractions. Under these conditions, the fermentation produced acetic acid as the only VFA. Based on the results, the mass balance of the whole process (from pretreatment and fermentation) showed the possibility to obtain 30.4 kg acetic acid and 20.3 kg lignin with a 70% purity from 100 kg OPEFB.
1. Introduction
The increasing global demand for vegetable oils promotes palm oil production since it has the lowest production cost [1] and a relatively high productivity [2]. According to the United States Department of Agriculture (USDA), global palm oil production was about 72 million tons in 2019, and Indonesia had contributed to about 43 million tons [3,4]. For every kilogram of palm oil produced, 1.3 kg of oil palm empty fruit bunches (OPEFB) are generated [5]. Thus, 55.9 million tons of OPFEB were produced in Indonesia in 2019. The typical treatment for OPEFB includes composting [6], combustion [7], or dumping in a landfill [8]. The composting of OPEFB is a relatively slow biological process, and hence it is inadequate to manage the enormous amount of OPEFB produced. The combustion of OPEFB generates fly ash as an air pollutant [9] and produces corrosive materials that damage the superheater [10]. Moreover, due to its large quantity, open dumping of OPEFB requires vast land, and the decomposition process releases a foul odor [11]. Therefore, the handling and valorization of OPEFB are pivotal. As a starting point, the fact that palm oil production in Indonesia is concentrated in a few provinces [12] can be used as an advantage for valorization in view of reduced transportation costs.
‘Oil palm empty fruit bunch’ (OPEFB), a lignocellulosic biomass, mainly consists of cellulose, hemicellulose, and lignin, which account for 24–65 wt%, 21–34 wt%, and 14–31 wt% of the material, respectively [13]. OPEFB’s composition makes it an attractive substrate for conversion into biofuels and biochemicals [14,15]. For this purpose, pretreatment is essential to deconstruct the compact lignocellulosic structure of OPEFB to make carbohydrate polymers more available and accessible for their subsequent utilization. Organosolv pretreatment is a promising method, as it provides good purity and recovery of glucan while hemicellulosic compounds can also be recovered. In addition, lignin is recovered reasonably well with relatively high purity (Mondylaksita, et al. [16]. The resulting glucan and hemicellulosic compounds after delignification are potential substrates for the production of various biochemical compounds. One of the promising strategies is their bioconversion into platform compounds, such as volatile fatty acids (VFAs), through the anaerobic digestion process. VFAs include monocarboxylic acids of C2-C6 carbon atoms such as acetic acid, propionic acid, butyric acid, valeric acid, and caproic acid [17]. VFAs are produced through the stages of hydrolysis and acidogenesis among the four biochemical reactions (hydrolysis–acidogenesis–acetogenesis–methanogenesis) taking place in anaerobic digestion. Therefore, VFAs production is known as acidogenic fermentation. As platform compounds, VFAs have a broad range of applications in the production of value-added products. Furthermore, the production process of VFAs does not need additional enzymatic hydrolysis and sterilization since it is based on the concerted action of a diversified microbial community. Hence, mixed microbial cultures can also provide energy savings with a positive impact on the overall economy of the process [18].
Anaerobic digestion is a biological process that has been used to treat organic wastes. The performance of anaerobic digestion, such as process stability, biogas production, organic reduction, and inhibition, depends on the substrates’ characteristics [19]. In anaerobic digestion, substrates with a high degree of acidification (e.g., starch and whey) and high accumulation of VFAs can be expected, and methanogenesis is usually the rate-limiting stage. However, for the case of lignocellulosic materials, the rate-limiting step is the hydrolysis process [20]. This means that if lignocelluloses have passed through degradation in the hydrolysis process, methane will be quickly produced as the end-product (since VFAs are not accumulated). Therefore, methanogenesis needs to be inhibited to obtain a high accumulation of VFAs. For this purpose, physicochemical conditions (e.g., C/N ratio and medium supplementation) can be optimized to boost hydrolysis-acidogenesis-acetogenesis stages. At the same time, methanogens are inhibited by, for example, the addition of chemicals or heat-shock treatment [21,22,23,24,25].
A proper C/N ratio in the substrate is one of the crucial factors to boost acidogenic activity. Generally, an optimum C/N ratio range for acidogenic fermentation ranges from 20–30 [26]. Trace elements (such as cobalt, nickel, molybdenum, and iron) play an essential role in activating and maintaining enzymatic activities in the acidogenic fermentation process [23,27]. A commonly used method to inhibit the methanogens is the addition of methanogen inhibitors such as 2-bromoethanesulfonate (BES). BES is coenzyme M analog. Unlike the fermentative acidogenic bacteria, methanogens have a coenzyme M that carries a methyl group to produce methane with the help of methyl-CoM reductase [28]. Coenzyme M analogs act as competitive inhibitors in methyl transfer reactions at concentrations between 5–50 mM [25,28]. In addition, acidogenic bacteria can form spores when subjected to high temperatures, whereas methanogens lack that functionality [28,29,30]. Thus, heat shock at around 80–100 °C for 15–120 min can be employed to deactivate the methanogens [24,28].
This study aimed to evaluate the effect of C/N ratio adjustment, medium supplementation, and inoculum preparation (through adding chemicals and heat treatment) to obtain high VFAs accumulation using glucan-rich fraction and hemicellulosic compounds-rich fraction from OPEFB pretreatment with organosolv as the substrates. A biorefinery concept of OPEFB based on the OPEFB fractionation and VFAs production was also proposed.
2. Materials and Methods
2.1. The Oil Palm Empty Fruit Bunch
Oil palm empty palm fruit bunch (OPEFB) was collected from a palm oil industry in Medan, Indonesia. It was sun-dried to achieve a 7% moisture content. The OPEFB contains glucan, hemicellulose, lignin, and ash that account for 40.09 ± 0.01 wt%, 23.94 ± 0.02 wt%, 21.77 ± 0.27 wt%, and 3.72 ± 0.07 wt% of the total material, respectively. The dried OPEFB was milled using a cutting mill (Retsch SM 100, Haan, Germany) with a pore size of 300 μm, which resulted in the following particle size distribution: 44.24% of >500 μm, 17.96% of 250–500 μm, 23.81% of 100–250 μm, and 13.99% of 63–125 μm.
2.2. Organosolv-Derived OPEFB Fractions
Organosolv-derived OPEFB fractions were obtained through ethanol-organosolv pretreatment of OPEFB at the best conditions found in a previously published study [16]. The OPEFB was firstly dried and milled. The OPEFB powder and 50% ethanol solution, adjusted to pH 3.0 with 2 M H2SO4 (corresponding to 0.07% of pure acid per substrate dry weight), were added to 150 mL stainless steel reactors (Swagelok, El Paso, TX, USA) at a solid-to-liquid ratio of 1:10. The reactors were then sealed and placed into an oil bath (Julabo, Seelbach Germany) at 210 °C for 90 min under static conditions. After that, the mixture was filtered using a 250 μm sieve for solid-liquid separation. The solid cellulose (glucan)-rich fraction was obtained through sieving and then washed with 28.3 mL ethanol solution per gram of dry-untreated OPEFB. As much as 56.6 mL water was added (per 1 g dry-untreated OPEFB) into the remaining black liquor to precipitate and separate lignin from hemicellulosic compounds-rich fraction. The precipitation was followed by centrifugation (Thermo Scientific Heraeus Megafuge 8, Waltham, MA, USA) at 3360× g for 5 min. The glucan-rich fraction, hemicellulosic compounds-rich fraction, and mixed fractions (a mixture of glucan- and hemicellulosic compounds-rich fraction) were evaporated using a rotary evaporator (LABO ROTA 20, Heidolph, Germany) at 110 °C, 40 rpm, and at a vacuum pressure of 100 mPa, to eliminate the ethanol and water. The residue was then used as the substrate for acidogenic fermentation. The total solids (TS), volatile solids (VS), and nitrogen contents of each fraction are presented in Table 1.

Table 1.
The total solid (TS), volatile solids (VS), and nitrogen (TKN) contents of untreated OPEFB and organosolv pretreatment-derived OPEFB fractions.
2.3. Inoculum
The inoculum used in the acidogenic fermentation process was obtained from an upflow anaerobic sludge blanket (UASB) reactor treating wastewater (Hammarby Sjöstad, Stockholm, Sweden) and contained flocculated bacteria. The UASB was operated at 20 °C. The inoculum was stored for five days in an incubator at 37 °C before being used. The inoculum comprised 9.54 ± 0.32% of total solids (TS) and 6.50 ± 0.15% of volatile solids (VS). The microbial community analysis of the inoculum is according to Owusu-Agyeman, et al. [31].
2.4. Acidogenic Fermentation
The untreated OPEFB, glucan-rich fraction, hemicellulosic compounds-rich fraction, and mixed fractions were used at an inoculum-to-substrate ratio of 1:1 (0.3 g VS inoculum and 0.3 g VS substrate) in 120 mL serum bottles. Water was added so that the working volume in each bottle was 50 mL. The initial pH of all samples was adjusted to pH 5.5 using 2 M HCl and 2 M NaOH; there was no pH adjustment during the acidogenic fermentation. The bottles were then sealed and sparged with a gas mixture of 80% N2 and 20% CO2 for 2 min to ensure anaerobic condition. Lastly, the bottles were incubated at 37 °C in a water bath, shaking at 100 rpm for 14 days. The experiments were carried out in triplicate. Glucose, Avicel, and samples containing only inoculum were used as control.
To study the effect of substrate type and medium adjustment on VFA production, a 5 × 22 full factorial experimental design was planned and conducted using MINITAB® 17 (Minitab Ltd., Coventry, UK). Factors examined were substrate type (Avicel as control, untreated OPEFB, glucan-rich fraction, hemicellulosic compounds-rich fraction, and mixed fraction), C/N ratio adjustment, and trace metals supplementation. The measured responses were VFAs yield and methane gas. The C/N ratio adjustment used in this experiment was 22, which was adjusted with urea. The CoCl2·7H2O, NiCl2·6H2O, FeSO4·7H2O, and Na2MoO4·2H2O, was added at 0.076, 0.159, 0.296, and 0.082 mg/L, respectively, to mimic the commercial supplement [23].
Another full factorial experimental design of 5 × 3 was performed to study the effect of substrate type and methanogenesis inhibition on VFAs and methane gas production. Factors investigated included substrate type (Avicel as control, untreated OPEFB, glucan-rich fraction, hemicellulosic compounds-rich fraction, and mixed fractions) and methanogenesis inhibition strategy (addition of 2-bromoethanesulfonate (BES) and application of heat shock). The responses were VFAs yield and methane gas. The heat shock inoculum treatment was carried out in a water bath which was shaken at 100 rpm at 80 °C for 15 min, while the BES treatment was carried out by adding 10 mmol/L to each bottle [25].
2.5. Analytical Methods
The total solids (TS), tCOD (total chemical oxygen demand), and volatile solids (VS) were determined according to biomass analytical procedures by APHA-AWWA-WEF [32]. The samples’ TS and VS were analyzed with the thermogravimetric method. The samples were dried in an oven at 105 ± 3 °C until the constant weights were achieved (TS). Oven-dried samples were then ignited at 550 ± 25 °C to obtain a fixed solid. Volatile solid (VS) was then calculated from the subtraction of total solid with fixed solid.
The total Kjeldahl nitrogen (TKN) content of all the fractions was determined using the Kjeldahl method according to Mahboubi, et al. [33]. The total carbon was calculated by correcting the total dry weight carbon value for the ash content according to Haug [34] and Zhou, et al. [35].
Gas samples were withdrawn using a 0.25 mL gas-tight syringe (VICI, Precision Sampling Inc., Baton Rouge, LA, USA). Gas analysis was conducted to determine the methane content using a Perkin-Elmer gas chromatograph (Clarus 550; Perkin Elmer, Norwalk, CT, USA). The gas chromatograph was equipped with a packed column (CarboxenTM 1000, 6′ × 1.8″ OD, 60/80 Mesh, Supelco, Shelton, CT, USA) and a thermal conductivity detector (Perkin-Elmer, Norwalk, CT, USA). The injection temperature was 200 °C. The carrier gas was N2 with a flow rate of 30 mL/min at 75 °C.
The VFAs were measured using a gas chromatograph (Perkin-Elmer, Shelton, CT, USA), equipped with a capillary column (Elite-WAX ETR, 30 m × 0.32 mm × 1.00 µm, Perkin-Elmer, Shelton, CT, USA) and a flame-ionized detector (PerkinElmer, Shelton, CT, USA). The injection and detection temperatures were 250 °C and 300 °C, respectively. The carrier gas was nitrogen at a 2 mL/min flow rate and a pressure of 20 psi. The volume of each injection was 1 μL. The stock solution of caproic acid, iso-valeric acid, valeric acid, iso-butyric acid, butyric acid, propionic acid, and acetic acid was prepared by dissolving each of these VFAs in water. These solutions were subsequently mixed to prepare a 10 g/L mixed stock standard solution. For quantification and calibration, the concentration of the mixed standard solution was diluted to 5.00, 2.50, 1.25, 0.62 and 0.31 g/L in distilled water. Butanol at the concentration of 1 g/L was used as an internal standard. During intermittent injection, a clean-up procedure was performed before each injection of a new sample. Distilled water was used for the clean-up procedure.
2.6. Statistical Analysis
MINITAB® 17 (Minitab Ltd., Coventry, UK) was used for statistical analysis. A general linear model with a confidence interval of 95% was applied to analyze variance (ANOVA) and factor interaction; statistical differences were identified at p-value < 0.05. Pairwise comparisons were also carried out according to Tukey’s test. All intervals and error bars reported represent two times the standard deviation.
3. Results and Discussion
Glucan and hemicellulosic compound fractions are potential substrates for VFAs production. To obtain high VFAs production, acidogenic fermentation in which VFAs are produced needs to be boosted by inhibiting methanogenesis through adding chemicals or heat-shock treatment. The first stage of the work was focused on the substrate type, C/N ratio, and medium supplementation on the yield of VFAs and methane. In the second stage, the focus was on methanogenesis inhibition by adding a methanogen inhibitor or applying a heat-shock treatment.
3.1. Effect of C/N Ratio and Medium Supplementation
Acidogenic fermentation was performed using OPEFB fractions as the substrates after ethanol-organosolv pretreatment. In addition, the untreated OPEFB and Avicel were used as controls. As shown in Figure 1a, the highest production of VFAs (0.40 ± 0.02 g VFA/g VS) from Avicel was obtained when the C/N ratio was adjusted. However, there was a simultaneous consumption of VFAs for methane production (Figure 1f), although the methane yield was relatively small (Table 2), ca. 13% of the theoretical value, which was calculated based on modified Buswell equation [36]. Medium supplementation with trace metals had a detrimental effect on the VFAs yield since it led to an increase in methane yield, which was 80.29 ± 0.75 mL/g VS (26% of theoretical value). As for the untreated OPEFB, negligible VFAs accumulation was observed (Figure 2b and Table 2). When trace metals were added, the produced VFAs appeared to be consumed directly for methane production (Figure 2g), even though the methane yield was low (less than 20% of the theoretical value).
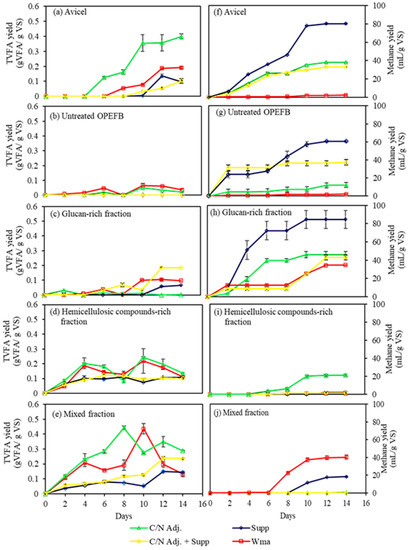
Figure 1.
Total volatile fatty acid (TVFA) (a–e) and methane gas (f–j) yield during batch acidogenic fermentation of Avicel, untreated OPEFB, glucan-rich fraction, hemicellulosic compounds-rich fraction, and mixed fraction with or without C/N ratio adjustment and medium supplementation. The experiments were done in triplicate. Wma: without medium adjustment; C/N adj: C/N ratio adjustment; Sup: supplementation with trace metals.

Table 2.
Maximum VFAs yield from different substrates with different medium adjustments. The experiments were carried out in triplicate.
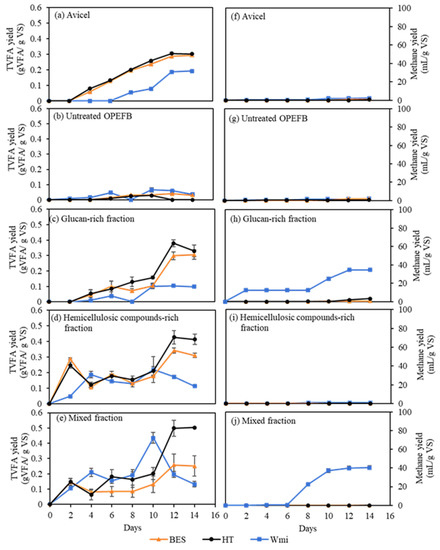
Figure 2.
Total volatile fatty acid (TVFA) (a–e) and methane gas (f–j) yield during batch acidogenic fermentation of Avicel, untreated OPEFB, glucan-rich fraction, hemicellulosic compounds-rich fraction, and mixed fraction with or without methanogenesis inhibition. The experiments were done in triplicate. The abbreviations of the medium adjustment are: Wmi: without methanogenesis inhibition, BES: 2-bromoethanesulfonate, HT: heat-shock treatment.
When glucan-rich fraction was used as a substrate, C/N ratio adjustment or trace metals supplementation alone did not accumulate VFAs. The VFAs yields were even lower than those without medium adjustment (Table 2 and Figure 1c). The highest VFAs accumulation of 0.19 ± 0.00 g VFA/g VS was obtained when the C/N ratio adjustment and the trace metals supplementation were conducted simultaneously. However, the results presented in Figure 1h suggest that the VFAs were being used for methane production. Table 2 shows that glucan-rich fraction with trace metals supplementation alone resulted in the highest methane yield of 84.58 ± 9.89 mL/g VS (27% of the theoretical value). For 1 g of VS, 84.58 mL CH4 is produced from 0.23 g of acetic acid conversion. Hence, if methane formation was successfully inhibited, then 0.23 g acetic acid/g VS could be accumulated instead of converted into CH4.
In the acidogenic fermentation of hemicellulosic compounds-rich fraction, the medium adjustment did not positively affect the VFAs yield. After all, the results in Figure 1d showed that there were ups and downs in the VFAs accumulation. The VFAs were accumulated on the sixth day, but it was then followed by VFAs consumption on the eighth day. VFAs accumulation occurred again on the tenth day, and it started to be consumed afterward. This phenomenon indicates that VFAs accumulation slightly inhibited methane production. The highest methane yield of 21.33 ± 1.15 mL/g VS was achieved when the C/N ratio was adjusted. However, the highest methane yield was only 7% of the theoretical value.
In the experiment with mixed fraction, C/N ratio adjustment and trace metals supplementation did not enhance VFA yield (Table 2). It can be seen in Figure 1e that the VFAs were accumulated on the eighth day when the C/N ratio adjustment was applied. The VFA accumulation was faster than the VFAs accumulation without C/N ratio adjustment, which happened on the tenth day. In all conditions, the accumulated VFAs were followed by methane production (Figure 1e,j). Like the hemicellulosic compounds-rich fraction results, accumulation of VFAs seemed to have a slight inhibition effect on methane production since a delay in its production was observed (Figure 1j).
The results showed that a C/N ratio adjustment was needed to achieve the highest yield of VFAs during acidogenic fermentation of Avicel. During acidogenic fermentation of a somewhat similar substrate, namely glucan-rich fraction, C/N ratio adjustment and supplementation of trace metals led to the highest yield of VFAs. Previous studies also reported that a C/N ratio of 20–30 effectively increased VS degradation of textile wastewater sludge and during co-digestion of algal sludge and waste paper [37,38]. In the untreated OPEFB, it is more likely that the slow digestion of the substrate resulted in the slow VFAs production, which was immediately converted into methane. Hence, no accumulation of VFAs occurred.
Nevertheless, C/N ratio adjustment and trace metals supplementation did not completely inhibit methane production. The results from this study are in agreement with those reported by Karlsson, et al. [27], where supplementation of trace metals (500 mg/L of Fe, 0.5 mg/L of Co, and 0.25 g/L of Ni) on anaerobic digestion of food industry and household waste caused acetate production with a concomitant increase in biogas production. Similarly, a study carried out by Menon, et al. [39] suggested that medium supplementation with 303 mg/L of Ca, 777 mg/L of Mg, 7 mg/L of Co, and 3 mg/L of Ni resulted in VFAs production, which was directly followed by an increase of methane gas yield by 40%. In addition, trace metals such as Fe, Co, Ni, and Mo are essential for the activity of many hydrogenase enzymes, which affect both acidogenic and methanogenic microorganisms [40]. Hence, the micronutrients helped to increase methane production. The results showed that the VFAs produced were not high enough to suppress the methane production. For this reason, inhibition of methanogenesis with other methods such as heat-shock treatment or adding methanogen inhibitor was necessary to be conducted to produce a high accumulation of VFAs.
3.2. Effect of Methanogenesis Inhibition
When Avicel was used as the substrate, the addition of methanogen inhibitor (BES) and application of heat-shock treatment of inoculum increased VFAs yield (Figure 2a) and decreased methane yield (Figure 2f). The enhancement of VFAs yield was 50% compared to without methanogenesis inhibition (Table 3).

Table 3.
Maximum VFAs yield from different substrates with different methanogenesis inhibition methods. The experiments were carried out in triplicate.
The results suggested that methanogenesis inhibitions, by adding a methanogen inhibitor and applying a heat-shock treatment, successfully increased the accumulation of VFAs and inhibited methane production on Avicel. On the other hand, for the untreated OPEFB, negligible VFAs and methane were produced for all conditions (Figure 2b,g). Heat-shock treatment and addition of BES did not affect VFAs enhancement since the untreated OPEFB was hard to digest. Therefore, no VFAs production was observed (Table 3).
As for glucan-rich fraction, VFAs production started to accelerate after the tenth day of the fermentation (Figure 2c). It can be seen in Figure 2h that the addition of BES and heat-shock treatment were able to inhibit methane production, and as a result, VFAs could be accumulated. Compared to the conditions without methanogenesis inhibition, the increase in VFAs yield was three-fold for the addition of BES and four-fold for heat-shock treatment (Table 3). For the condition without methanogenesis inhibition, VFAs accumulation was relatively low, and methane started directly to be produced on the second day. Similar results were obtained from acidogenic fermentation of hemicellulosic compounds-rich fraction. Both BES addition and heat shock-treatment led to VFAs accumulation, with no methane production (Figure 2d,i). The VFAs yields for hemicellulosic compounds-rich fraction without methanogenesis inhibition, with the addition of BES, and with heat-shock treatment were 0.22 ± 0.08, 0.34 ± 0.02, and 0.43 ± 0.04 g VFA/g VS, respectively (Table 3). The increase of VFAs yield from the condition with heat-shock treatment was almost 100%, compared to that without any methanogenesis inhibition.
In comparison, it was only a 55% increase of VFAs yield from the condition when BES was added. Unlike fermentation of glucan-rich fraction, which mainly was cellulose, acidogenic fermentation of the hemicellulosic compounds-rich fraction did not have a lag phase in producing VFAs. The reason for this is most likely since hemicellulosic compounds are more easily degraded than cellulose. In the mixed fraction, VFAs were accumulated, and no methane production was observed for the conditions with the addition of BES or application of heat-shock treatment (Figure 2e,j). However, the addition of BES gave a lower VFAs yield than those without methanogenesis inhibition and heat-shock treatment. In the mixed fraction without heat-shock application, although the VFA yield was 0.45 g VFA/g VS, VFA could not accumulate since it was used for methane gas production (Figure 2e,j). Meanwhile, with the application of heat shock, the highest VFA yield was 0.50 ± 0.00 g VFA/g VS with acetic acid as the only VFA produced. In addition, there was no methane production observed on this condition.
Overall, the addition of BES and heat-shock treatment enhanced VFAs yield. 2-bromoethanesulfonate (BES) is the chemical most commonly used as a methane inhibitor [41]. A dosage of 10 mmol BES/L successfully inhibited 100% of methanogens in anaerobic digestion of cow dung and municipal wastewater treatment sludge [25]. In this study, when glucan, hemicellulosic compounds and mixed-fractions were used as substrates for acidogenic fermentation, the addition of BES resulted in a lower VFAs yield compared to that of heat-shock treatment. Studies by Liu, et al. [42] and Lu, et al. [43] showed that BES might inhibit the growth of other microorganisms responsible for VFAs or hydrogen biosynthesis. This finding indicates that BES might harm the acidogens.
On the other hand, the ability of acidogens to form spores under extreme conditions, such as high temperature, can be used as a strategy to kill methanogens. The genera Clostridium and Bacillus of acidogens, which were responsible for producing VFAs, can form spores that are resilient to heat shock [28]. The microbes’ resilience could be the reason for heat-shock treatment to be more effective than the addition of BES in inhibiting methane production in this study. Moreover, the inoculum used in this work was taken from a UASB reactor operated at 20 °C, so that a short time of heat-shock treatment (15 min) at 80 °C effectively inhibited methane production. A previous study by Mei, et al. [24] also showed that after heat-shocking methanogens at 60 and 70 °C for 15 min, methane production was inhibited entirely throughout 90 days of incubation.
3.3. Profile of VFAs
Acidogenic fermentation of mixed fraction with heat-shock treatment (referred to as the best condition in this work) resulted in acetic acid as the only type of VFA produced. Without heat-shock treatment, the percentage of acetic acid produced from mixed fractions was 77%. For other conditions, other types of VFAs were produced, and the percentages were in the range of 44–100% acetic acid, 3.75–56% propionic acid, and 5.55–26.75% butyric acid (data not shown). Methane gas was not produced under the best condition. These results might suggest that the methanogens were completely inhibited by heat-shock treatment and the anaerobic digestion stopped at the acidogenesis stage. Heat-shock treatment was reported to inhibit the acetoclastic methanogens’ activity [44]. A study by Penning and Conrad [45] showed that there was a linear accumulation of acetic acid when the acetoclastic methanogen was inhibited by methyl fluoride. As it was previously reported, the most abundant methanogens in the inoculum used in this work were acetoclastic methanogens [31]. Thus, it is possible that when acetoclastic methanogens were inhibited by heat-shock treatment, acetic acid could be solely produced.
Furthermore, the inoculum used in this work was flocculated bacteria from the UASB reactor in the wastewater treatment (described in the Section 2). According to Wainaina, et al. [41] and Owusu-Agyeman, et al. [31], who used the same inoculum, in this flocculated bacteria, at the phylum level, Actinobacteria and Firmicutes were dominating (5–8%) [41], whereas, at the family level, Anaerolinaceae was the predominant family with a relative abundance of 16.2–17.5%, followed by Syntrophaceae with a relative abundance of 7.9–10.1%. Other bacterial genera found in the granular sludge of the reactor were Paludibacter, Desulfomicrobium, and Proteiniclasticum [31]. Based on the previous studies [46,47,48,49], these bacteria were fermentative bacteria with acetate as the main fermentation product of several substrates. This could be the reason why under all conditions acetic acid was more dominantly produced than the other VFAs.
3.4. OPEFB Based Biorefinery
To increase the economic feasibility of lignocellulosic bioconversion, a biorefinery concept in which all lignocellulosic components are utilized to produce value-added products, is an excellent strategy to be implemented. The construction of a cost-effective lignocellulosic biorefinery will be significantly influenced by the selling price of the final value-added product. Based on the results of previous work by Mondylaksita, et al. [16] and current work, a new concept of OPEFB biorefinery is proposed (Figure 3). In this study, the yield of acetic acid was 0.50 g/g VS with a titer of 3%. The titer of acetic acid from the commercial fermentation ranges from 3.9–10% [49], while the yield ranges from 0.31–0.96 g of acetic acid/g of the substrate [50]. The yield of acetic acid from this study is comparable to the yield of the commercial fermentation process. Acetic acid is quite popularly used in industries such as food, paint, paper, textile, pharmaceutical, adhesive, and plastics industries [51,52]. Acetic acid has the highest market size among other VFAs of about 3,500,000 tons/year with a price per tonne of 800$ compared to the other VFAs [53]. In addition to glucan and hemicellulosic compounds, lignin with relatively high recovery and purity can be generated from organosolv pretreatment [16]. Lignin has a high value, and lignin derivatives such as resins, flavoring compounds, and nanofibers with antioxidant activity, which are helpful in many applications, can compensate for the costs incurred in the pretreatment process.
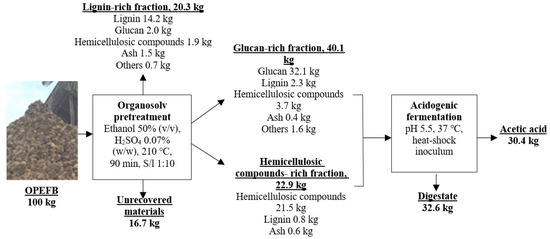
Figure 3.
Mass balance on OPEFB biorefinery concept to produce lignin and acetic acid.
From 100 kg OPEFB (dry basis), 38.5 kg of glucan-rich fraction and 22.9 kg of hemicellulosic compounds-rich fraction were obtained [16]. The total solid was 63.0 kg (including lignin and ash). According to Table 1, the VS content of the mixed fraction was 96.55 ± 0.05%. Hence, volatile solid (VS) was assumed as the total amount of organic materials from the pretreated OPEFB. Using the VS value, the number of organic materials in the mixed fraction was calculated to be 60.8 kg. Our previous results also showed that a high lignin purity of 70% and 65% lignin recovery were obtained [16]. Using these values, it can be calculated that from 100 kg of OPEFB (dry basis) with a lignin content of 21.77%, 30.4 kg of acetic acid, and 20.3 kg of lignin with 70% purity can be produced (Figure 3). With 55.9 million tons of OPEFB produced in 2019, scaling up by multiplication, 17 million tons of acetic acid and 11.3 million tons of lignin can be produced.
This acetic acid production through the acetogenesis process could be an alternative to the existing process. The Monsanto process for the carbonylation of methanol to acetic acid has been used as the method of choice for acetic acid production [54]. However, this method uses high temperatures between 150 and 200 °C, requires an expensive (rhodium) catalyst, and the equipment is prone to severe corrosion due to the iodide co-catalyst used [54]. Other than the Monsanto process, acetic acid has been produced through biological processes. According to food purity law, a food-grade acetic acid should preferably originate from biological processes [55]. Acetic acid is produced through a two-stage fermentation, namely, alcoholic and acetous fermentation [56]. On alcoholic fermentation, yeast Saccharomyces cerevisiae is mainly used, while on the acetous fermentation, the acetic acid bacteria used are mainly Acetobacter [50]. Using two different microbes requires two different fermentation conditions and fermenters. Hence it is quite laborious. Therefore, a one-step biological process, i.e., acidogenic fermentation, which offers milder conditions, can be considered.
4. Conclusions
The fractions of oil palm empty fruit from organosolv pre-treatment were used as substrates for acidogenic fermentation to produce volatile fatty acids. Adjusting the C/N ratio and adding trace metals did not result in high VFAs accumulation, which was expected to inhibit methane production. Meanwhile, the addition of methanogen inhibitor (BES) and application of heat-shock treatment succeeded in inhibiting methane production, where heat-shock treatment was more effective than the BES addition. The mixed fraction with heat-shock treatment produced the highest VFA yield of 0.50 ± 0.00 g VFA/g VS with acetic acid as the only detected VFA. From the integrated process in the biorefinery concept, bioconversion of OPEFB through acidogenic fermentation can produce valuable products (i.e., acetic acid and lignin).
Author Contributions
Conceptualization, W.B., M.J.T. and R.M.; methodology, K.M., J.A.F., M.J.T. and R.M.; validation, J.A.F., W.B., M.J.T. and R.M.; formal analysis, K.M. and J.A.F.; investigation, K.M.; writing—original draft preparation, K.M., J.A.F. and R.M.; writing—review and editing, W.B., C.N., M.J.T. and R.M.; supervision, J.A.F., W.B., C.N., M.J.T. and R.M.; project administration, C.N., M.J.T. and R.M.; funding acquisition, C.N., M.J.T. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Research and Technology/National Research and Innovation Agency of the Republic of Indonesia (RISTEK-BRIN) (grant number 3194/UN1.DITLIT/DIT-LIT/PT/2020) and the Swedish Research Council.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Ministry of Education and Culture, the Republic of Indonesia, for the scholarship under Pendidikan Magister menuju Doktor untuk Sarjana Unggul (PMDSU) Batch III. PT London Sumatra Indonesia Tbk is gratefully acknowledged for providing the OPEFB.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pacheco, P.; Gnych, S.; Dermawan, A.; Komarudin, H.; Okarda, B. The Palm Oil Global Value Chain: Implications for Economic Growth and Socialand Environmental Sustainability; CIFOR: Bogor, Indonesia, 2017; Volume 220. [Google Scholar]
- Barcelos, E.; Rios Sde, A.; Cunha, R.N.; Lopes, R.; Motoike, S.Y.; Babiychuk, E.; Skirycz, A.; Kushnir, S. Oil palm natural diversity and the potential for yield improvement. Front. Plant Sci. 2015, 6, 190. [Google Scholar] [CrossRef]
- USDA. Oilseeds: World Markets and Trades. Availabe online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed on 23 September 2020).
- USDA. Indonesia: Oilseeds and Product Update. Availabe online: https://www.fas.usda.gov/data/indonesia-oilseeds-and-products-update-15 (accessed on 23 September 2021).
- Yusoff, S. Renewable energy from palm oil—Innovation on effective utilization of waste. J. Clean. Prod. 2006, 14, 87–93. [Google Scholar] [CrossRef]
- Geng, A. Conversion of Oil Palm Empty Fruit Bunch to Biofuels. In Liquid, Gaseous and Solid Biofuels—Conversion Techniques; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Husain, Z.; Zainal, Z.A.; Abdullah, M.Z. Analysis of biomass-residue-based cogenerationsystem in palm oil mills. Biomass Bioenergy 2003, 24, 117–124. [Google Scholar] [CrossRef]
- Tahir, A.A.; Mohd Barnoh, N.F.; Yusof, N.; Mohd Said, N.N.; Utsumi, M.; Yen, A.M.; Hashim, H.; Mohd Noor, M.J.M.; Akhir, F.N.M.D.; Mohamad, S.E.; et al. Microbial Diversity in Decaying Oil Palm Empty Fruit Bunches (OPEFB) and Isolation of Lignin-degrading Bacteria from a Tropical Environment. Microbes Environ. 2019, 34, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Latip, N.A.; Sofian, A.H.; Ali, M.F.; Ismail, S.N.; Idris, D.M.N.D. Structural and morphological studies on alkaline pre-treatment of oil palm empty fruit bunch (OPEFB) fiber for composite production. Mater. Today Proc. 2019, 17, 1105–1111. [Google Scholar] [CrossRef]
- Okoro, S.C.; Kiamehr, S.; Montgomery, M.; Frandsen, F.J.; Pantleon, K. Effect of flue gas composition on deposit induced high temperature corrosion under laboratory conditions mimicking biomass firing. Part I: Exposures in oxidizing and chlorinating atmospheres. Mater. Corros. 2017, 68, 499–514. [Google Scholar] [CrossRef]
- Novianti, S.; Biddinika, M.K.; Prawisudha, P.; Yoshikawa, K. Upgrading of Palm Oil Empty Fruit Bunch Employing Hydrothermal Treatment in Lab-scale and Pilot Scale. Procedia Environ. Sci. 2014, 20, 46–54. [Google Scholar] [CrossRef]
- Ditjenbun. Tree Crop Estate Statistics of Indonesia Palm Oil (2017–2019); Ministry of Agriculture of Indonesia: Jakarta, Indonesia, 2018.
- Chang, S.H. An overview of empty fruit bunch from oil palm as feedstock for bio-oil production. Biomass Bioenergy 2014, 62, 174–181. [Google Scholar] [CrossRef]
- Gnansounou, E.; Dauriat, A. Techno-economic analysis of lignocellulosic ethanol: A review. Bioresour. Technol. 2010, 101, 4980–4991. [Google Scholar] [CrossRef]
- Noomtim, P.; Cheirsilp, B. Production of Butanol from Palm Empty Fruit Bunches Hydrolyzate by Clostridium Acetobutylicum. Energy Procedia 2011, 9, 140–146. [Google Scholar] [CrossRef]
- Mondylaksita, K.; Ferreira, J.A.; Millati, R.; Budhijanto, W.; Niklasson, C.; Taherzadeh, M.J. Recovery of High Purity Lignin and Digestible Cellulose from Oil Palm Empty Fruit Bunch Using Low Acid-Catalyzed Organosolv Pretreatment. Agronomy 2020, 10, 674. [Google Scholar] [CrossRef]
- Chang, H.N.; Kim, N.-J.; Kang, J.; Jeong, C.M. Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol. Bioprocess. Eng. 2010, 15, 1–10. [Google Scholar] [CrossRef]
- Kim, N.-J.; Lim, S.-J.; Chang, H. Volatile Fatty Acid Platform: Concept and Application. In Emerging Areas in Bioengineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 173–190. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H.; Li, J. Effects of organic composition on mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2017, 244, 213–224. [Google Scholar] [CrossRef]
- Annamalai, N.; Elayaraja, S.; Oleskowicz-Popiel, P.; Sivakumar, N.; Bahry, S.A. Volatile fatty acids production during anaerobic digestion of lignocellulosic biomass. In Recent Developments in Bioenergy Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 237–251. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Chen, Y.; Du, G.; Chen, J. Effects of organic matter and initial carbon–nitrogen ratio on the bioconversion of volatile fatty acids from sewage sludge. J. Chem. Technol. Biotechnol. 2008, 83, 1049–1055. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Yan, Y.; Feng, L. Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Appl. Energy 2013, 102, 1197–1204. [Google Scholar] [CrossRef]
- Kumi, P.J.; Henley, A.; Shana, A.; Wilson, V.; Esteves, S.R. Volatile fatty acids platform from thermally hydrolysed secondary sewage sludge enhanced through recovered micronutrients from digested sludge. Water Res. 2016, 100, 267–276. [Google Scholar] [CrossRef]
- Mei, R.; Narihiro, T.; Nobu, M.K.; Liu, W.T. Effects of heat shocks on microbial community structure and microbial activity of a methanogenic enrichment degrading benzoate. Lett. Appl. Microbiol. 2016, 63, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.M.; Smith, A.L.; Reddy, R.R.; Pinto, A.J.; Hayes, K.F.; Raskin, L. Anaerobic microbial community response to methanogenic inhibitors 2-bromoethanesulfonate and propynoic acid. Microbiologyopen 2016, 5, 537–550. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Karlsson, A.; Einarsson, P.; Schnurer, A.; Sundberg, C.; Ejlertsson, J.; Svensson, B.H. Impact of trace element addition on degradation efficiency of volatile fatty acids, oleic acid and phenyl acetate and on microbial populations in a biogas digester. J. Biosci. Bioeng. 2012, 114, 446–452. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Poggi-Varaldo, H.M. Hydrogen production by fermentative consortia. Renew. Sustain. Energy Rev. 2009, 13, 1000–1013. [Google Scholar] [CrossRef]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Eyice, O.; Cetecioglu, Z.; Plaza, E. The study of structure of anaerobic granules and methane producing pathways of pilot-scale UASB reactors treating municipal wastewater under sub-mesophilic conditions. Bioresour. Technol. 2019, 290, 121733. [Google Scholar] [CrossRef]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2017, 59, 518–525. [Google Scholar] [CrossRef]
- Haug, R. The Practical Handbook of Compost Engineering; Routledge: Boca Raton, FL, USA, 2018. [Google Scholar]
- Zhou, C.; Liu, Z.; Huang, Z.L.; Dong, M.; Yu, X.L.; Ning, P. A new strategy for co-composting dairy manure with rice straw: Addition of different inocula at three stages of composting. Waste Manag. 2015, 40, 38–43. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Theoretical analysis of biogas potential prediction from agricultural waste. Resour.-Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, J.; Fan, J.; Wang, J.; Liu, H. Control of C/N ratio for butyric acid production from textile wastewater sludge by anaerobic digestion. Water Sci. Technol. 2012, 65, 883–889. [Google Scholar] [CrossRef]
- Liu, M.; Ren, N.Q.; Chen, Y.; Zhu, W.F.; Ding, J. Conversion regular patterns of acetic acid, propionic acid and butyric acid in UASB reactor. J. Environ. Sci. 2004, 16, 387–391. [Google Scholar]
- Menon, A.; Wang, J.Y.; Giannis, A. Optimization of micronutrient supplement for enhancing biogas production from food waste in two-phase thermophilic anaerobic digestion. Waste Manag. 2017, 59, 465–475. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, X.; Zhang, P.; Wan, J.; Guo, H.; Ghasimi, D.S.M.; Morera, X.C.; Zhang, T. Overview of key operation factors and strategies for improving fermentative volatile fatty acid production and product regulation from sewage sludge. J. Environ. Sci. 2020, 87, 93–111. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Horváth, I.S.; Taherzadeh, M.J. Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew. Energy 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, A.; Chen, J.; Ding, J. Chemical inhibitors of methanogenesis and putative applications. Appl. Microbiol. Biotechnol. 2011, 89, 1333–1340. [Google Scholar] [CrossRef]
- Lu, L.; Ren, N.; Zhao, X.; Wang, H.; Wu, D.; Xing, D. Hydrogen production, methanogen inhibition and microbial community structures in psychrophilic single-chamber microbial electrolysis cells. Energy Environ. Sci. 2011, 4, 1329–1336. [Google Scholar] [CrossRef]
- Hernández, C.; Alamilla-Ortiz, Z.L.; Escalante, A.E.; Navarro-Díaz, M.; Carrillo-Reyes, J.; Moreno-Andrade, I.; Valdez-Vazquez, I. Heat-shock treatment applied to inocula for H2 production decreases microbial diversities, interspecific interactions and performance using cellulose as substrate. Int. J. Hydrog. Energy 2019, 44, 13126–13134. [Google Scholar] [CrossRef]
- Penning, H.; Conrad, R. Effect of inhibition of acetoclastic methanogenesis on growth of archaeal populations in an anoxic model environment. Appl. Environ. Microbiol. 2006, 72, 178–184. [Google Scholar] [CrossRef][Green Version]
- McIlroy, S.J.; Kirkegaard, R.H.; Dueholm, M.S.; Fernando, E.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. Culture-independent analyses reveal novel anaerolineaceae as abundant primary fermenters in anaerobic digesters treating waste activated sludge. Front. Microbiol. 2017, 8, 1134. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, Gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef]
- Copeland, A.; Spring, S.; Göker, M.; Schneider, S.; Lapidus, A.; Del Rio, T.G.; Tice, H.; Cheng, J.-F.; Lucas, S.; Chen, F. Complete genome sequence of Desulfomicrobium baculatum type strain (XT). Stand. Genom. Sci. 2009, 1, 29–37. [Google Scholar] [CrossRef]
- Zhang, K.; Song, L.; Dong, X. Proteiniclasticum ruminis gen. nov., sp. nov., a strictly anaerobic proteolytic bacterium isolated from yak rumen. Int. J. Syst. Evol. Microbiol. 2010, 60, 2221–2225. [Google Scholar] [CrossRef]
- Németh, Á.; Vidra, A. Bio-produced Acetic Acid: A Review. Period. Polytech. Chem. Eng. 2017, 62, 245–256. [Google Scholar] [CrossRef]
- Boer, E.; Łukaszewska, A.; Kluczkiewicz, W.; Lewandowska, D.; King, K.; Reijonen, T.; Kuhmonen, T.; Suhonen, A.; Jääskeläinen, A.; Heitto, A.; et al. Volatile fatty acids as an added value from biowaste. Waste Manag. 2016, 58, 62–69. [Google Scholar] [CrossRef]
- Christodoulou, X.; Velasquez-Orta, S.B. Microbial Electrosynthesis and Anaerobic Fermentation: An Economic Evaluation for Acetic Acid Production from CO2 and CO. Environ. Sci. Technol. 2016, 50, 11234–11242. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation—The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Kalck, P.; Le Berre, C.; Serp, P. Recent advances in the methanol carbonylation reaction into acetic acid. Coord. Chem. Rev. 2020, 402, 213078. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Luzon-Quintana, L.M.; Castro, R.; Duran-Guerrero, E. Biotechnological Processes in Fruit Vinegar Production. Foods 2021, 10, 945. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).