Screening of Gas Substrate and Medium Effects on 2,3-Butanediol Production with C. ljungdahlii and C. autoethanogenum Aided by Improved Autotrophic Cultivation Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Medium Compositions
2.2. Batch Gas Fermentation Experiments
2.3. Calculation of Biomass Concentration
2.4. Calculation of Batch Fermentation Metrics
2.5. Analytics
2.6. Data Analysis
3. Results and Discussion
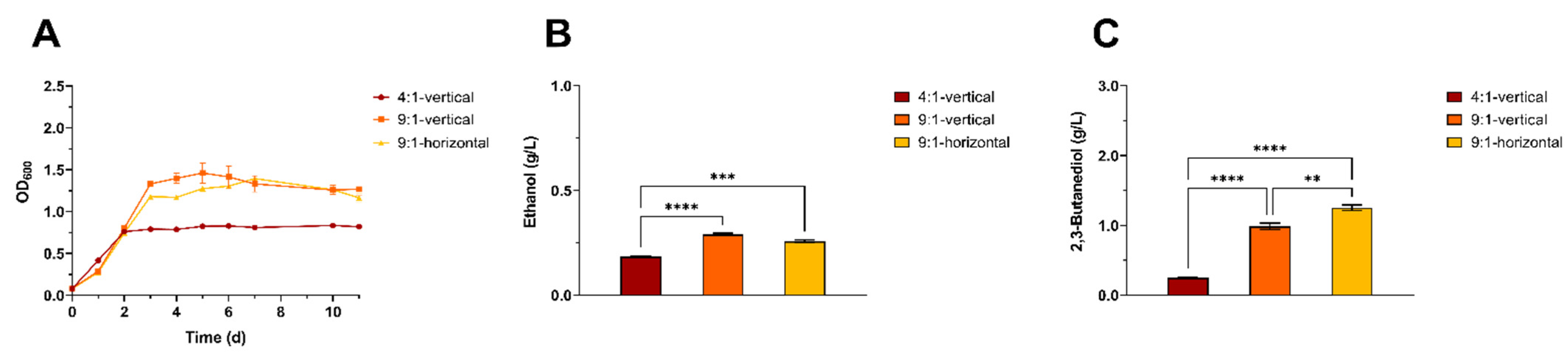
3.1. Cultivation Technique Optimization for the Autotrophic Growth of a Model Acetogen
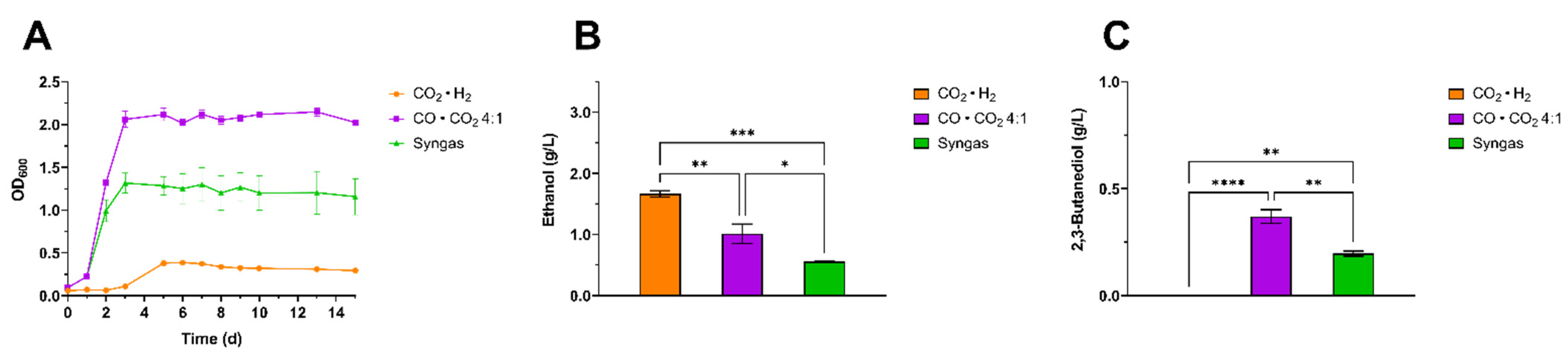
3.2. Screening of Gaseous Substrates for Enhanced 2,3-BDO Production Using Clostridium ljungdhalii and Clostridium autoethanogenum
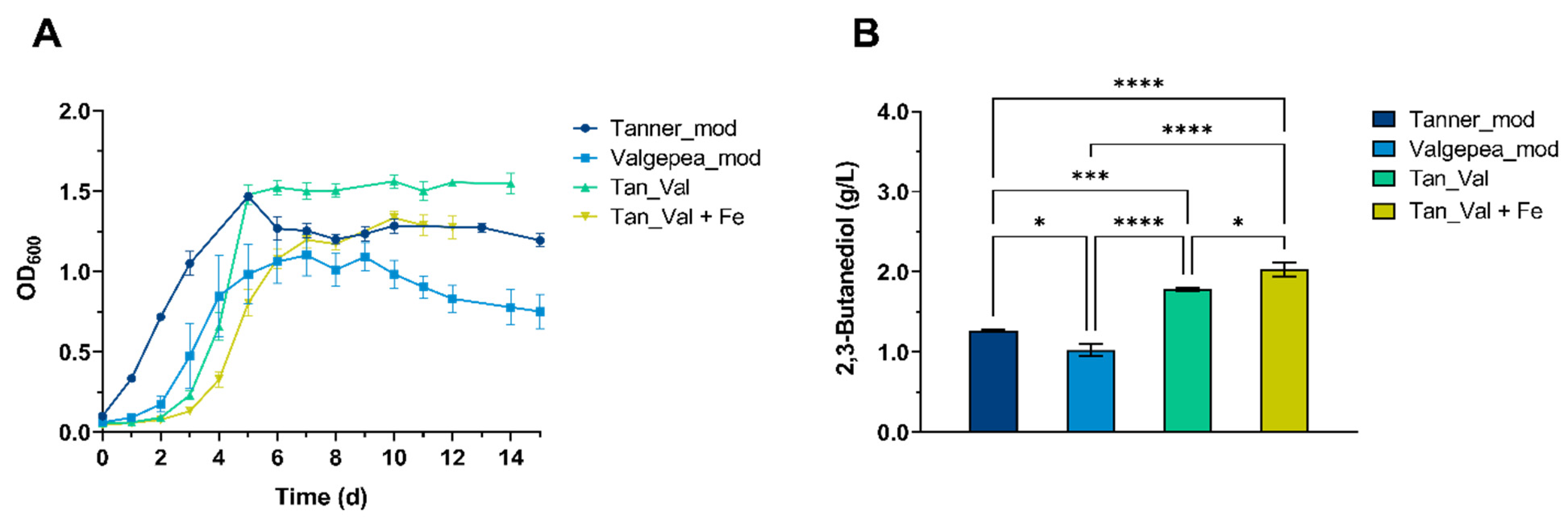
3.3. Influence of Cultivation Medium on 2,3-BDO Production from CO · CO2 using Clostridium ljungdahlii
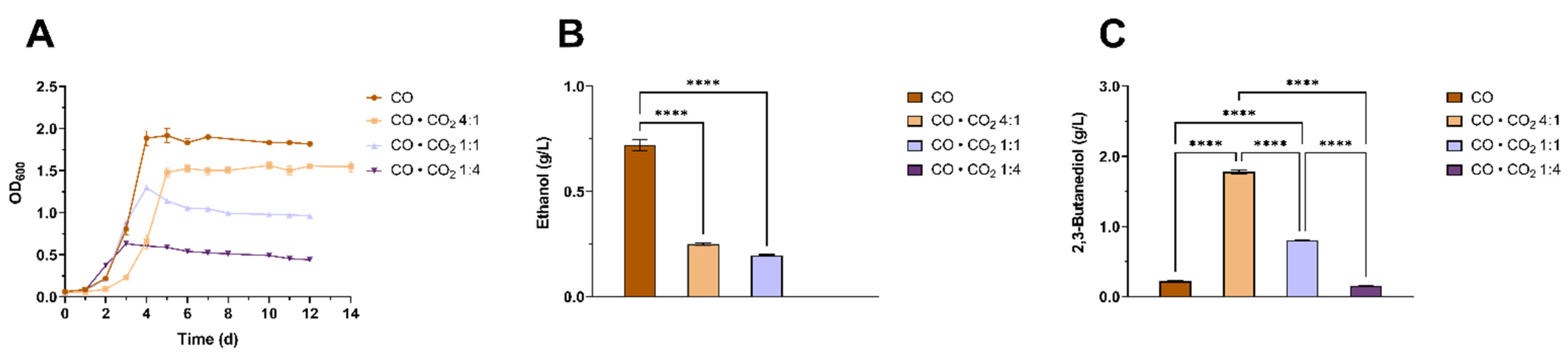
3.4. Characterization of the Effects of Different CO to CO2 Ratios on 2,3-BDO Production by Clostridium ljungdahlii
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liew, F.M.; Martin, M.E.; Tappel, R.C.; Heijstra, B.D.; Mihalcea, C.; Köpke, M. Gas fermentation-A flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front. Microbiol. 2016, 7, 694. [Google Scholar] [CrossRef]
- Köpke, M.; Simpson, S. Pollution to products: Recycling of ‘above ground’ carbon by gas fermentation. Curr. Opin. Biotechnol. 2020, 65, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Takors, R.; Kopf, M.; Mampel, J.; Bluemke, W.; Blombach, B.; Eikmanns, B.; Bengelsdorf, F.R.; Weuster-Botz, D.; Dürre, P. Using gas mixtures of CO, CO2 and H2 as microbial substrates: The do’s and don’ts of successful technology transfer from laboratory to production scale. Microb. Biotechnol. 2018, 11, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Song, Y.; Lee, H.; Shin, J.; Jin, S.; Kang, S.; Cho, B.K. Valorization of C1 gases to value-added chemicals using acetogenic biocatalysts. Chem. Eng. J. 2022, 428, 131325. [Google Scholar] [CrossRef]
- European Commission. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 14 October 2021).
- Infantes, A.; Kugel, M.; Raffelt, K.; Neumann, A. Side-by-side comparison of clean and as substrate for acetogenic fermentation with Clostridium ljungdahlii. Fermentation 2020, 6, 84. [Google Scholar] [CrossRef]
- Zhu, H.F.; Liu, Z.Y.; Zhou, X.; Yi, J.H.; Lun, Z.M.; Wang, S.N.; Tang, W.Z.; Li, F.L. Energy conservation and carbon flux distribution during fermentation of CO or H2/CO2 by Clostridium ljungdahlii. Front. Microbiol. 2020, 11, 416. [Google Scholar] [CrossRef]
- Hermann, M.; Teleki, A.; Weitz, S.; Niess, A.; Freund, A.; Bengelsdorf, F.R.; Takors, R. Electron availability in CO2, CO and H2 mixtures constrains flux distribution, energy management and product formation in Clostridium ljungdahlii. Microb. Biotechnol. 2020, 13, 1831–1846. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Jia, D.C.; Zhang, K.D.; Zhu, H.F.; Zhang, Q.; Jiang, W.H.; Gu, Y.; Li, F.L. Ethanol metabolism dynamics in Clostridium ljungdahlii grown on carbon monoxide. Appl. Environ. Microbiol. 2020, 86, 1–14. [Google Scholar] [CrossRef]
- Katsyv, A.; Müller, V. Overcoming energetic barriers in acetogenic C1 conversion. Front. Bioeng. Biotechnol. 2020, 8, 621166. [Google Scholar] [CrossRef]
- Mock, J.; Zheng, Y.; Mueller, A.P.; Ly, S.; Tran, L.; Segovia, S.; Nagaraju, S.; Köpke, M.; Dürre, P.; Thauer, R.K. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation. J. Bacteriol. 2015, 197, 2965–2980. [Google Scholar] [CrossRef]
- Heffernan, J.K.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Casini, I.; Plan, M.; Tappel, R.; Simpson, S.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-valorization using clostridium autoethanogenum for sustainable fuel and chemicals production. Front. Bioeng. Biotechnol. 2020, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Valgepea, K.; de Souza Pinto Lemgruber, R.; Meaghan, K.; Palfreyman, R.W.; Abdalla, T.; Heijstra, B.D.; Behrendorff, J.B.; Marcellin, E. Maintenance of ATP homeostasis triggers metabolic shifts in gas-fermenting acetogens. Cell Syst. 2017, 4, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, E.; Behrendorff, J.B.; Nagaraju, S.; DeTissera, S.; Segovia, S.; Palfreyman, R.W.; Daniell, J.; Licona-Cassani, C.; Quek, L.; Speight, R.; et al. Low carbon fuels and commodity chemicals from waste gases-systematic approach to understand energy metabolism in a model acetogen. Green Chem. 2016, 18, 3020–3028. [Google Scholar] [CrossRef]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and product formation of Clostridium ljungdahlii in presence of cyanide. Front. Microbiol. 2018, 9, 1213. [Google Scholar] [CrossRef]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on syngas fermentation with Clostridium carboxidivorans in stirred-tank reactors with defined gas impurities. Front. Microbiol. 2021, 12, 655390. [Google Scholar] [CrossRef]
- Stoll, I.K.; Boukis, N.; Sauer, J. Syngas fermentation to alcohols: Reactor technology and application perspective. Chem. Ing. Tech. 2020, 92, 125–136. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Straub, M.; Dürre, P. Bacterial synthesis gas (syngas) fermentation. Environ. Technol. 2013, 34, 1639–1651. [Google Scholar] [CrossRef]
- ArcelorMittal. Available online: https://belgium.arcelormittal.com/en/arcelormittal-and-lanzatech-break-ground-on-e150million-project-to-.revolutionise-blast-furnace-carbon-emissions-capture/ (accessed on 14 October 2021).
- Lanzatech 2020. Available online: https://www.lanzatech.com/2020/09/15/advanced-biofuel-facility-gets-green-light-in-india/ (accessed on 14 October 2021).
- Sekisui Chemical 2020. Available online: https://www.sekisuichemical.com/news/2020/1364066_38530.html (accessed on 14 October 2021).
- Simpson, S.; Collet, C.; Mihalcea, C.D.; Conolly, J.J.; Waters, G.W. Fermentation Process for Controlling Butanediol Production. U.S. Patent No. US 2012/0252082 A1, 4 October 2012. [Google Scholar]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryn, B. New compounds for production of polyurethane foams. J. Appl. Polym. Sci. 2006, 102, 5918–5926. [Google Scholar] [CrossRef]
- Köpke, M.; Mihalcea, C.; Liew, F.M.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S. 2,3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Poehlein, A.; Linder, S.; Erz, C.; Hummel, T.; Hoffmeister, S.; Daniel, R.; Dürre, P. Industrial acetogenic biocatalysts: A comparative metabolic and genomic analysis. Front. Microbiol. 2016, 7, 1036. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridium ljungdahlii Sp-Nov, an acetogenic species in clostridial ribosomal-RNA homology group-I. Int. J. Syst. Bacteriol. 1993, 43, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Köpke, M.; Held, C.; Hujer, S.; Liesegang, H.; Wiezer, A.; Wollherr, A.; Ehrenreich, A.; Liebl, W.; Gottschalk, G.; Durre, P. Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc. Natl. Acad. Sci. USA 2010, 107, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Abrini, J.; Naveau, H.; Nyns, E.J. Clostridium autoethanogenum, Sp. Nov., an anaerobic bacterium that produces ethanol from carbon monoxide. Arch. Microbiol. 1994, 161, 345–351. [Google Scholar] [CrossRef]
- Brown, S.D.; Nagaraju, S.; Utturkar, S.; De Tissera, S.; Segovia, S.; Mitchell, W.; Land, M.L.; Dassanayake, A.; Köpke, M. Comparison of single-molecule sequencing and hybrid approaches for finishing the genome of clostridium autoethanogenum and analysis of CRISPR systems in industrial relevant clostridia. Biotechnol. Biofuels 2014, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Henstra, A.M.; Winzer, K.; Köpke, M.; Simpson, S.D.; Minton, N.P. Insights into CO2 fixation pathway of Clostridium autoethanogenum by targeted mutagenesis. MBio 2016, 7, e00427-16. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liang, C.; Yuan, Z.; Xu, J.; Hua, Q.; Guo, Y. A study of CO/Syngas bioconversion by Clostridium autoethanogenum with a flexible gas-cultivation system. Enzyme Microb. Technol. 2017, 101, 24–29. [Google Scholar] [CrossRef]
- Liew, F.; Henstra, A.M.; Köpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Valgepea, K.; De Souza Pinto Lemgruber, R.; Abdalla, T.; Binos, S.; Takemori, N.; Takemori, A.; Tanaka, Y.; Marcellin, E. H2 drives metabolic rearrangements in gas-fermenting Clostridium autoethanogenum. Biotechnol. Biofuels 2018, 11, 55. [Google Scholar] [CrossRef]
- Jack, J.; Lo, J.; Maness, P.-C.; Ren, Z.J. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas. Biomass Bioenerg. 2019, 124, 95–101. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.W.; Chae, C.G.; Kwon, S.J.; Kim, Y.J.; Lee, J.H.; Lee, H.S. Domestication of the novel alcohologenic acetogen Clostridium sp. AWRP: From isolation to characterization for syngas fermentation. Biotechnol. Biofuels 2019, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Richter, H.; Saha, S.; Angenent, L.T. Traits of selected clostridium strains for syngas fermentation to ethanol. Biotechnol. Bioeng. 2016, 113, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.L.; Chinn, M.S.; Grunden, A.M. Influence of process parameters on growth of Clostridium ljungdahlii and Clostridium autoethanogenum on synthesis gas. Enzyme Microb. Technol. 2009, 44, 281–288. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid. Bioresour. Technol. 2015, 186, 122–127. [Google Scholar] [CrossRef]
- Gao, J.; Atiyeh, H.K.; Phillips, J.R.; Wilkins, M.R.; Huhnke, R.L. Development of low cost medium for ethanol production from syngas by Clostridium ragsdalei. Bioresour. Technol. 2013, 147, 508–515. [Google Scholar] [CrossRef]
- Tanner, R.S. Cultivation of bacteria and fungi. In Manual of Environmental Microbiology; Hurst, C.J., Crawford, R.L., Garland, J.L., Lipson, D.A., Mills, A.L., Stetzenbach, L.D., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 69–78. [Google Scholar]
- Infantes, A.; Kugel, M.; Neumann, A. evaluation of media components and process parameters in a sensitive and robust fed-batch syngas fermentation system with Clostridium ljungdahlii. Fermentation 2020, 6, 61. [Google Scholar] [CrossRef]
- Yasin, M.; Jeong, Y.; Park, S.; Jeong, J.; Lee, E.Y.; Lovitt, R.W.; Kim, B.H.; Lee, J.; Chang, I.S. Microbial synthesis gas utilization and ways to resolve kinetic and mass-transfer limitations. Bioresour. Technol. 2015, 177, 361–374. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Coelho, F.; Ferreira, T.F.; Amaral, P.F.F. A new strategy for acetogenic bacteria cell growth and metabolites production using syngas in lab scale. IOSR J. Biotechnol. Biochem. 2017, 3, 27–30. [Google Scholar] [CrossRef]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef]
- Norman, R.; Millat, T.; Schatschneider, S.; Henstra, A.M.; Breitkopf, R.; Pander, B.; Annan, F.; Piatek, P.; Hartman, H.B.; Hodgman, C. A genome-scale model of Clostridium autoethanogenum reveals optimal bioprocess conditions for high-value commodity chemical production from carbon monoxide. Eng. Biol. 2019, 3, 32–40. [Google Scholar] [CrossRef]
- Bertsch, J.; Müller, V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria. Biotechnol. Biofuels 2015, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Stoll, K.I.; Herbig, S.; Zwick, M.; Boukis, N.; Sauer, J.; Neumann, A.; Oswald, F. Fermentation of H2 and CO2 with Clostridium ljungdahlii at elevated process pressure—first experimental results. Chem. Eng. Trans. 2018, 64, 151–156. [Google Scholar] [CrossRef]
- Simpson, S.D.; Köpke, M.; Smart, K.F.; Tran, L.P.; Sechrist, P. A System and a Method for Controlling Metabolite Production in a Microbial Fermentation. World Intellectual Property. Organization Patent No. WO 2014/151158 A1, 25 September 2014. [Google Scholar]
- Saxena, J.; Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei. J. Ind. Microbiol. Biotechnol. 2011, 38, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Martin, M.E.; Angenent, L.T. A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies 2013, 6, 3987–4000. [Google Scholar] [CrossRef]
- Park, S.; Ahn, B.; Kim, Y.K. Growth enhancement of bioethanol-producing microbe Clostridium autoethanogenum by changing culture medium composition. Bioresour. Technol. Rep. 2019, 6, 237–240. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Bender, G.; Pierce, E.; Hill, J.A.; Darty, J.E.; Ragsdale, S.W. Metal centers in the anaerobic microbial metabolism of CO and CO2. Metallomics 2011, 3, 797–815. [Google Scholar] [CrossRef][Green Version]
- Smart, K.F.; Ly, B.S. Fermentation Process for the Production and Control of Pyruvate-Derived Products. U.S. Patent No. US 9,701,987 B2, 11 July 2017. [Google Scholar]
- Ragsdale, S.W. Catalysis of methyl group transfers involving tetrahydrofolate and B12. Vitam. Horm. 2008, 79, 293–324. [Google Scholar] [CrossRef]
- Korkhin, Y.; Kalb (Gilboa), A.J.; Peretz, M.; Bogin, O.; Burstein, Y.; Frolow, F. NADP-dependent bacterial alcohol dehydrogenases: Crystal structure, cofactor-binding and cofactor specificity of the ADHs of Clostridium Beijerinckii and Thermoanaerobacter Brockii. J. Mol. Biol. 1998, 278, 967–981. [Google Scholar] [CrossRef]
- Charubin, K.; Papoutsakis, E.T. Direct cell-to-cell exchange of matter in a synthetic Clostridium syntrophy enables CO2 fixation, superior metabolite yields, and an expanded metabolic space. Metab. Eng. 2019, 52, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Enzymology of the wood-ljungdahl pathway of acetogenesis. Ann. N. Y. Acad. Sci. 2008, 1125, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Drennan, C.L.; Doukov, T.I.; Ragsdale, S.W. The metalloclusters of carbon monoxide Dehydrogenase/Acetyl-CoA Synthase: A Story in Pictures. J. Biol. Inorg. Chem. 2004, 9, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Lee, J.; Lee, H.S. Enhanced alcohol production from synthesis gas using a CO-resistant mutant of Clostridium sp. AWRP. Microbiol. Biotechnol. Lett. 2019, 47, 581–584. [Google Scholar] [CrossRef]
- Scopes, R.K. An Iron-activated alcohol Dehydrogenase. FEBS Lett. 1983, 156, 303–306. [Google Scholar] [CrossRef]
- Simpson, S.; Tran, P.L.; Mihalcea, C.D.; Fung, J.M.Y.; Liew, F.M. Production of Butanediol by Anaerobic Microbial Fermentation. World Intellectual Property. Organization Patent No. WO/2009/151342, 17 December 2009. [Google Scholar]
- Simpson, S.; Fleming, S.E.; Havill, A.M.; Trevethick, S.R. Process for Producing Chemicals Using Microbial Fermentation of Substrates Comprising Carbon Monoxide. U.S. Patent No. US 2012/0045807 A1, 23 February 2012. [Google Scholar]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Impact of cyclic pH shifts on carbon monoxide fermentation to ethanol by Clostridium autoethanogenum. Fuel 2016, 178, 56–62. [Google Scholar] [CrossRef]


| Medium Composition (Per Liter) | |||||
| Tanner_Mod | Valgepea_Mod | Tan_Val | Tan_Val + Fe | ||
| MES | 10 | 10 | 10 | 10 | g |
| yeast extract | 0.5 | / | / | / | g |
| resazurin (1 g/L) | 1 | 1 | 1 | 1 | mL |
| cysteine-HCl*H2O | 1 | 1 | 1 | 1 | g |
| Mineral solution | 25 | / | 25 | 25 | mL |
| Trace element solution | 10 | 10 | 10 | 10 | mL |
| Vitamin solution | 10 | 10 | 10 | 10 | mL |
| NaCl | * | 0.2 | * | * | g |
| NH4Cl | * | 2.5 | * | * | g |
| KCl | * | 0.5 | * | * | g |
| NaH2PO4*H2O | * | 2.3439 | * | * | g |
| MgCl2*6H2O | * | 0.5 | * | * | g |
| CaCl2*2H2O | * | 0.1324 | * | * | g |
| FeCl3*6H2O | * | 0.017 | * | 0.017 | g |
| Mineral Solution (Per Liter) | |||||
| Tanner_Mod | Valgepea_Mod | Tan_Val | Tan_Val + Fe | ||
| NaCl | 80 | ** | 80 | 80 | g |
| NH4Cl | 100 | ** | 100 | 100 | g |
| KCl | 10 | ** | 10 | 10 | g |
| KH2PO4 | 10 | ** | 10 | 10 | g |
| MgSO4*7H2O | 20 | ** | 20 | 20 | g |
| CaCl2*2H2O | 4 | ** | 4 | 4 | g |
| Trace Element Solution (Per Liter) | |||||
| Tanner_Mod | Valgepea_Mod | Tan_Val | Tan_Val + Fe | ||
| Nitrilotriacetic acid | 2 | 1.5 | 1.5 | 1.5 | g |
| MnSO4*H2O | 1 | 0.5 | 0.5 | 0.5 | g |
| Fe(SO4)2(NH4)2*6H2O | 0.8 | / | / | / | g |
| FeSO4*7H2O | / | 0.667 | 0.667 | 0.667 | g |
| CoCl2*6H2O | 0.2 | 0.2 | 0.2 | 0.2 | g |
| ZnSO4*7H2O | 0.001 | 0.2 | 0.2 | 0.2 | g |
| CuCl2*2H2O | 0.02 | 0.02 | 0.02 | 0.02 | g |
| NiCl2*6H2O | 0.02 | 0.02 | 0.02 | 0.02 | g |
| NaMoO4*2H2O | 0.02 | 0.03 | 0.03 | 0.03 | g |
| Na2SeO3*5H2O | 0.02 | / | / | / | g |
| Na2SeO3 | / | 0.02 | 0.02 | 0.02 | g |
| Na2WO4*2H2O | 0.02 | 0.02 | 0.02 | 0.02 | g |
| H3BO3 | / | 0.3 | 0.3 | 0.3 | g |
| MgSO4*7H2O | / | 3 | 3 | 3 | g |
| NaCl | / | 1 | 1 | 1 | g |
| AlK(S2O8)*12H2O | / | 0.0199 | 0.0199 | 0.0199 | g |
| Vitamin Solution (Per Liter) | |||||
| Tanner_Mod | Valgepea_Mod | Tan_Val | Tan_Val + Fe | ||
| pyridoxine-HCl | 10 | 10 | 10 | 10 | mg |
| thiamine-HCl*2H2O | 5 | 50 | 50 | 50 | mg |
| riboflavine | 5 | 50 | 50 | 50 | mg |
| calcium pantothenate | 5 | 50 | 50 | 50 | mg |
| thioctic acid | 5 | 50 | 50 | 50 | mg |
| 4-aminobenzoic acid | 5 | 50 | 50 | 50 | mg |
| nicotinic acid | 5 | 50 | 50 | 50 | mg |
| vitamin B12 | 0.1 | 50 | 50 | 50 | mg |
| biotin | 2 | 20 | 20 | 20 | mg |
| folic acid | 2 | 20 | 20 | 20 | mg |
| Mineral Solution (mM) | ||||
| Tanner_Mod | Valgepea_Mod | Tan_Val | Tan_Val + Fe | |
| NaCl | 34.22 | 3.59 | 34.39 | 34.39 |
| NH4Cl | 46.74 | 46.74 | 46.74 | 46.74 |
| KCl | 3.35 | 6.71 | 3.35 | 3.35 |
| PO4 | 1.84 | 16.99 | 1.84 | 1.84 |
| Mg | 2.03 | 2.58 | 2.15 | 2.15 |
| Ca | 0.68 | 0.90 | 0.68 | 0.68 |
| Trace Element (Metal) Solution (µM) | ||||
| Tanner_Mod | Valgepea_Mod | Tan_Val | Tan_Val + Fe | |
| Mn | 59.17 | 29.58 | 29.58 | 29.58 |
| Fe | 20.40 | 86.89 | 23.99 | 86.89 |
| Co | 8.41 | 8.41 | 8.41 | 8.41 |
| Zn | 0.03 | 6.96 | 6.96 | 6.96 |
| Cu | 1.17 | 1.17 | 1.17 | 1.17 |
| Ni | 0.84 | 0.84 | 0.84 | 0.84 |
| Mo | 0.83 | 1.24 | 1.24 | 1.24 |
| Se | 0.76 | 1.16 | 1.16 | 1.16 |
| W | 0.61 | 0.61 | 0.61 | 0.61 |
| B | / | 48.52 | 48.52 | 48.52 |
| Al | / | 0.84 | 0.84 | 0.84 |
| Vitamin Solution (µM) | ||||
| Tanner_Mod | Valgepea_Mod | Tan_Val | Tan_Val + Fe | |
| pyridoxine | 486.29 | 486.29 | 486.29 | 486.29 |
| thiamine | 148.24 | 1482.36 | 1482.36 | 1482.36 |
| riboflavine | 132.85 | 1328.52 | 1328.52 | 1328.52 |
| pantothenate | 104.93 | 1049.32 | 1049.32 | 1049.32 |
| thioctic acid | 242.33 | 2423.30 | 2423.30 | 2423.30 |
| 4-aminobenzoic acid | 364.59 | 3645.91 | 3645.91 | 3645.91 |
| nicotinic acid | 406.14 | 4061.41 | 4061.41 | 4061.41 |
| vitamin B12 | 0.74 | 368.63 | 368.63 | 368.63 |
| biotin | 81.86 | 818.63 | 818.63 | 818.63 |
| folic acid | 45.31 | 453.10 | 453.10 | 453.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, L.; Agostino, V.; Fino, D.; Re, A. Screening of Gas Substrate and Medium Effects on 2,3-Butanediol Production with C. ljungdahlii and C. autoethanogenum Aided by Improved Autotrophic Cultivation Technique. Fermentation 2021, 7, 264. https://doi.org/10.3390/fermentation7040264
Ricci L, Agostino V, Fino D, Re A. Screening of Gas Substrate and Medium Effects on 2,3-Butanediol Production with C. ljungdahlii and C. autoethanogenum Aided by Improved Autotrophic Cultivation Technique. Fermentation. 2021; 7(4):264. https://doi.org/10.3390/fermentation7040264
Chicago/Turabian StyleRicci, Luca, Valeria Agostino, Debora Fino, and Angela Re. 2021. "Screening of Gas Substrate and Medium Effects on 2,3-Butanediol Production with C. ljungdahlii and C. autoethanogenum Aided by Improved Autotrophic Cultivation Technique" Fermentation 7, no. 4: 264. https://doi.org/10.3390/fermentation7040264
APA StyleRicci, L., Agostino, V., Fino, D., & Re, A. (2021). Screening of Gas Substrate and Medium Effects on 2,3-Butanediol Production with C. ljungdahlii and C. autoethanogenum Aided by Improved Autotrophic Cultivation Technique. Fermentation, 7(4), 264. https://doi.org/10.3390/fermentation7040264







