Characterization of Antioxidant Peptides from Thai Traditional Semi-Dried Fermented Catfish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pla Duk Ra Sample
2.3. Peptide Extraction, Deproteinization, and Fractionation
2.4. Amino Acid Composition
2.5. Peptide Identification Using LC-MS/MS
2.6. Antioxidant Activities
2.7. Effects of Heating Temperature, pH, and In Vitro Digestion on DPPH● Scavenging Activity of Selected Peptide Fraction
2.8. Statistical Analysis
3. Results and Discussion
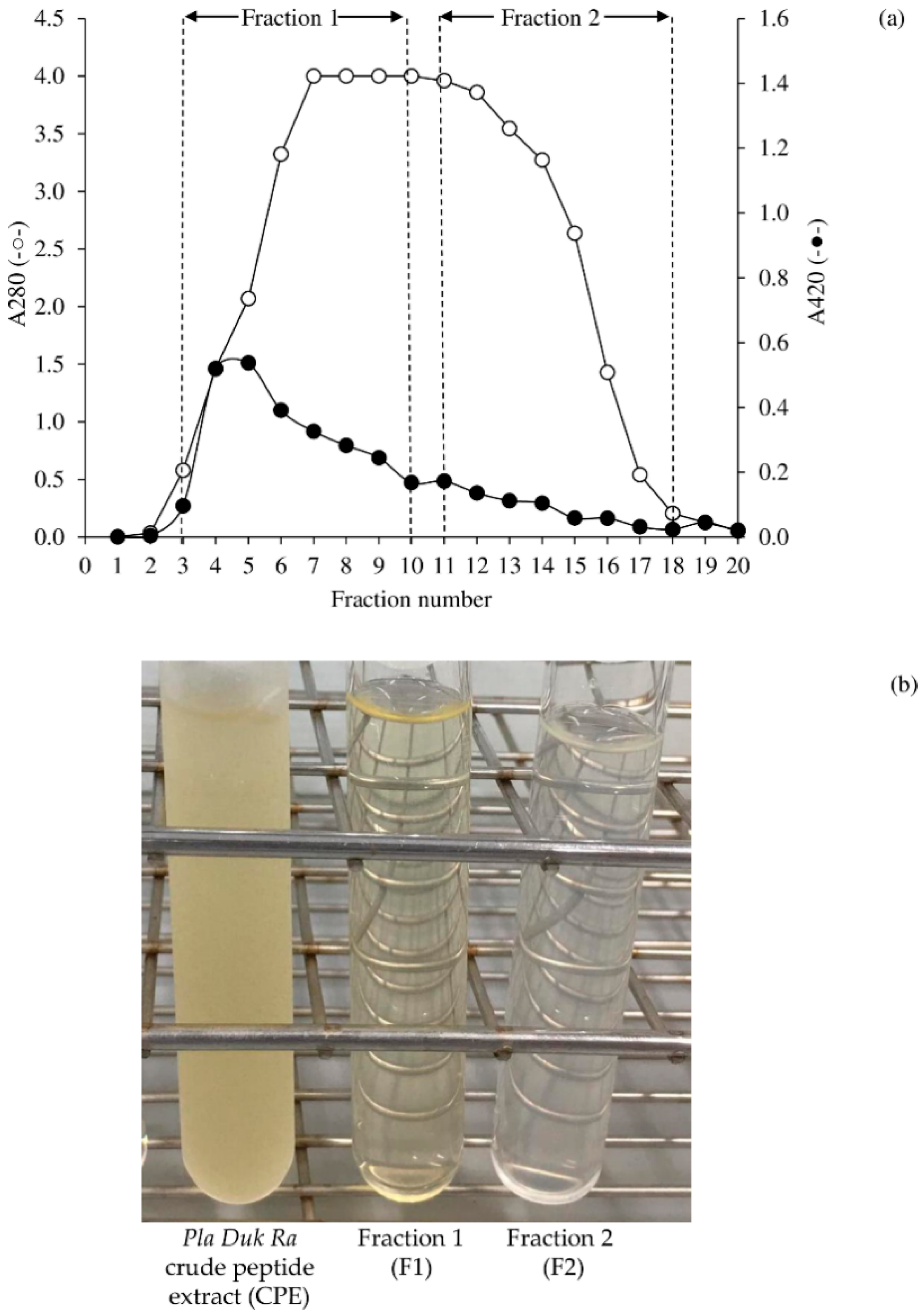
3.1. Peptide Fractionation Using Hitrap Sephadex-G25 Columns
3.2. Amino Acid Composition
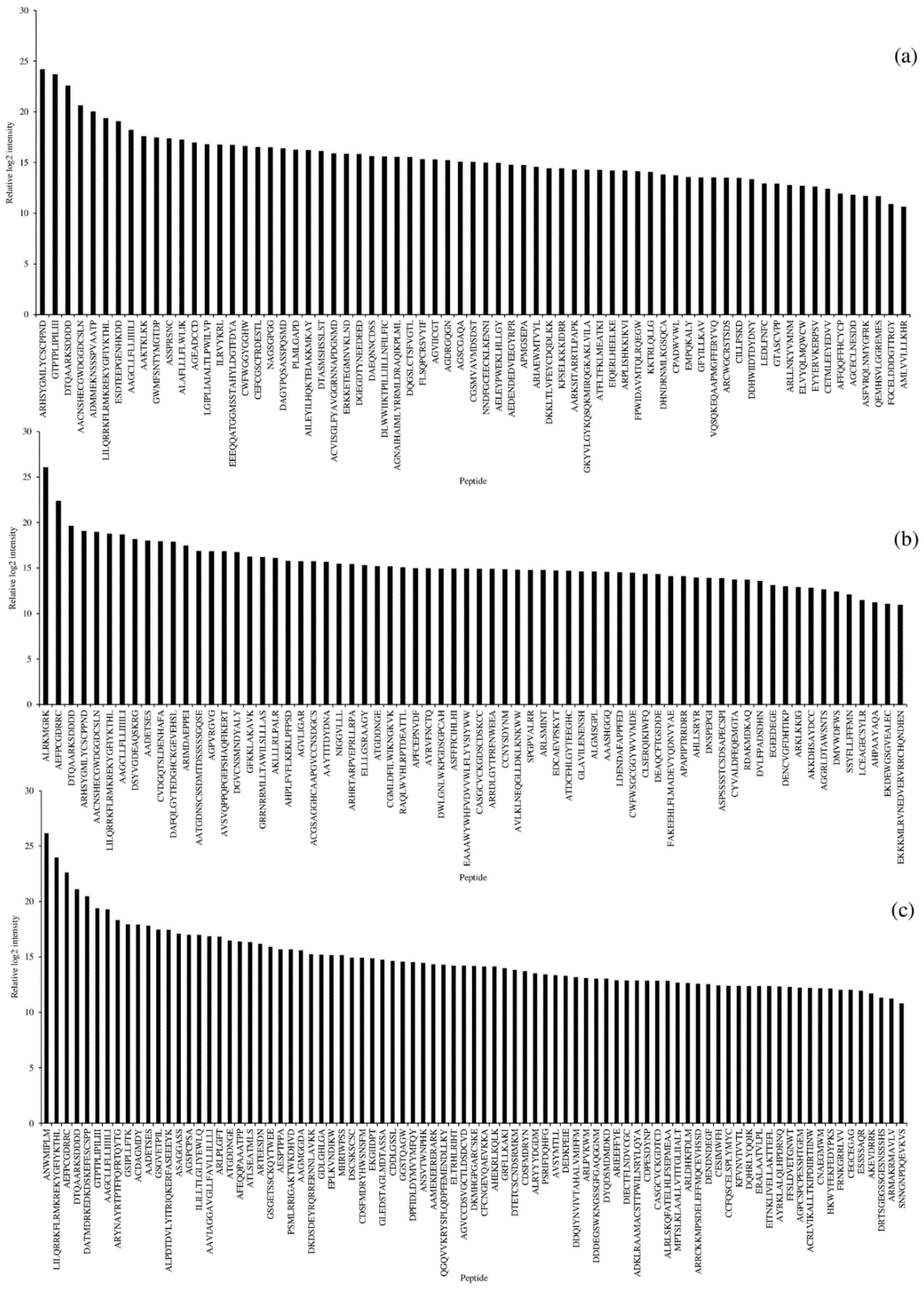
3.3. Peptide Profiles
3.4. Antiradical Scavenging Activities
3.5. Inhibitory Activity against ROS (OH●, H2O2, and 1O2)
3.6. Metal Chelating Activity
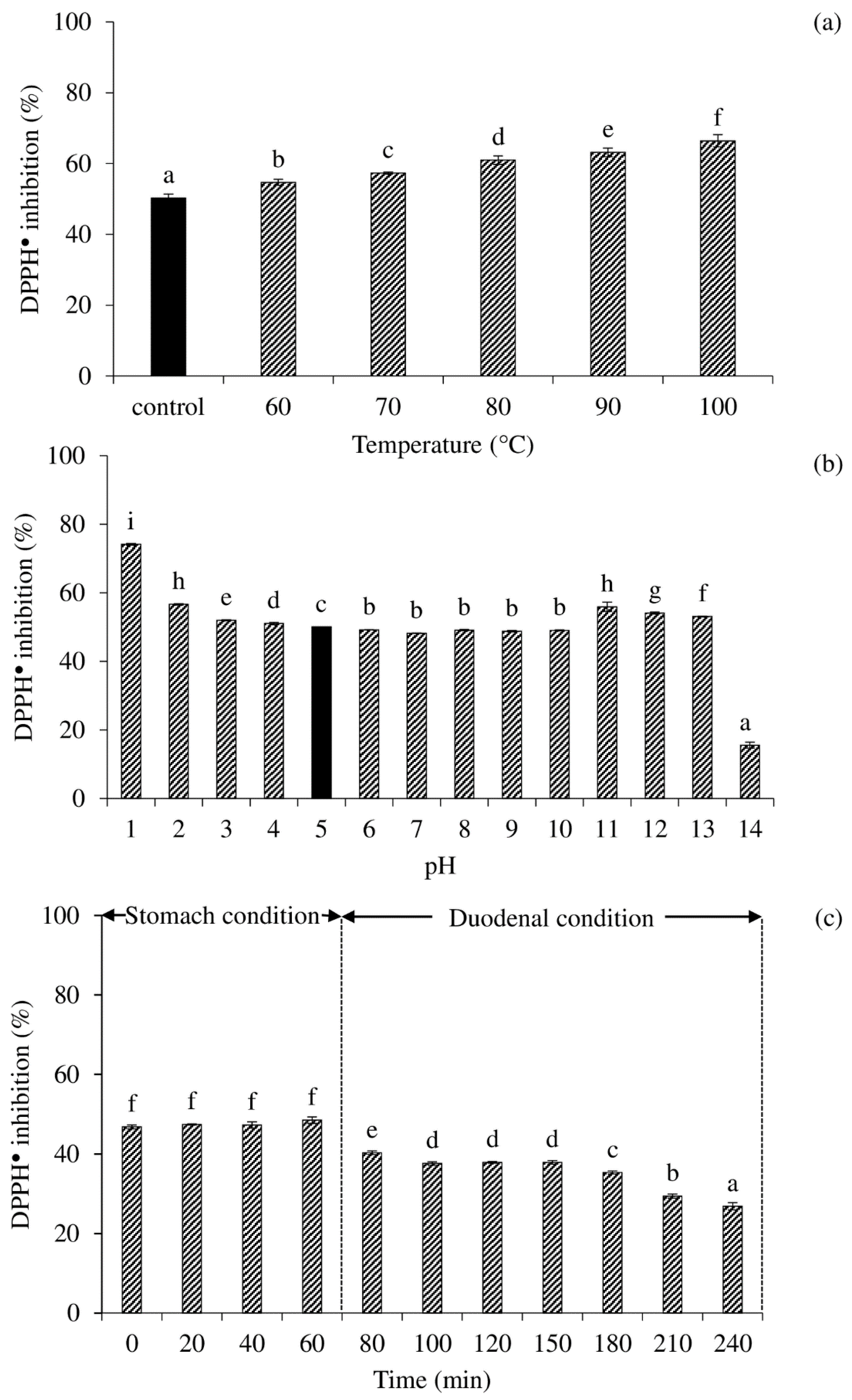
3.7. Effects of Heating Temperature, pH, and In Vitro Digestion on DPPH● Scavenging Activity of Selected Peptide Fraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pongsetkul, J.; Benjakul, S. Development of modified atmosphere packaging (MAP) on shelf-life extension of pla-duk-ra (dried fermented catfish) stored at room temperature. Food Control 2021, 124, 107882. [Google Scholar] [CrossRef]
- Phetsang, H.; Panpipat, W.; Panya, A.; Phonsatta, N.; Chaijan, M. Occurrence and development of off-odor compounds in farmed hybrid catfish (Clarias macrocephalus × Clarias gariepinus) muscle during refrigerated storage: Chemical and volatilomic analysis. Foods 2021, 10, 1841. [Google Scholar] [CrossRef] [PubMed]
- Thai Community Product Standard. Thai Community Product Standard No. TCPS 1029/2018; Thai Industrial Standards Institute: Bangkok, Thailand, 2018. [Google Scholar]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.M. Quality, functionality, and microbiology of fermented fish: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1228–1242. [Google Scholar] [CrossRef]
- Choksawangkarn, W.; Phiphattananukoon, S.; Jaresitthikunchai, J.; Roytrakul, S. Antioxidative peptides from fish sauce by-product: Isolation and characterization. Agric. Nat. Resour. 2018, 52, 460–466. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [CrossRef] [Green Version]
- Adessi, C.; Soto, C. Converting a peptide into a drug: Strategies to improve stability and bioavailability. Curr. Med. Chem. 2002, 9, 963–978. [Google Scholar] [CrossRef]
- Xiao, C.; Toldrá, F.; Zhou, F.; Gallego, M.; Zhao, M.; Mora, L. Effect of cooking and in vitro digestion on the peptide profile of chicken breast muscle and antioxidant and alcohol dehydrogenase stabilization activity. Food Res. Int. 2020, 136, 109459. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Chitsomboon, B.; Roytrakul, S.; Yongsawadigul, J. Isolation and identification of antioxidative peptides from hydrolysate of threadfin bream surimi processing byproduct. J. Funct. Foods 2013, 5, 1654–1664. [Google Scholar] [CrossRef]
- Yin, L.-J.; Tong, Y.-L.; Jiang, S.-T. Improvement of the Functionality of Minced Mackerel by Hydrolysis and Subsequent Lactic Acid Bacterial Fermentation. J. Food Sci. 2006, 70, M172–M178. [Google Scholar] [CrossRef]
- Khositanon, P.; Panya, N.; Roytrakul, S.; Krobthong, S.; Chanroj, S.; Choksawangkarn, W. Effects of fermentation periods on antioxidant and angiotensin I-converting enzyme inhibitory activities of peptides from fish sauce by-products. LWT 2020, 135, 110122. [Google Scholar] [CrossRef]
- Hamzeh, A.; Noisa, P.; Yongsawatdigul, J. Characterization of the antioxidant and ACE-inhibitory activities of Thai fish sauce at different stages of fermentation. J. Funct. Foods 2019, 64, 103699. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Zhou, A.; Miao, J.; Liu, J.; Benjakul, S. A novel antioxidant peptide purified from defatted round scad (Decapterus maruadsi) protein hydrolysate extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103907. [Google Scholar] [CrossRef]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, Z.-R.; Luo, H.-Y. Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Farvin, K.S.; Andersen, L.L.; Otte, J.; Nielsen, H.H.; Jessen, F.; Jacobsen, C. Antioxidant activity of cod (Gadus morhua) protein hydrolysates: Fractionation and characterisation of peptide fractions. Food Chem. 2016, 204, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafian, L.; Babji, A.S. Purification and Identification of Antioxidant Peptides from Fermented Fish Sauce (Budu). J. Aquat. Food Prod. Technol. 2018, 28, 14–24. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chinarak, K.; Chaijan, M.; Panpipat, W. Farm-raised sago palm weevil (Rhynchophorus ferrugineus) larvae: Potential and challenges for promising source of nutrients. J. Food Compos. Anal. 2020, 92, 103542. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Basic composition, antioxidant activity and nanoemulsion behavior of oil from mantis shrimp (Oratosquilla nepa). Food Biosci. 2019, 31, 100448. [Google Scholar] [CrossRef]
- Benjakul, S.; Kittiphattanabawon, P.; Shahidi, F.; Maqsood, S. Antioxidant activity and inhibitory effects of lead (Leucaena leucocephala) seed extracts against lipid oxidation in model systems. Food Sci. Technol. Int. 2013, 19, 365–376. [Google Scholar] [CrossRef]
- Hamzeh, A.; Benjakul, S.; Senphan, T. Comparative study on antioxidant activity of hydrolysates from splendid squid (Loligo formosana) gelatin and protein isolate prepared using protease from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). J. Food Sci. Technol. 2016, 53, 3615–3623. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.-Z.; Zhang, W.-G.; Kang, Z.-L.; Zhou, G.-H.; Xu, X.-L. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014, 96, 783–789. [Google Scholar] [CrossRef]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. ReV. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; McClements, D.J.; Decker, E.A. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase β-lactoglobulin in oil-in-water emulsions. J. Agric. Food Chem. 2005, 53, 10248–10253. [Google Scholar] [CrossRef]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Lee, J.-H.; Samarakoon, K.; Kim, J.-S.; Jeon, Y.-J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2012, 52, 113–120. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Fractionation and identification of novel antioxidant peptides from fermented fish (pekasam). J. Food Meas. Charact. 2018, 12, 2174–2183. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, A.N.; Kim, S.-K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2004, 53, 581–587. [Google Scholar] [CrossRef]
- Langner, E.; Rzeski, W. Biological Properties of Melanoidins: A Review. Int. J. Food Prop. 2013, 17, 344–353. [Google Scholar] [CrossRef]
- Nie, X.; Xu, D.; Zhao, L.; Meng, X. Antioxidant activities of chicken bone peptide fractions and their Maillard reaction products: Effects of different molecular weight distributions. Int. J. Food Prop. 2017, 20, S457–S466. [Google Scholar] [CrossRef]
- Misra, B.R.; Misra, H.P. Vasoactive intestinal peptide, a singlet oxygen quencher. J. Biol. Chem. 1990, 265, 15371–15374. [Google Scholar] [CrossRef]
- Matheson, I.B.C.; Etheridge, R.D.; Kratowich, N.R.; Lee, J. The quenching of singlet oxygen by amino acids and proteins. Photochem. Photobiol. 1975, 21, 165–171. [Google Scholar] [CrossRef]
- Sjöberg, B.; Foley, S.; Staicu, A.; Pascu, A.; Pascu, M.; Enescu, M. Protein reactivity with singlet oxygen: Influence of the solvent exposure of the reactive amino acid residues. J. Photochem. Photobiol. B Biol. 2016, 159, 106–110. [Google Scholar] [CrossRef]
- Sharp, J.S.; Becker, J.M.; Hettich, R.L. Analysis of Protein Solvent Accessible Surfaces by Photochemical Oxidation and Mass Spectrometry. Anal. Chem. 2003, 76, 672–683. [Google Scholar] [CrossRef]
- Xu, K.; Liu, G.; Fu, C. The Tryptophan Pathway Targeting Antioxidant Capacity in the Placenta. Oxidative Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Weiss, G.; Diez-Ruiz, A.; Murr, C.; Theur, I.; Fuchs, D. Tryptophan Metabolites as Scavengers of Reactive Oxygen and Chlorine Species. Pteridines 2002, 13, 140–143. [Google Scholar] [CrossRef]
- Christen, S.; Peterhans, E.; Stocker, R. Antioxidant activities of some tryptophan metabolites: Possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. USA 1990, 87, 2506–2510. [Google Scholar] [CrossRef] [Green Version]
- Tsopmo, A.; Diehl-Jones, B.W.; Aluko, R.E.; Kitts, D.D.; Elisia, I.; Friel, J.K. Tryptophan released from mother’s milk has antioxidant properties. Pediatr. Res. 2009, 66, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.N.; Buttar, H.S. Evaluation of the antioxidant properties of tryptophan and its metabolites in in vitro assay. J. Complement. Integr. Med. 2016, 13, 129–136. [Google Scholar] [CrossRef]
- Jung, M.; Kim, S.K.; Kim, S.Y. Riboflavin-sensitized photooxidation of ascorbic acid: Kinetics and amino acid effects. Food Chem. 1995, 53, 397–403. [Google Scholar] [CrossRef]
- Ihara, H.; Kakihana, Y.; Yamakage, A.; Kai, K.; Shibata, T.; Nishida, M.; Yamada, K.-I.; Uchida, K. 2-Oxo-histidine–containing dipeptides are functional oxidation products. J. Biol. Chem. 2019, 294, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Du, R.; Liu, K.; Zhao, S.; Chen, F. Changes in antioxidant activity of peptides identified from brown rice hydrolysates under different conditions and their protective effects against AAPH-induced oxidative stress in human erythrocytes. ACS Omega 2020, 5, 12751–12759. [Google Scholar] [CrossRef]
- Mallamace, D.; Fazio, E.; Mallamace, F.; Corsaro, C. The Role of Hydrogen Bonding in the Folding/Unfolding Process of Hydrated Lysozyme: A Review of Recent NMR and FTIR Results. Int. J. Mol. Sci. 2018, 19, 3825. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Frenkel-Pinter, M.; Liotta, C.L.; Grover, M.A. The pH dependent mechanisms of non-enzymatic peptide bond cleavage reactions. Phys. Chem. Chem. Phys. 2019, 22, 107–113. [Google Scholar] [CrossRef]
- Ohashi, Y.; Onuma, R.; Naganuma, T.; Ogawa, T.; Naude, R.; Nokihara, K.; Muramoto, K. Antioxidant Properties of Tripeptides Revealed by a Comparison of Six Different Assays. Food Sci. Technol. Res. 2015, 21, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Gallego, M.; Mauri, L.; Aristoy, M.C.; Toldrá, F.; Mora, L. Antioxidant peptides profile in dry-cured ham as affected by gastrointestinal digestion. J. Funct. Foods 2020, 69, 103956. [Google Scholar] [CrossRef]
| Amino Acid (%) | Crude Extract | Fraction 1 | Fraction 2 |
|---|---|---|---|
| Alanine * | 7.68 ± 0.18 c | 7.07 ± 0.02 b | 3.03 ± 0.00 a |
| Glycine * | 5.46 ± 0.11 a | 6.22 ± 0.03 b | 7.67 ± 0.00 c |
| Valine * | 4.59 ± 0.06 c | 4.01 ± 0.04 b | 1.45 ± 0.00 a |
| Leucine * | 6.80 ± 0.20 b | 5.24 ± 0.05 a | ND |
| Isoleucine * | 3.79 ± 0.08 c | 2.83 ± 0.03 b | 0.23 ± 0.00 a |
| Proline * | 3.03 ± 0.09 a | 3.15 ± 0.03 b | 3.39 ± 0.00 c |
| Methionine * | 2.62 ± 0.04 a | 3.38 ± 0.00 b | 4.37 ± 0.00 c |
| Serine | 2.66 ± 0.09 a | 4.34 ± 0.04 b | 8.30 ± 0.00 c |
| Threonine | 2.59 ± 0.05 a | 3.43 ± 0.02 b | 5.63 ± 0.00 c |
| Phenylalanine *,■ | 2.16 ± 0.02 b | 1.81 ± 0.02 a | 1.81 ± 0.00 a |
| Aspartic acid | 5.66 ± 0.11 a | 7.66 ± 0.06 b | 13.15 ± 0.01 c |
| Hydroxyproline | 3.39 ± 0.21 a | 5.12 ± 0.09 b | 9.05 ± 0.00 c |
| Cysteine | 3.19 ± 0.10 a | 5.31 ± 0.03 b | ND |
| Glutamic acid | 14.78 ± 0.08 a | 15.36 ± 0.08 b | 16.38 ± 0.00 c |
| Lysine | 13.00 ± 0.86 b | 0.39 ± 0.29 a | ND |
| Arginine | 1.05 ± 0.02 a | 1.31 ± 0.02 b | ND |
| Histidine | 7.11 ± 0.21 a | 10.36 ± 0.07 b | ND |
| Tyrosine *,■ | 2.13 ± 0.06 a | 3.32 ± 0.02 b | 6.20 ± 0.00 c |
| Hydroxylysine | 3.04 ± 0.14 | ND | ND |
| Tryptophan *,■ | 5.25 ± 0.26 a | 9.69 ± 0.90 b | 19.30 ± 0.01 c |
| Total hydrophobic amino acid | 43.51 | 46.72 | 47.45 |
| Total aromatic amino acid | 9.54 | 14.82 | 27.31 |
| Antioxidant Activity (IC50; mg/mL) | Crude Extract | Fraction 1 | Fraction 2 |
|---|---|---|---|
| Free radical scavenging activity | |||
| DPPH radical scavenging activity | 2.21 ± 0.67 c | 0.69 ± 0.01 b | 0.23 ± 0.00 a |
| ABTS radical scavenging activity | 3.45 ± 0.78 c | 1.12 ± 0.08 b | 0.37 ± 0.06 a |
| Inhibitory activity against reactive oxygen species | |||
| Hydroxyl radical scavenging activity | 8.27 ± 0.17 c | 4.92 ± 0.52 b | 0.54 ± 0.21 a |
| Hydrogen peroxide scavenging activity | 3.35 ± 0.05 c | 2.12 ± 0.20 b | 0.35 ± 0.10 a |
| Singlet oxygen scavenging activity | 6.86 ± 0.87 c | 1.87 ± 0.07 b | 0.32 ± 0.11 a |
| Metal chelating activity | 4.74 ± 0.07 c | 2.18 ± 0.04 a | 3.21 ± 0.32 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaijan, M.; Rodsamai, T.; Charoenlappanit, S.; Roytrakul, S.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Panpipat, W. Characterization of Antioxidant Peptides from Thai Traditional Semi-Dried Fermented Catfish. Fermentation 2021, 7, 262. https://doi.org/10.3390/fermentation7040262
Chaijan M, Rodsamai T, Charoenlappanit S, Roytrakul S, Panya A, Phonsatta N, Cheong L-Z, Panpipat W. Characterization of Antioxidant Peptides from Thai Traditional Semi-Dried Fermented Catfish. Fermentation. 2021; 7(4):262. https://doi.org/10.3390/fermentation7040262
Chicago/Turabian StyleChaijan, Manat, Tanutchaporn Rodsamai, Sawanya Charoenlappanit, Sittiruk Roytrakul, Atikorn Panya, Natthaporn Phonsatta, Ling-Zhi Cheong, and Worawan Panpipat. 2021. "Characterization of Antioxidant Peptides from Thai Traditional Semi-Dried Fermented Catfish" Fermentation 7, no. 4: 262. https://doi.org/10.3390/fermentation7040262
APA StyleChaijan, M., Rodsamai, T., Charoenlappanit, S., Roytrakul, S., Panya, A., Phonsatta, N., Cheong, L.-Z., & Panpipat, W. (2021). Characterization of Antioxidant Peptides from Thai Traditional Semi-Dried Fermented Catfish. Fermentation, 7(4), 262. https://doi.org/10.3390/fermentation7040262








