Microbial Exopolysaccharides in Traditional Mexican Fermented Beverages
Abstract
1. Introduction
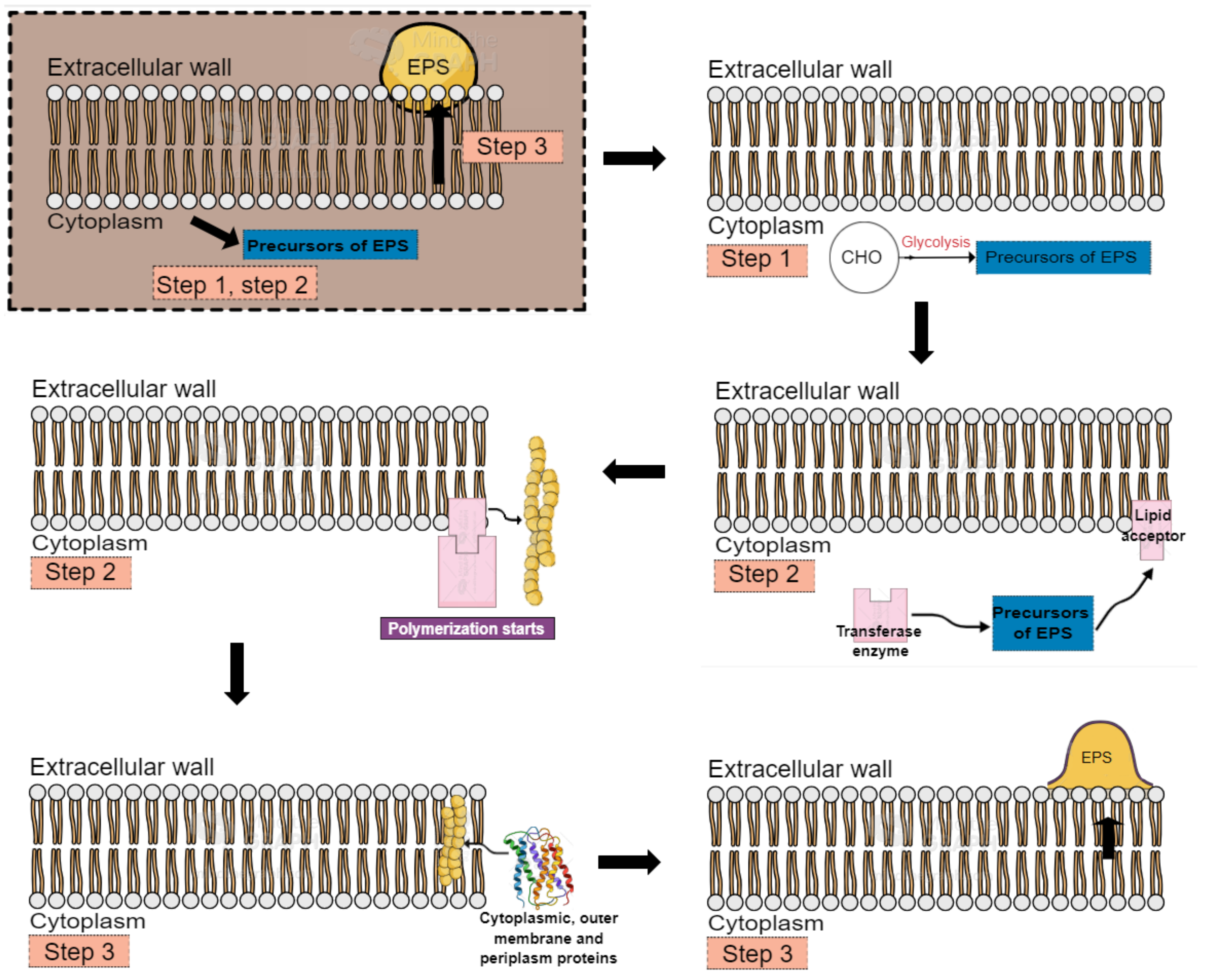
2. Exopolysaccharides
3. Microbial EPS in Traditionally Fermented Regional Beverages
3.1. Tavern
3.2. Tuba
3.3. Sotol
3.4. Aguamiel
4. Lactic Acid Bacteria and Their Influence on the Functional Qualities of Traditional Mexican Drinks
5. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rana, S.; Upadhyay, L.S. Microbial Exopolysaccharides: Synthesis Pathways, Types and Their Commercial Applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.G.; Regenstein, J.M.; Seesuriyachan, P. Microbial Exopolysaccharides for Immune Enhancement: Fermentation, Modifications and Bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Garcia, C.; Fessard, A.; Barba, F.; Munekata, P.; Lorenzo, J.; Remize, F. Nutritional and Microbiological Quality of Tiger Nut Tubers (Cyperus esculentus), Derived Plant-Based and Lactic Fermented Beverages. Fermentation 2018, 5, 3. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of Lactic Acid Bacteria for the Biopreservation of Meat Products: A Systematic Review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Joshi, S.R.; Koijam, K. Exopolysaccharide Production by a Lactic Acid Bacteria, Leuconostoc Lactis Isolated from Ethnically Fermented Beverage. Natl. Acad. Sci. Lett. 2014, 37, 59–64. [Google Scholar] [CrossRef]
- İspirli, H.; Demirbaş, F.; Dertli, E. Glucan Type Exopolysaccharide (EPS) Shows Prebiotic Effect and Reduces Syneresis in Chocolate Pudding. J. Food Sci. Technol. 2018, 55, 3821–3826. [Google Scholar] [CrossRef]
- Welman, A.D. Exopolysaccharides from Fermented Dairy Products and Health Promotion. Adv. Fermented Foods Beverages 2015, 23–38. [Google Scholar]
- García, C.; Rendueles, M.; Díaz, M. Liquid-Phase Food Fermentations with Microbial Consortia Involving Lactic Acid Bacteria: A Review. Food Res. Int. 2019, 119, 207–220. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Lynch, K.M.; Arendt, E.K.; Coffey, A. Isolation, Characterisation and Exploitation of Lactic Acid Bacteria Capable of Efficient Conversion of Sugars to Mannitol. Int. J. Food Microbiol. 2020, 321, 108546. [Google Scholar] [CrossRef]
- Escobar-Ramírez, M.C.; Jaimez-Ordaz, J.; Escorza-Iglesias, V.A.; Rodríguez-Serrano, G.M.; Contreras-López, E.; Ramírez-Godínez, J.; Castañeda-Ovando, A.; Morales-Estrada, A.I.; Felix-Reyes, N.; González-Olivares, L.G. Lactobacillus Pentosus Abheau-05: An in Vitro Digestion Resistant Lactic Acid Bacterium Isolated from a Traditional Fermented Mexican Beverage. Rev. Argent. Microbiol. 2020, 52, 305–314. [Google Scholar] [CrossRef]
- Gerwig, G.J. Structural Analysis of Exopolysaccharides from Lactic Acid Bacteria. Lact. Acid Bact. 2018, 67–84. [Google Scholar]
- Ispirli, H.; Dertli, E. Isolation and Characterisation of Lactic Acid Bacteria from Traditional KOUMISS and Kurut. Int. J. Food Prop. 2017, 20 (Suppl. S3), S2441–S2449. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Sun, L.; Sadiq, F.A.; Yang, Y.; Gao, J.; Sang, Y. Preparation Screening, Production Optimization and Characterization of Exopolysaccharides Produced by Lactobacillus Sanfranciscensis LS-1001 Isolated from Chinese Traditional Sourdough. Int. J. Biol. Macromol. 2019, 139, 1295–1303. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rather, I.A.; Majumder, R.; Shukla, S.; Aeron, A.; Kim, K.; Kang, S.C.; Dubey, R.C.; Maheshwari, D.K.; Lim, J.; et al. Exopolysaccharide and Lactic Acid Bacteria: Perception, Functionality and Prospects. Bangladesh J. Pharmacol. 2015, 11, 1. [Google Scholar] [CrossRef]
- Hooshdar, P.; Khosravi-Darani, K.; Ghadam, P.; Kermanshahi, R.K. A Review on Production of Exopolysaccharide and Biofilm in Probiotics like Lactobacilli and Methods of Analysis. Biointerface Res. Appl. Chem. 2020, 10, 6058–6075. [Google Scholar]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical Review of EPS Production, Synthesis and Composition for Sludge Flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from Lactic Acid Bacteria: Techno-Functional Application in the Food Industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria: Structures, Physiochemical Functions and Applications in the Food Industry. Food Hydrocoll. 2019, 94, 475–499. [Google Scholar] [CrossRef]
- Coutiño, B.; Flores, A.C.; Vela-Gutiérrez, G.; Sepúlveda, L.; Aguilar, C.N.; Chavez-Gonzalez, M.; Rodríguez, R. Tavern or Coyol Wine: A Beverage from Palm Sap with Biotechnological Potential. Biotechnol. Prog. Beverage Consum. 2020, 233–252. [Google Scholar]
- Coutiño, B.; Rodríguez, R.; Belmares, R.; Aguilar, C.N.; Ruelas, X. Selección de la bebida “Taberna” obtenida de la palma Acrocomia aculeata y análisis químico proximal. Multiciencias 2015, 15, 397–409. [Google Scholar]
- Santiago-Urbina, J.A.; Verdugo-Valdez, A.G.; Ruiz-Terán, F. Physicochemical and Microbiological Changes during Tapping of Palm Sap to Produce an Alcoholic Beverage Called “Taberna”, Which Is Produced in the South East of Mexico. Food Control 2013, 33, 58–62. [Google Scholar] [CrossRef]
- Alcántara-Hernández, R.J.; Rodríguez-Álvarez, J.A.; Valenzuela-Encinas, C.; Gutiérrez-Miceli, F.A.; Castañón-González, H.; Marsch, R.; Ayora-Talavera, T.; Dendooven, L. The Bacterial Community in ‘Taberna’ a Traditional Beverage of Southern Mexico. Lett. Appl. Microbiol. 2010, 51, 558–563. [Google Scholar] [CrossRef]
- Song, J.-O.; Kim, T.-J.; Kim, Y.-H. Inhibitory Effect on Rotavirus by Exopolysaccharides Extracted from Kefir. Korean J. Food Sci. Anim. Resour. 2007, 27, 538–542. [Google Scholar] [CrossRef][Green Version]
- Giovani, G.; Rosi, I.; Bertuccioli, M. Quantification and Characterization of Cell Wall Polysaccharides Released by Non-Saccharomyces Yeast Strains during Alcoholic Fermentation. Int. J. Food Microbiol. 2012, 160, 113–118. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, Partial Characterization and Biological Activity of Exopolysaccharides Produced from Lactobacillus Fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional Fermented Beverages in Mexico: Biotechnological, Nutritional, and Functional Approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef]
- Flores-Gallegos, A.C.; Vázquez-Vuelvas, O.F.; López-López, L.L.; Sainz-Galindo, A.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Rodriguez-Herrera, R. Tuba: A Fermented and Refreshing Beverage from Coconut Palm Sap. Non-Alcohol. Beverages 2019, 163–184. [Google Scholar]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic Potential and Bioactivity Spectrum of an Exopolysaccharide from a Probiotic Isolate Lactobacillus Paracasei M7. Bioact. Carbohydr. Diet. Fibre 2019, 19, 100191. [Google Scholar] [CrossRef]
- La China, S.; Zanichelli, G.; De Vero, L.; Gullo, M. Oxidative Fermentations and Exopolysaccharides Production by Acetic Acid Bacteria: A Mini Review. Biotechnol. Lett. 2018, 40, 1289–1302. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Q.; Liang, L.; Zhang, L.; Ping, Z.; Hu, B.; Ma, N. Research on Sugarcane Juice Fermentation by Ganoderma Lucidum and Assay of Antioxidant Activity of Exopolysaccharide. J. Food Process. Preserv. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Ionela Istrati, D.; Mihaela Pricop, E.; Georgiana Profir, A.; Vizireanu, C. Fermented Functional Beverages. In Functional Foods; IntechOpen: London, UK, 2019. [Google Scholar]
- Flores-Gallegos, A.C.; Cruz-Requena, M.; Castillo-Reyes, F.; Rutiaga-Quiñones, O.M.; Torre, L.S.; Paredes-Ortíz, A.; Soto, O.N.; Rodriguez-Herrera, R. Sotol, an Alcoholic Beverage with Rising Importance in the Worldwide Commerce. In Alcoholic Beverages; Woodhead Publishing: Sawston, UK, 2019; pp. 141–160. [Google Scholar]
- Atputharajah, J.D.; Widanapathirana, S.; Samarajeewa, U. Microbiology and Biochemistry of Natural Fermentation of Coconut Palm Sap. Food Microbiol. 1986, 3, 273–280. [Google Scholar] [CrossRef]
- Kalaiyarasi, K.; Sangeetha, K.; Rajarajan, S. A comparative study on the microbial flora of the fresh sap from cut inflorescence and fermented sap (toddy) of Borrassus flabellifer Linn (Palmyrah tree) and of Cocos nucifera Linn (Coconut tree) to identify the microbial fermenters. Int. J. Res. Pure Appl. Microbiol. 2013, 3, 43–47. [Google Scholar]
- Dos Santos, L.F.; De Melo, F.B.C.; Paiva, W.M.; Borsato, D.; Da Silva, M.C.C.; Celligoi, M.P.C. Characterization and optimization of levan production by Bacillus subtilis NATTO. Rom. Biotechnol. Lett. 2013, 18, 8413–8422. [Google Scholar]
- Ma’unatin, A.; Harijono, H.; Zubaidah, E.; Rifa’i, M. The isolation of exopolysaccharide-producing lactic acid bacteria from lontar (Borassus flabellifer L.) sap. Iran. J. Microbiol. 2020, 12, 437–444. [Google Scholar] [CrossRef]
- Abo Saif, F.A.A.; Sakr, E.A.E. Characterization and Bioactivities of Exopolysaccharide Produced from Probiotic Lactobacillus Plantarum 47fe and Lactobacillus Pentosus 68fe. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100231. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and Bioactivities of the Exopolysaccharide from a Probiotic Strain of Lactobacillus Plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Min, W.-H.; Fang, X.-B.; Wu, T.; Fang, L.; Liu, C.-L.; Wang, J. Characterization and Antioxidant Activity of an Acidic Exopolysaccharide from Lactobacillus Plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Cao, C.; Zhu, X.Y.; Wang, C.; Wu, R.; Wu, J. Extraction and Biological Activity of Exopolysaccharide Produced by Leuconostoc Mesenteroides Sn-8. Int. J. Biol. Macromol. 2020, 157, 36–44. [Google Scholar] [CrossRef]
- Fang, L.; Catchmark, J.M. Characterization of Cellulose and Other Exopolysaccharides Produced from Gluconacetobacter Strains. Carbohydr. Polym. 2015, 115, 663–669. [Google Scholar] [CrossRef]
- Ramírez-Guzmán, K.N.; Torres-León, C.; Martinez-Medina, G.A.; de la Rosa, O.; Hernández-Almanza, A.; Alvarez-Perez, O.B.; Araujo, R.; González, L.R.; Londoño, L.; Ventura, J.; et al. Traditional Fermented Beverages in Mexico. In Fermented Beverages; Woodhead Publishing: Sawston, UK, 2019; pp. 605–635. [Google Scholar]
- Jansson, P.-E.; Lindberg, J.; Wimalasiri, K.M.S.; Dankert, M.A. Structural Studies of Acetan, an Exopolysaccharide Elaborated by Acetobacter Xylinum. Carbohydr. Res. 1993, 245, 303–310. [Google Scholar] [CrossRef]
- Fang, L.; Catchmark, J.M. Characterization of Exopolysaccharides from Certain Strains of Gluconacetobacter Xylinus. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, Louisville, KY, USA, 7–10 August 2011; p. 1111152. [Google Scholar]
- Villarreal-Morales, S.L.; Muñiz-Márquez, D.B.; Michel-Michel, M.; González-Montemayor, Á.-M.; Escobedo-García, S.; Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Aguamiel a Fresh Beverage from Agave Spp. SAP with Functional Properties. In Nature Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 179–208. [Google Scholar]
- Cervantes-Elizarrarás, A.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Vega-Sánchez, V.; Velázquez-Guadarrama, N.; Zafra-Rojas, Q.; Piloni-Martini, J. In Vitro Probiotic Potential of Lactic Acid Bacteria Isolated from Aguamiel and Pulque and Antibacterial Activity against Pathogens. Appl. Sci. 2019, 9, 601. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Pourcelly, G.; Doco, T.; Williams, P.; Dornier, M.; Belleville, M.-P. Analysis of the Main Components of the Aguamiel Produced by the Maguey-Pulquero (Agave mapisaga) throughout the Harvest Period. J. Agric. Food Chem. 2008, 56, 3682–3687. [Google Scholar] [CrossRef]
- Escobedo-García, S.; Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Contreras-Esquivel, J.C.; González-Montemayor, Á.M.; López, M.G.; Rodríguez-Herrera, R. Functionality of Agave Bagasse as Supplement for the Development of Prebiotics-Enriched Foods. Plant Foods Hum. Nutr. 2019, 75, 96–102. [Google Scholar] [CrossRef]
- Huang, M.L.; Huang, J.Y.; Kao, C.Y.; Fang, T.J. Fermented Soymilk and Soy and Cow Milk Mixture, Supplemented with Orange Peel Fiber Ortremella Flavafermented Powder as Prebiotics for High Exopolysaccharide-Producinglactobacillus Pentosus SLC 13. J. Sci. Food Agric. 2019, 99, 4373–4382. [Google Scholar] [CrossRef]
- Torres-Rodríguez, I.; Rodríguez-Alegría, M.E.; Miranda-Molina, A.; Giles-Gómez, M.; Conca Morales, R.; López-Munguía, A.; Bolívar, F.; Escalante, A. Screening and Characterization of Extracellular Polysaccharides Produced by Leuconostoc kimchii Isolated from Traditional Fermented Pulque Beverage. SpringerPlus 2014, 3, 583. [Google Scholar] [CrossRef]
- Castro-Rodríguez, D.; Hernández-Sánchez, H.; Yáñez Fernández, J. Structural Characterization and Rheological Properties of Dextran Produced by Native Strains Isolated of Agave Salmiana. Food Hydrocoll. 2019, 90, 1–8. [Google Scholar]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR Spectroscopic Analysis of Exopolysaccharides Produced by Leuconostoc citreum and Weissella confusa. Carbohydr. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef]
- Parolis, L.A.S.; Parolis, H.; Kenne, L.; Meldal, M.; Bock, K. The Extracellular Polysaccharide of Pichia (Hansenula) Holstii NRRL Y-2448: The Phosphorylated Side Chains. Carbohydr. Res. 1998, 309, 77–87. [Google Scholar] [CrossRef]
- Li, H.; Huang, L.; Zhang, Y.; Yan, Y. Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula Mucilaginosa YL-1 Isolated from Sea Salt Field. Mar. Drugs 2020, 18, 595. [Google Scholar] [CrossRef]
- Ernandes, F.M.; Cruz, C.H. Uso De Caldo De Cana-De-Açúcar Para Produção De Levana Por Zymomonas Mobilis CCT4494. Ciênc. Agrotecnol. 2011, 35, 354–360. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented Beverages with Health-Promoting Potential: Past and Future Perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Hittinger, C.T.; Steele, J.L.; Ryder, D.S. Diverse Yeasts for Diverse Fermented Beverages and Foods. Curr. Opin. Biotechnol. 2018, 49, 199–206. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Banerjee, U.C. Traditional Bio-Preservation in Beverages: Fermented Beverages. Preserv. Preserv. Approaches Beverages 2019, 69–113. [Google Scholar]
- Ordóñez, J.L.; Troncoso, A.M.; García-Parrilla, M.D.; Callejón, R.M. Recent Trends in the Determination of Biogenic Amines in Fermented Beverages—A Review. Anal. Chim. Acta 2016, 939, 10–25. [Google Scholar] [CrossRef]
- Astudillo-Melgar, F.; Ochoa-Leyva, A.; Utrilla, J.; Huerta-Beristain, G. Bacterial Diversity and Population Dynamics during the Fermentation of Palm Wine from Guerrero Mexico. Front. Microbiol. 2019, 10, 531. [Google Scholar] [CrossRef]
- Leathers, T.D.; Côté, G.L. Biofilm Formation by Exopolysaccharide Mutants of Leuconostoc mesenteroides Strain NRRL B-1355. Appl. Microbiol. Biotechnol. 2008, 78, 1025–1031. [Google Scholar] [CrossRef]
- Semjonovs, P.; Zikmanis, P. Evaluation of Novel Lactose-Positive and Exopolysaccharide-Producing Strain of Pediococcus pentosaceus for Fermented Foods. Eur. Food Res. Technol. 2007, 227, 851–856. [Google Scholar] [CrossRef]
- Soumiya, S.; Santhiagu, A.; Manjusha Chemmattu, M. Optimization of Cultural Conditions of Gellan Gum Production from Recombinant Sphingomonas paucimobilis ATCC 31461 and Its Characterization. J. Appl. Biol. Biotechnol. 2021, 9, 58–67. [Google Scholar]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, Purification, and Characterization of Exopolysaccharide Produced by Leuconostoc Citreum N21 from Dried Milk Cake. Trans. Tianjin Univ. 2018, 25, 161–168. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, H.C. Isolation and characterization of exopolysaccharide producing lactic acid bacteria from kimchi. Korean J. Microbiol. Biotechnol. 2006, 34, 196–203. [Google Scholar]
- Oh, Y.J.; Jung, D.S. Evaluation of Probiotic Properties of Lactobacillus and Pediococcus Strains Isolated from Omegisool, a Traditionally Fermented Millet Alcoholic Beverage in Korea. LWT-Food Sci. Technol. 2015, 63, 437–444. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Du, R.; Guo, S.; Ping, W.; Ling, H.; Ge, J. Purification and Characterization of an Exopolysaccharide from Leuconostoc Lactis L2. Int. J. Biol. Macromol. 2019, 139, 1224–1231. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.; Zhu, Y.; Yang, Y.; Wang, C. Purification and Characterization of an Exopolysaccharide Produced by Lactobacillus Plantarum Hy Isolated from Home-Made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide Producing Lactic Acid Bacteria: Their Techno-Functional Role and Potential Application in Gluten-Free Bread Products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, B.; Prasad Uday, U.S.; Oinam, G.; Mondal, A.; Bandyopadhyay, T.K.; Tiwari, O.N. Characterization, Genetic Regulation and Production of Cyanobacterial Exopolysaccharides and Its Applicability for Heavy Metal Removal. Carbohydr. Polym. 2018, 179, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Park, H.-S.; Uhm, T.-B. Production of Fermented Apple Juice Using Lactobacillus Plantarum JBE245 Isolated from Korean Traditional Meju. Korean J. Food Sci. Technol. 2016, 48, 445–453. [Google Scholar] [CrossRef]
- Bancalari, E.; Castellone, V.; Bottari, B.; Gatti, M. Wild Lactobacillus Casei Group Strains: Potentiality to Ferment Plant Derived Juices. Foods 2020, 9, 314. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An Overview of Plant-Autochthonous Microorganisms and Fermented Vegetable Foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Mitra, S.; Ghosh, B.C. Quality Characteristics of Kefir as a Carrier for Probiotic Lactobacillus Rhamnosus GG. Int. J. Dairy Technol. 2019, 73, 384–391. [Google Scholar] [CrossRef]
- Mituniewicz–Małek, A.; Zielińska, D.; Ziarno, M. Probiotic Monocultures in Fermented Goat Milk Beverages—Sensory Quality of Final Product. Int. J. Dairy Technol. 2019, 72, 240–247. [Google Scholar] [CrossRef]
- Grom, L.C.; Coutinho, N.M.; Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Freitas, M.Q.; Duarte, M.C.; Pimentel, T.C.; Esmerino, E.A.; et al. Probiotic Dairy Foods and Postprandial Glycemia: A Mini-Review. Trends Food Sci. Technol. 2020, 101, 165–171. [Google Scholar] [CrossRef]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, Formulations, Advanced Delivery and Health Benefits of Probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Elizaquível, P.; Carrasco, P.; Espinosa, J.; Reyes, D.; Wacher, C.; Aznar, R. Diversity and Dynamics of Lactic Acid Bacteria in Atole Agrio, a Traditional Maize-Based Fermented Beverage from South-Eastern Mexico, Analysed by High Throughput Sequencing and Culturing. Antonie Leeuwenhoek 2017, 111, 385–399. [Google Scholar] [CrossRef]
- Chiou, T.-Y.; Suda, W.; Oshima, K.; Hattori, M.; Matsuzaki, C.; Yamamoto, K.; Takahashi, T. Lactobacillus Kosoi Sp. Nov., a Fructophilic Species Isolated from Kôso, a Japanese Sugar-Vegetable Fermented Beverage. Antonie Leeuwenhoek 2018, 111, 1149–1156. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Dahunsi, S.O.; Olayanju, A. Synergistic Microbial Interactions between Lactic Acid Bacteria and Yeasts during Production of Nigerian Indigenous Fermented Foods and Beverages. Food Control 2020, 110, 106963. [Google Scholar] [CrossRef]
- Ripari, V. Techno-Functional Role of Exopolysaccharides in Cereal-Based, Yogurt-like Beverages. Beverages 2019, 5, 16. [Google Scholar] [CrossRef]
- Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C. Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-like Beverages. Foods 2018, 7, 51. [Google Scholar] [CrossRef]
- Llamas-Arriba, M.G.; Hernández-Alcántara, A.M.; Yépez, A.; Aznar, R.; Dueñas, M.T.; López, P. Functional and Nutritious Beverages Produced by Lactic Acid Bacteria. Nutr. Beverages 2019, 419–465. [Google Scholar]

| Acetobacter | Saccharomyces | Other Microorganisms |
|---|---|---|
| A. tropicalis | S. cerevisiae | Cyberlindnera jadinii |
| A. estunensis | S. chevalieri | Kodamaea ohmeri |
| A. aceti | S. bayanus | Meyerozyma guilliermondii |
| A. lovaniensis | S. uvarum | Millerozyma farinosa |
| Saccharomycodes ludwigii | ||
| Pichia | Candida | Wickerhamiella paraugosa |
| P. membranifaciens | C. tropicalis | Zygosaccharomyces bailii |
| P. kudriavzevii | C. parapsilosis |
| Fermented Beverage | Microbiota | EPS | Beneficial Effects | References |
|---|---|---|---|---|
| Tavern | Acetobacter tropicalis, A. estunensis, A. aceti and, A. lovaniensis; Arthroascus spp; Candida tropicalis and C. parapsilosis; Cyberlindnera jadinii; Pichia kudriavzevii;Wickerhamiella paraugosa; Fructilactobacillus fructosus *; Gluconobacter oxydans, and Gluconacetobacter saccharivorans; Hanseniaspora uctum; Limosilactobacillus fermentum *; Liquorilactobacillus sucicola *; Leuconostoc mesenteroides *; Kloeckera; Millerozyma farinosa; Pichia membranifaciens, Kodamaea ohmeri * and, Meyerozyma guilliermondii; Saccharomyces cerevisiae, S. bayanus, S. uvarum, and S. chevalieri; Saccharomycodes ludwigii *, Zygosaccharomyces bailii, Schizosaccharomyces pombe *; Zymomonas mobilis * | Levan Dextran HOP (Mw 400 kDa, composed of repeating units of d-glucose) HEP (Mw < 2 kDa) HEPs (Mw 44,500 and 2820 kDa, composed of glucose, galactose, mannose and arabinose) HEP (Mw 200 kDa with the presence of monomers such as glucan and mannose) EPS with high mannose contents (93%) HEP (composed of galactose, mannose and glucose) | Antioxidant activity, improves gastrointestinal transit tolerance, antibiofilm activity against pathogens, great emulsification effect, could scavenge superoxide anion free radicals, and inhibitory effect on rotavirus | [20,23,24,25,26,27,37,41,56,57,58,59,60,61] |
| Tuba | Acetobacter *; Acidomonas; Bacillus *; Candida glabrata; Meyerozyma caribbica; Enterobacteriaceae; Fructilactobacillus fructosus *, Fructobacillus durionis; Gluconacetobacter; Gloconobacter *; Kloechera apiculata; Kluyveromyces marxianus; Lactiplantibacillus plantarum *; Leuconostoc mesenteroides *; Micrococcus; Pediococcus *; Pichia anghophorae; Saccharomyces chevalieri, S. cerevisiae; Saccharomycodes ludwigii; Schizosaccharomyces pombe; Sphingomonas *; Vibrio; Lachancea fermentati | Acetan Dextran Levan (Mw of 568 kDa and <50 kDa) Celullose Glucan (Mw 94 kDa) Gellan (HEP containing glucose, rhamnose, and glucuronic acid) HEP (Mw 66 kDa, consisting of xylose, glucose, and galactose) HEP (Mw 12.4 kDa consisting of arabinose, rhamnose, fucose, xylose, mannose, fructose, galactose, and glucose) HEP (Mw 2000 kDa composed of glucose and fructose) HEP (Mw 20 kDa with the presence of monomers such as glucan and mannose) | Antibacterial, anticoagulant, fibrinolytic activity, thermophile, great emulsification effect, and it could scavenge superoxide anion free radicals | [28,36,37,38,39,40,41,44,61,62,63,64,65] |
| Sotol | Acetobacter sp *; Bacillus licheniformis, B. subtilis *; Bradysia difformis; Candida glabrata; Prototheca sp; Saccharomyces cerevisiae | Acetan Celullose Levan (Mw of 568 kDa and <50 kDa) | [33,36,43,44,65] | |
| Aguamiel | Acetobacter malorum, Amycolatopsis orientalis; Bacillus *; Candida; Clavispora; Diplobacter; Hansenula; Kluyveromyces marxianus var. bulgaricus; Lactiplantibacillus plantarum *, Lacticaseibacillus casei *; Leuconostoc, L. citreum *, L. mesenteroides *; Companilactobacillus kimchii *; Mycoderma; Pichia *; Pseudomonas; Rhodotorula; Saccharomyces, S. cerevisiae; Sarcina; Streptococcus; Zymomonas mobilis. | Dextran (Mw 607 kDa composed of D-glucopyranose) Levan (Mw of 568 kDa and <50 kDa) Glucan (Mw 94 kDa) EPS (Mw 66 kDa consisting of xylose, glucose, and galactose) EPS (Mw 12.4 kDa consisting of arabinose, rhamnose, fucose, xylose, mannose, fructose, galactose, and glucose) EPS (Mw 306 kDa, containing only one monosaccharide, glucose) HEP (Mw 20 kDa with the presence of monomers such as glucan and mannose) EPS (composed of a phosphomannan core) HEP (Mw 1200 kDa composed of four neural monosaccharides: glucose, mannose, galactose, and fucose) HEP consists of glucose (75%) and rhamnose (15%) | Antibacterial anticoagulant, fibrinolytic activity, prebiotic source, thermophile, great emulsification effect, it could scavenge superoxide anion free radicals and immunomodulatory activity | [24,36,38,39,40,46,47,51,53,55,56,62,65,66] |
| Genus | Specie | Reported Exopolysaccharide | Reference |
|---|---|---|---|
| Acetobacter | A. xylinum G. xylinus | Acetan Cellulose, with large size when galactose served as a sole carbon source. | [44,45] |
| Bacillus | B. subtilis | Levan with two Mw of 568.000 Da and <50.000 Da | [36] |
| Fructilactobacillus | F. fructosus | It has been reported as EPS producer but has not been identified yet. | [37] |
| Leuconostoc | L. mesenteroides | Dextran by L. mesenteroides Heteropolisaccharide. Presence of monomers such as glucan and mannose. The Mw value is 2.0 × 105 Da, by L. mesenteroides. | [42,62] |
| L. citreum | Dextran. Contain about 11% α-(1-2) and about 3.5% α-(1-3)-linked branches. Glucooligosaccharides with α-(1-2) linked branches. | [53] | |
| Dextran with an Mw 6.07 × 106. (composed of D-glucopyranose units in a linear chain with consecutive α(1-6) linkages. No branching was found in the structure). | [65] | ||
| Companilactobacillus | C. kimchii | This polysaccharide contained only one monosaccharide, glucose, with an Mw of 306,606 Da | [67] |
| Dextran and Levan. Polymers produced by the soluble and cell-associated fractions are dextrans consisting of a linear backbone of linked α-(1-6) Glcp in the main chain with α-(1-2) and α-(1-3)linked branches. Polymer produced by the soluble fraction was identified as a class 1 dextran with a linear backbone containing consecutive α-(1-6) linked D-glucopyranosyl units with few α-(1-3)-linked branches, whereas the cell-associated EPS is a polymer mixture consisting of a levan composed of linear chains of (2-6) linked β-D-fructofuranosyl residues with β-(2-6) connections, and a class 1 dextran. | [51] | ||
| Limosilactobacillus | L. fermentum | Produces three different types of EPSs; the first homopolysaccharide (HOP) of an Mw of 400 kDa and two different low molecular weight heteropolysaccharides of less than 2 kDa. HOP is composed of repeating units of D-glucose linked by an α-1,4-glycosidic bond, where 20% of the glucose subunits is acetylated at C-3 | [26] |
| L. fermentum also produces EPS composed of glucose, galactose, mannose and arabinose with Mw of 4.45 × 106 and 2.82 × 106 Da. | [26] | ||
| Lacticaseibacillus | L. caseii | It produces a neutral heteropolysaccharide consisting of glucose (about 75%) and rhamnose (about 15%). | [40] |
| L. sucicola | Dextran from sucrose | [20] | |
| Lactiplantibacillus | L. plantarum | Glucan of 94 kDa, which possesses antibacterial, anticoagulant and fibrinolytic activity. EPS consisting of xylose, glucose, and galactose with an Mw 6.61 × 104 | [38,39] |
| EPS consists of arabinose, rhamnose, fucose, xylose, mannose, fructose, galactose, and glucose with an Mw of 12.4 kDa | [40] | ||
| Pediococcus | P. pentosaceus | Produce a complex of extracellular polysaccharides with an Mw of about 2000 kDa composed of glucose and fructose. | [63] |
| Kodamaea | K. ohmeri | EPS have not been identified. | [24] |
| P. holstii | The EPS produced is composed of a phosphomannan core to which oligosaccharide diester phosphate side chains are appended. | [53] | |
| Rhodotorula | R. mucilaginosa | This EPS comprises four neural monosaccharides: glucose, mannose, galactose, and fucose, with an average Mw of 1200 KDa. This polysaccharide is the first reported with immunomodulatory activity that is marine-derived. | [55] |
| Saccharomycodes | S. ludwigii | Exocellular polysaccharides produced by it revealed to essentially be mannoproteins with high mannose contents, ranging from 93% | [25] |
| Schizosaccharomyces | S. pombe | Have been reported as a non-identified EPS with the presence of galactose, mannose, and glucose | [25] |
| Sphingomonas | S. paucimobilis | Gellan. It is a heteropolymer containing glucose, rhamnose, and glucuronic acid This EPS produced by this strain has a high potential to be utilized in the pharmaceutical and food industry | [64] |
| Zymomonas | Z. mobilis | Levan | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cázares-Vásquez, M.L.; Rodríguez-Herrera, R.; Aguilar-González, C.N.; Sáenz-Galindo, A.; Solanilla-Duque, J.F.; Contreras-Esquivel, J.C.; Flores-Gallegos, A.C. Microbial Exopolysaccharides in Traditional Mexican Fermented Beverages. Fermentation 2021, 7, 249. https://doi.org/10.3390/fermentation7040249
Cázares-Vásquez ML, Rodríguez-Herrera R, Aguilar-González CN, Sáenz-Galindo A, Solanilla-Duque JF, Contreras-Esquivel JC, Flores-Gallegos AC. Microbial Exopolysaccharides in Traditional Mexican Fermented Beverages. Fermentation. 2021; 7(4):249. https://doi.org/10.3390/fermentation7040249
Chicago/Turabian StyleCázares-Vásquez, Martha L., Raúl Rodríguez-Herrera, Cristóbal N. Aguilar-González, Aidé Sáenz-Galindo, José Fernando Solanilla-Duque, Juan Carlos Contreras-Esquivel, and Adriana C. Flores-Gallegos. 2021. "Microbial Exopolysaccharides in Traditional Mexican Fermented Beverages" Fermentation 7, no. 4: 249. https://doi.org/10.3390/fermentation7040249
APA StyleCázares-Vásquez, M. L., Rodríguez-Herrera, R., Aguilar-González, C. N., Sáenz-Galindo, A., Solanilla-Duque, J. F., Contreras-Esquivel, J. C., & Flores-Gallegos, A. C. (2021). Microbial Exopolysaccharides in Traditional Mexican Fermented Beverages. Fermentation, 7(4), 249. https://doi.org/10.3390/fermentation7040249