The Effect of Microbial Transglutaminase on the Viscosity and Protein Network of Kefir Made from Cow, Goat, or Donkey Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Kefir Production Procedure
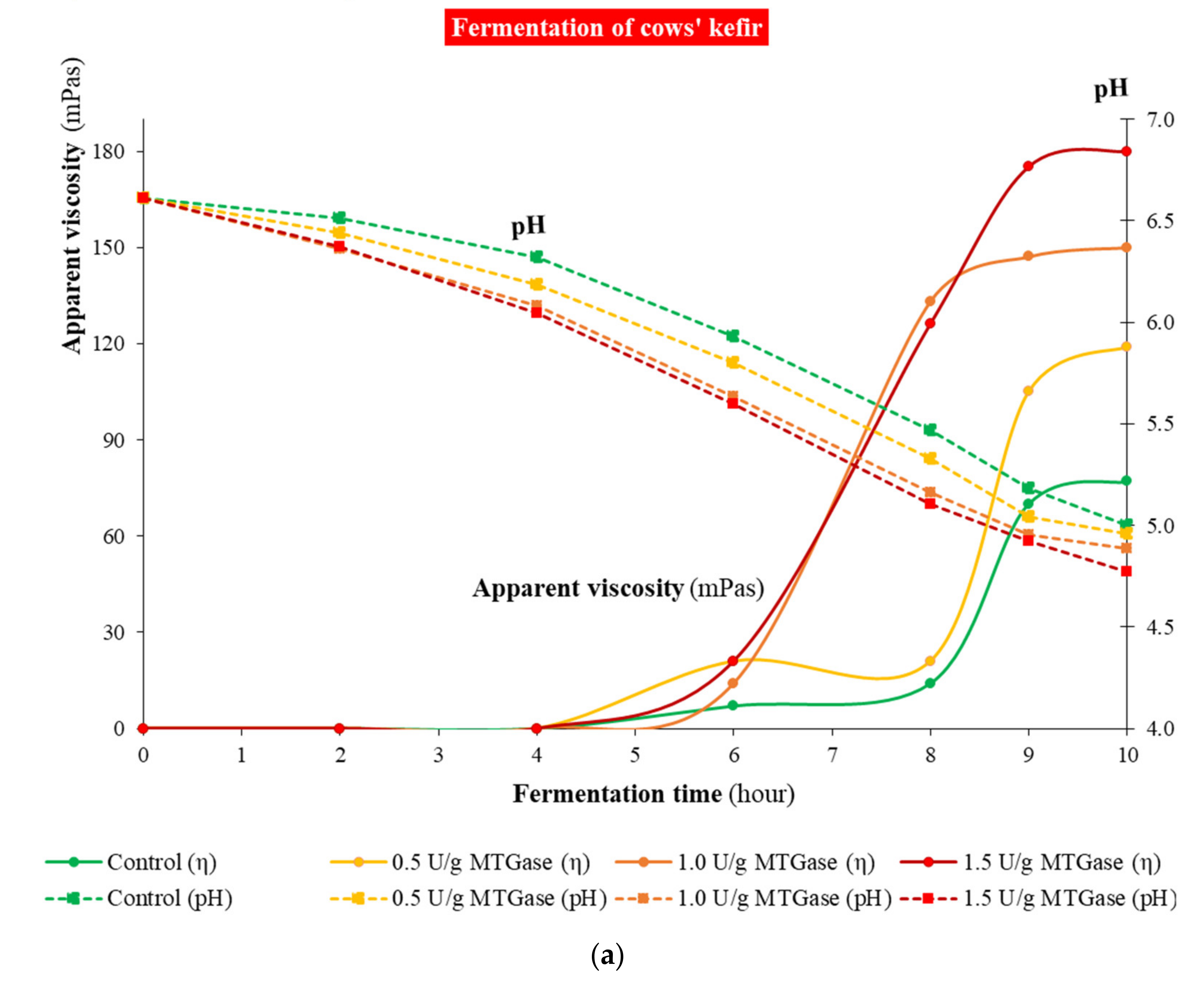
2.2. Monitoring Lactic Acid Fermentation
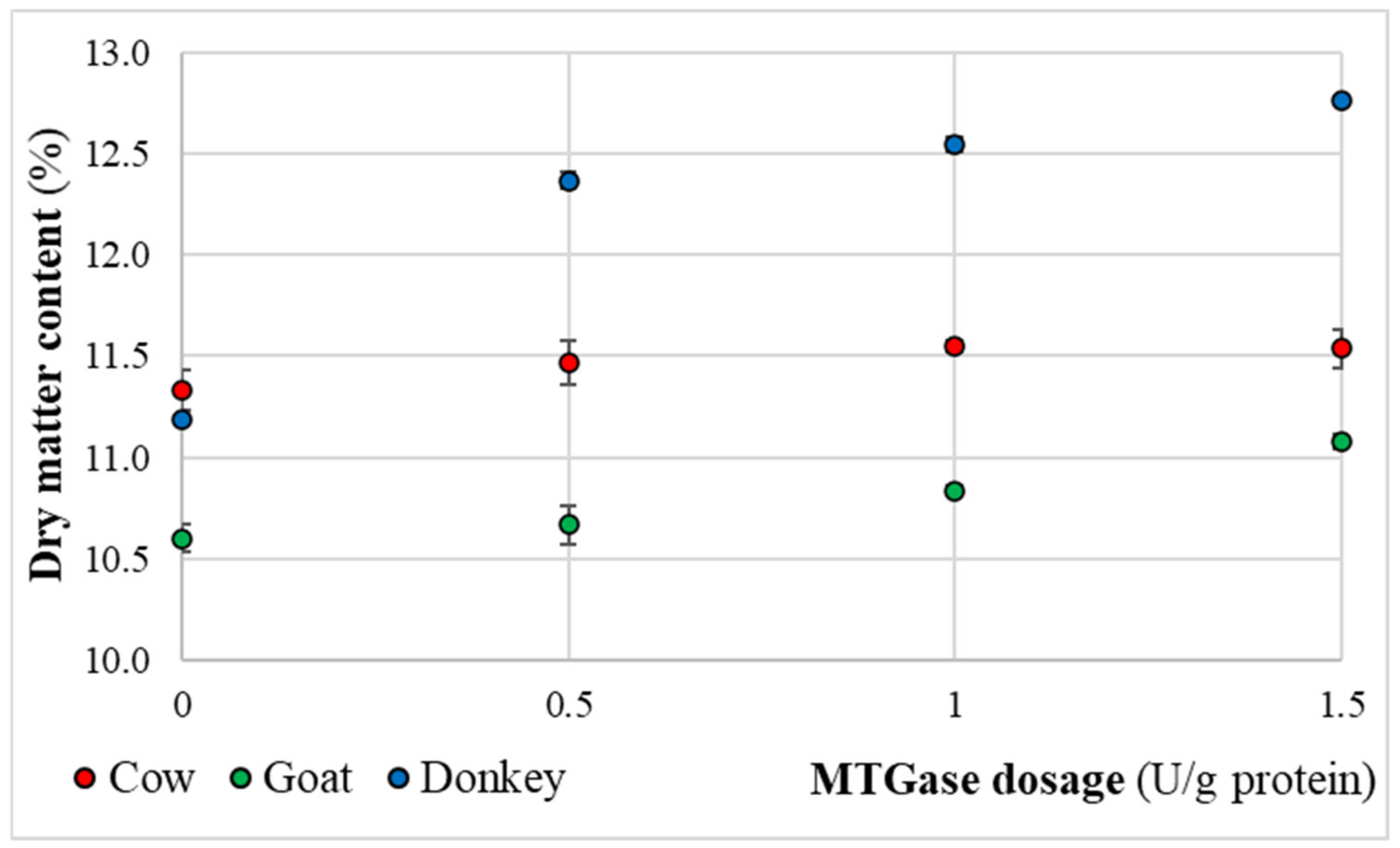
2.3. Dry Matter Content Measurements of the Final Kefir Products
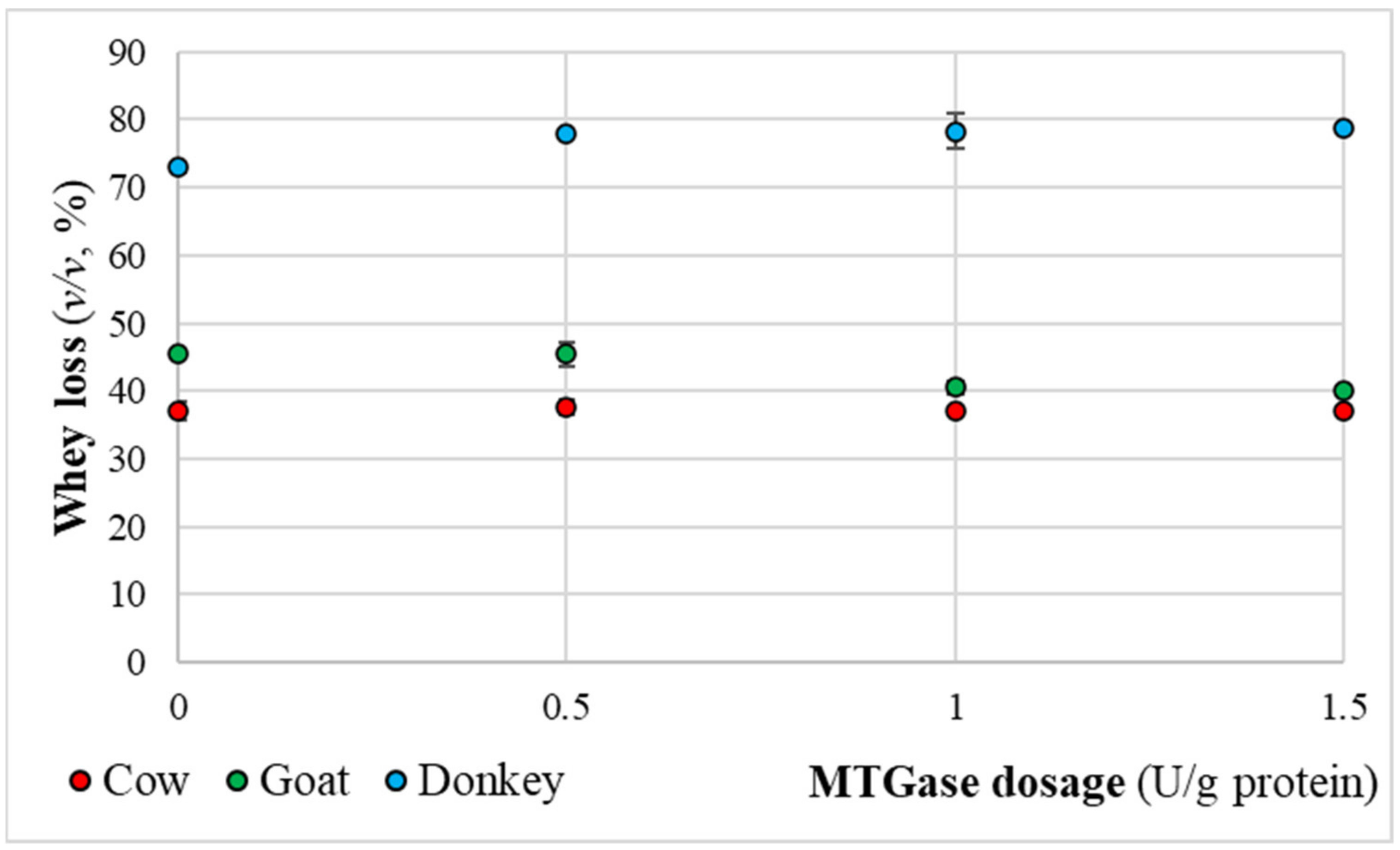
2.4. Syneresis-Whey Separation Measurements
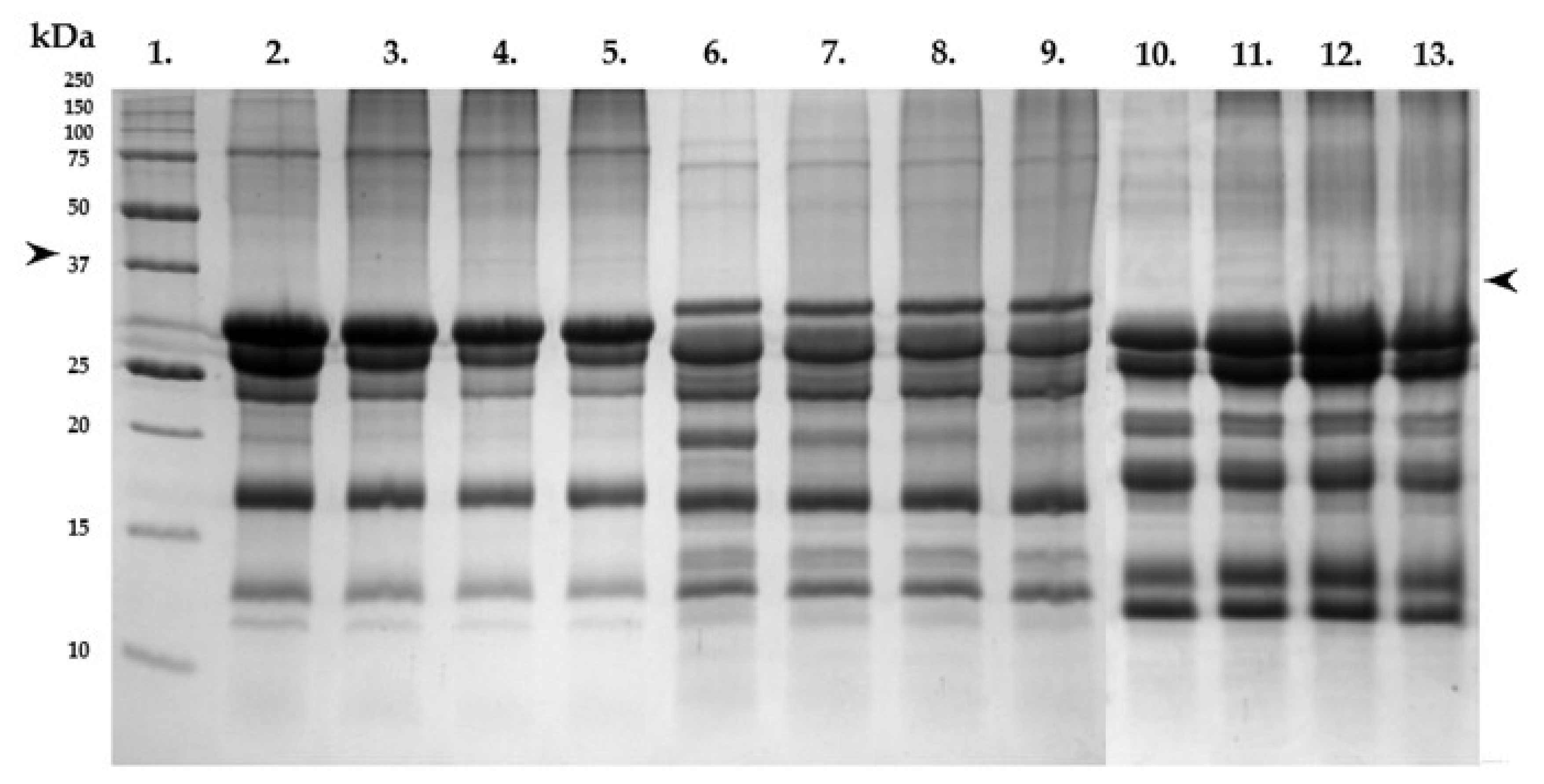
2.5. SDS-PAGE Analysis of the Final Kefir Products
2.6. Gel Firmness Measurements of the Final Kefir Products
2.7. Rheological Measurements of the Final Kefir Products
2.8. Statistical Analysis
3. Results
3.1. Analysis and Comparison of the Applied Milk Types for Kefir Production
3.2. Kinetics of Kefir Fermentation
3.3. Physical-Chemical Quality Characteristics of the Final Kefir Products
3.4. Protein Patterns of the Kefirs Based on Gel Electrophoresis
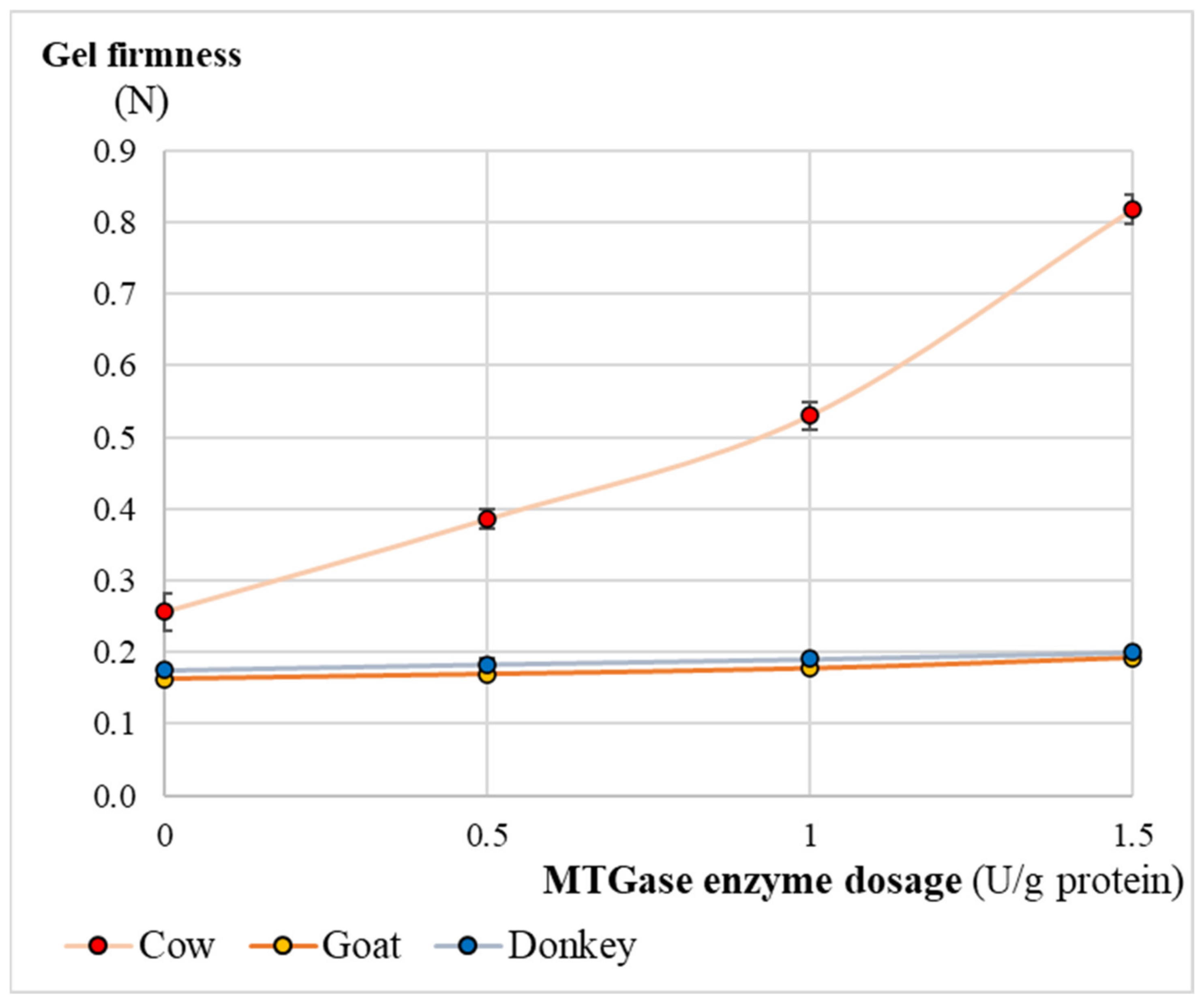
3.5. Gel Firmness of the Final Kefir Products
3.6. Rheological Characterization of the Final Kefir Products
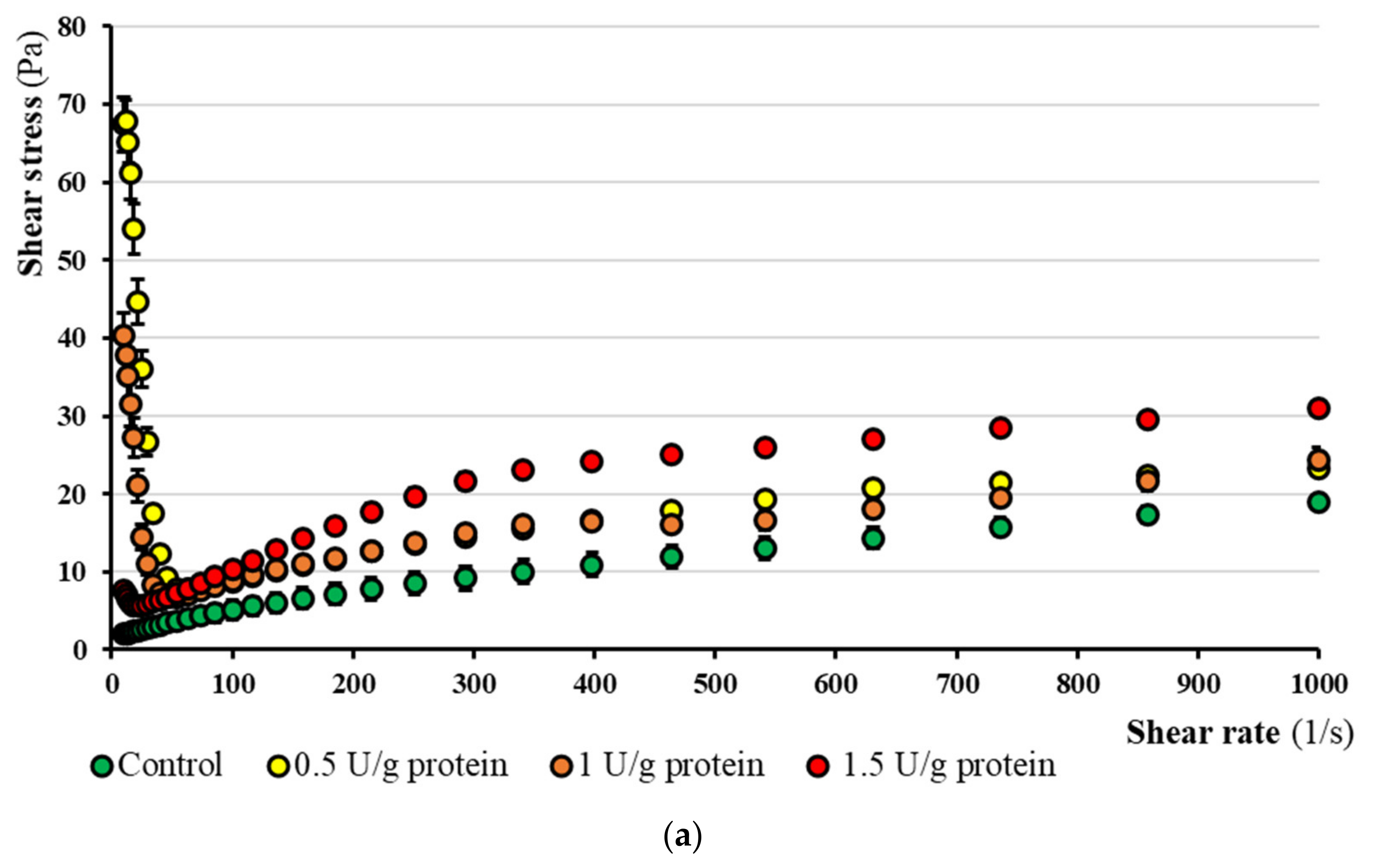
3.6.1. Rheological Flow Curves
3.6.2. Herschel–Bulkley Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Temiz, H.; Dağyıldız, K. Effects of Microbial Transglutaminase on Physicochemical, Microbial and Sensorial Properties of Kefir Produced by Using Mixture Cow’s and Soymilk. Korean J. Food Sci. Anim. Resour. 2017, 37, 606–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beirami-Serizkani, F.; Hojjati, M.; Jooyandeh, H. The effect of microbial transglutaminase enzyme and Persian gum on the characteristics of traditional kefir drink. Int. Dairy J. 2021, 112, 104843. [Google Scholar] [CrossRef]
- Bönisch, M.P.; Huss, M.; Weitl, K.; Kulozik, U. Transglutaminase cross-linking of milk proteins and impact on yoghurt gel properties. Int. Dairy J. 2007, 17, 1360–1371. [Google Scholar] [CrossRef]
- Guyot, C.; Kulozik, U. Effect of transglutaminase-treated milk powders on the properties of skim milk yoghurt. Int. Dairy J. 2011, 21, 628–635. [Google Scholar] [CrossRef]
- Romeih, E.A.; Abdel-Hamid, M.; Awad, A.A. The addition of buttermilk powder and transglutaminase improves textural and organoleptic properties of fat-free buffalo yoghurt. Dairy Sci. Technol. 2014, 94, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Pakseresht, S.; Mazaheri Tehrani, M.; Razavi, S.M.A. Optimization of low-fat set-type yoghurt: Effect of altered whey protein to casein ratio, fat content and microbial transglutaminase on rheological and sensorial properties. J. Food Sci. Technol. 2017, 54, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, B.; Kołakowski, P.; Pawlikowska, K.; Troszyńska, A.; Kaliszewska, A. Influence of the addition of transglutaminase on the immunoreactivit of milk proteins and sensory quality of kefir. Food Hydrocoll. 2009, 23, 2434–2445. [Google Scholar] [CrossRef]
- Beirami-Serizkani, F.; Hojjati, M.; Jooyandeh, H. Influence of the addition of transglutaminase on the immunoreactivity of milk proteins and sensory quality of kefir. In Proceedings of the 4th International Student Bioltechnology Congress, Tehran, Iran, 25–27 February 2019; Volume 23, pp. 238–239. [Google Scholar]
- Sabooni, P.; Pourahmad, R.; Adeli, H.R.M. Improvement of viability of probiotic bacteria, organoleptic qualities and physical characteristics in kefir using transglutaminase and xanthan. Acta Sci. Pol. Technol. Aliment. 2018, 17, 141–148. [Google Scholar] [CrossRef]
- Setyawardani, T.; Sumarmono, J.; Rahardjo, A.H.D.; Widayaka, K.; Santosa, R.S. Texture Profile and Sensory Characteristics of Kefir with Colostrum Addition. IOP Conf. Ser. Earth Environ. Sci. 2019, 372, 012036. [Google Scholar] [CrossRef]
- Baranowska, M.; Staniewski, B.; Bohdziewicz, K. Effect of transglutaminase on selected properties of kefir. Milchwissenschaft 2012, 67, 424–427. [Google Scholar]
- Kieliszek, M.; Misiewicz, A. Microbial transglutaminase and its application in the food industry. A Review. Folia Microbiol. 2014, 59, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnay, L.; Len, A.; Koncz, Á.; Friedrich, L. Small angle neutron scattering study of nanostructural changes in microbial transglutaminase-treated low-fat yogurt during fermentation. Food Sci. Biotechnol. 2015, 24, 2125–2128. [Google Scholar] [CrossRef]
- Hidas, K.I.; Németh, C.; Le Nguyen, P.F.; Visy, A.; Tóth, A.; Barkó, A.; Friedrich, L.; Nagy, A.; Nyulas-Zeke, I.C. Effect of cryogenic freezing on the rheological and calorimetric properties of pasteurized liquid egg yolk. Czech J. Food Sci. 2021, 2021. 39, 181–188. [Google Scholar] [CrossRef]
- Astrup, A.; Rica, N.; Geiker, W.; Magkos, F. Effects of Full-Fat and Fermented Dairy Products on Cardiometabolic Disease: Food Is More Than the Sum of Its Parts. Adv. Nutr. 2019, 10, 924S–930S. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.V.; Dowling, A.P.; Caldeo, V.; Kelly, A.L.; O’Mahony, J.A. Impact of α-lactalbumin:β-lactoglobulin ratio on the heat stability of model infant milk formula protein systems. Food Chem. 2016, 194, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mati, A.; Senoussi-Ghezali, C.; Si Ahmed Zennia, S.; Almi-Sebbane, D.; El-Hatmi, H.; Girardet, J.-M. Dromedary camel milk proteins, a source of peptides having biological activities–A review. Int. Dairy J. 2017, 73, 25–37. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Pucciarelli, S.; Polzonetti, V.; Polidori, P. Role of proteins and of some bioactive peptides on the nutritional quality of donkey milk and their impact on human health. Beverages 2017, 3, 34. [Google Scholar] [CrossRef]
- AJINOMOTO: ACTIVA General Information–Transglutaminase Basics. 1998. Available online: http://docplayer.net/20711824-Activa-general-information.html (accessed on 22 August 2021).
- Németh, C.; Tóth, A.; Hidas, K.I.; Surányi, J.; Friedrich, L. HHP treatment of liquid egg products. J. Phys. Conf. Ser. 2020, 1609, 012012. [Google Scholar] [CrossRef]


| Chemical Composition | Cow | Goat | Donkey |
|---|---|---|---|
| Fat (%) | 1.26 ± 0.17 | 1.34 ± 0.02 | 1.02 ± 0.14 * |
| Protein (%) | 3.29 ± 0.08 | 3.14 ± 0.14 | 3.45 ± 0.01 * |
| Lactose (%) | 4.90 ± 0.12 | 4.65 ± 0.18 | 5.23 ± 0.02 * |
| Solid nonfat (%) | 8.92 ± 0.22 | 8.45 ± 0.33 | 9.50 ± 0.03 * |
| pH | 6.64 ± 0.04 | 6.56 ± 0.02 | 7.14 ± 0.05 * |
| τ0 (Pa) | Cow | Goat | Donkey |
|---|---|---|---|
| Control | 0.94 ± 0.04 | 1.44 ± 0.04 | 0.00 ± 0.00 |
| 0.5 U/g protein | 0.34 ± 0.01 AB | 1.42 ± 0.04 AB | 0.00 ± 0.00 |
| 1 U/g protein | 13.82 ± 0.52 AB | 0.40 ± 0.01 AB | 0.01 ± 0.00 |
| 1.5 U/g protein | 19.66 ± 1.22 AB | 0.30 ± 0.01 AB | 1.36 ± 0.03 AB |
| C (Pas) | Cow | Goat | Donkey |
|---|---|---|---|
| Control | 0.22 ± 0.03 | 0.00 ± 0.00 | 0.06 ± 0.00 |
| 0.5 U/g protein | 1.38 ± 0.01 AB | 0.00 ± 0.00 | 0.05 ± 0.00 AB |
| 1 U/g protein | 2.69 ± 0.06 AB | 0.01 ± 0.00 B | 0.89 ± 0.02 AB |
| 1.5 U/g protein | 4.27 ± 0.07 AB | 0.06 ± 0.00 AB | 2.31 ± 0.00 AB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darnay, L.; Tóth, A.; Csehi, B.; Szepessy, A.; Horváth, M.; Pásztor-Huszár, K.; Laczay, P. The Effect of Microbial Transglutaminase on the Viscosity and Protein Network of Kefir Made from Cow, Goat, or Donkey Milk. Fermentation 2021, 7, 214. https://doi.org/10.3390/fermentation7040214
Darnay L, Tóth A, Csehi B, Szepessy A, Horváth M, Pásztor-Huszár K, Laczay P. The Effect of Microbial Transglutaminase on the Viscosity and Protein Network of Kefir Made from Cow, Goat, or Donkey Milk. Fermentation. 2021; 7(4):214. https://doi.org/10.3390/fermentation7040214
Chicago/Turabian StyleDarnay, Lívia, Adrienn Tóth, Barbara Csehi, Anna Szepessy, Martin Horváth, Klára Pásztor-Huszár, and Péter Laczay. 2021. "The Effect of Microbial Transglutaminase on the Viscosity and Protein Network of Kefir Made from Cow, Goat, or Donkey Milk" Fermentation 7, no. 4: 214. https://doi.org/10.3390/fermentation7040214
APA StyleDarnay, L., Tóth, A., Csehi, B., Szepessy, A., Horváth, M., Pásztor-Huszár, K., & Laczay, P. (2021). The Effect of Microbial Transglutaminase on the Viscosity and Protein Network of Kefir Made from Cow, Goat, or Donkey Milk. Fermentation, 7(4), 214. https://doi.org/10.3390/fermentation7040214





