Vitamin K in COVID-19—Potential Anti-COVID-19 Properties of Fermented Milk Fortified with Bee Honey as a Natural Source of Vitamin K and Probiotics
Abstract
1. Introduction
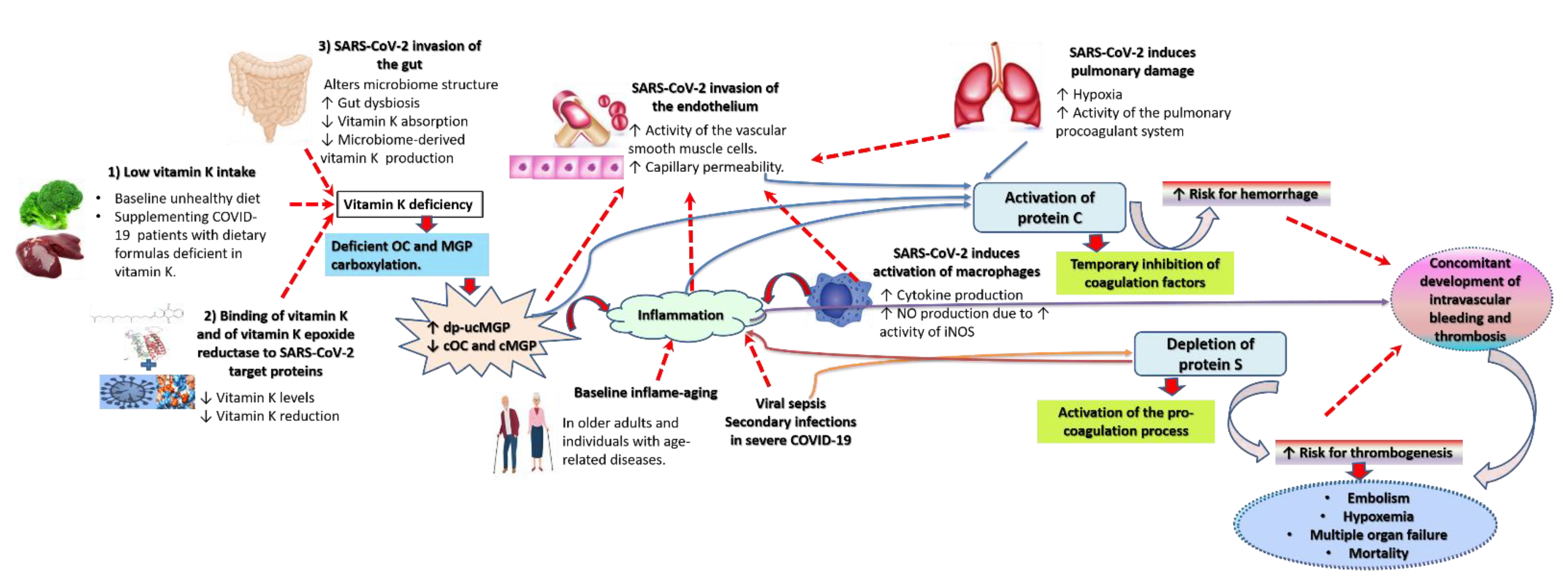
2. Possible Mechanisms for Increased Vulnerability to and Severity of COVID-19 in Vitamin K Deficient Groups
2.1. Lower Intake of Vitamin K Is Evident in Vulnerable Conditions
2.2. Gut Microbiome Disintegration Depletes Gut-Derived MK in COVID-19 Vulnerable Groups
2.3. The Cytokine Storm, Hypoxia Associated with Pulmonary Damage, and the Interaction of Vitamin K and Its Proteins with SARS-CoV-2 Deplete Active Vitamin K
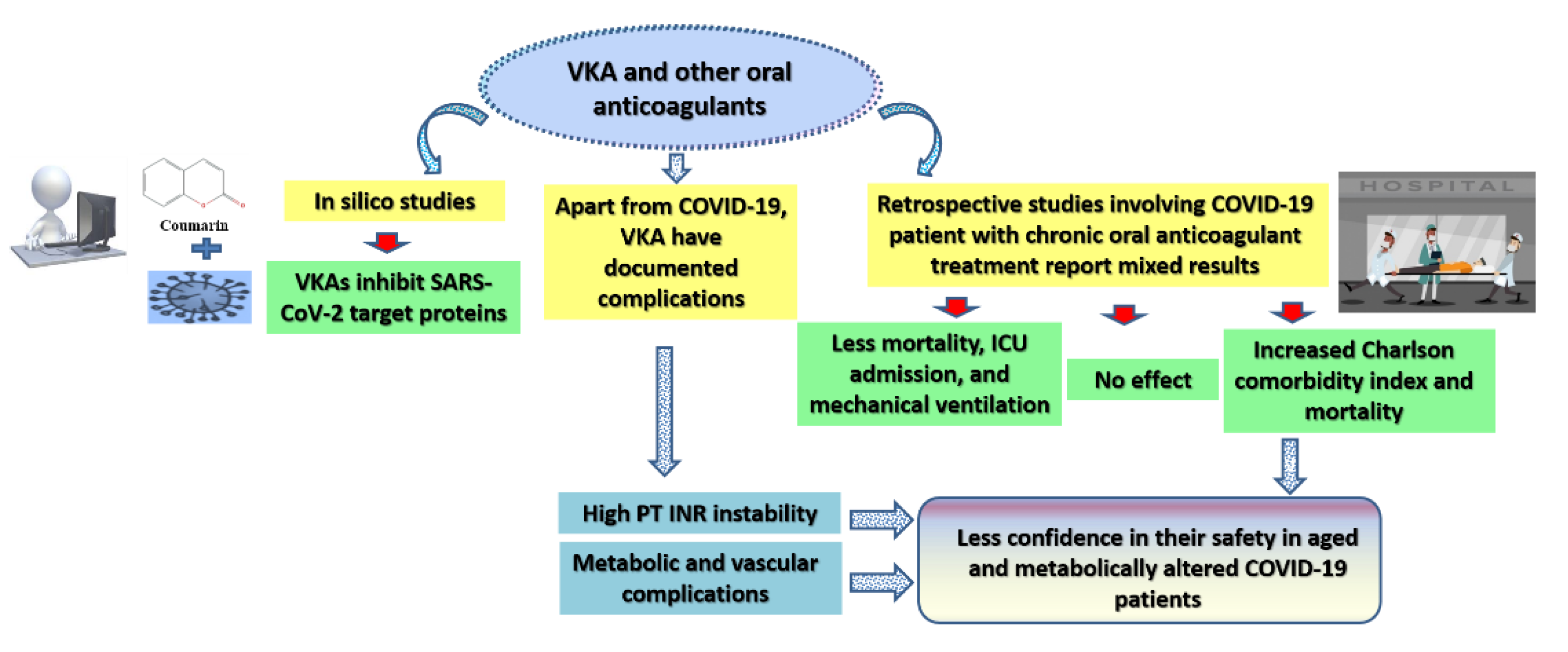
3. The Effect of Oral Anticoagulants, including Vitamin K Antagonists (VKA), on the Prognosis of COVID-19
4. Vitamin K Supplementation May Be Beneficial for Individuals Vulnerable to COVID-19
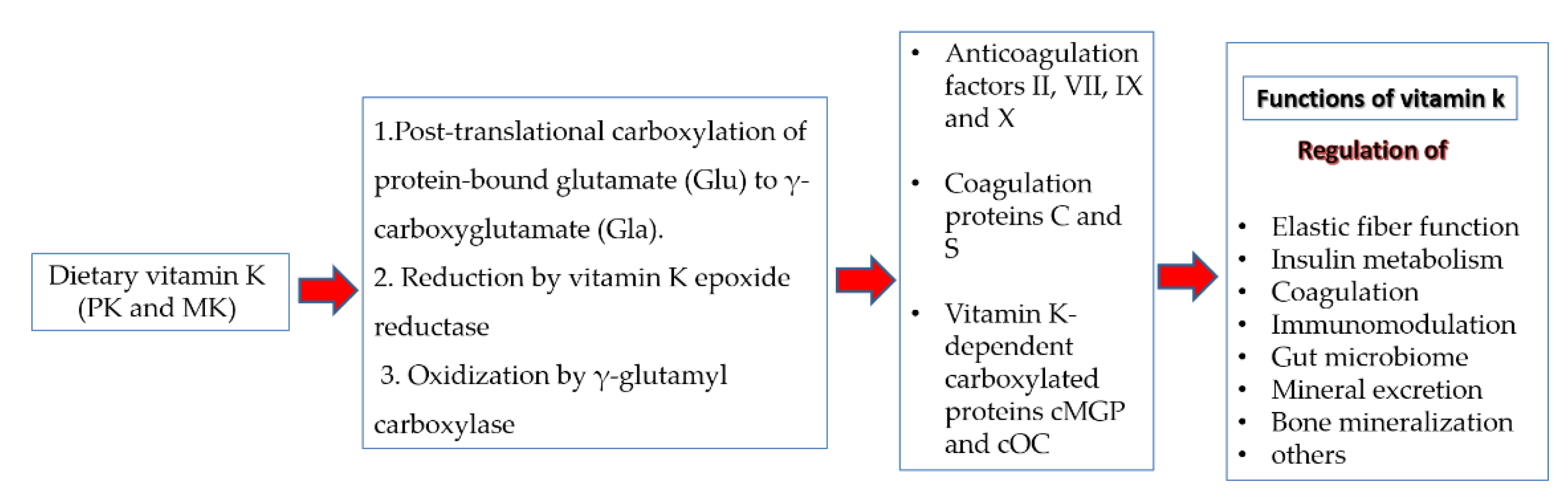
5. Possible Potentials of Vitamin K as an Anti-COVID-19 Agent
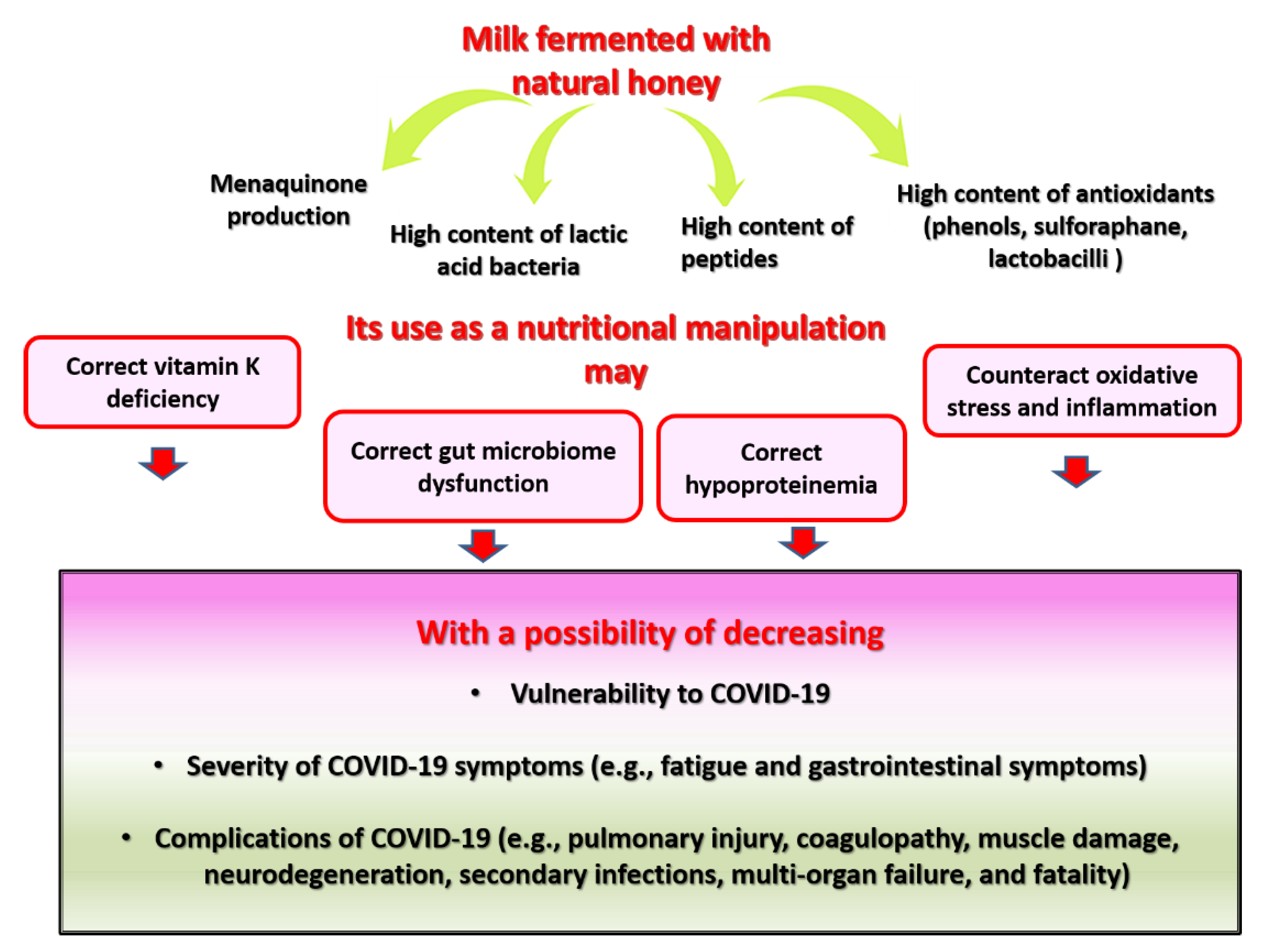
6. Fermented Milk-Fortified with Bee Honey as a Proposed Source of Dietary MK and Other Bioactive Compounds for COVID-19-Prone and COVID-19-Struck Individuals
6.1. Bee Honey as an Anti-COVID-19 Agent and a Natural Source of MK
6.2. Natural Milk as an Anti-COVID-19 Agent and a Natural Source of MK
6.3. Pharmacological Properties of Fermented Milk Fortified with Honey and Their Relevance to COVID-19
6.3.1. Fermented Milk Supplemented with Honey as a Source of Probiotics and Bacterial MK
6.3.2. Fermented Milk Supplemented with Honey as a Source of Antioxidants
6.3.3. Fermented Milk Supplemented with Honey as a Source of Antimicrobial Agents
6.3.4. Fermented Milk Supplemented with Honey as a Source of Bioactive Peptides and Proteins
6.3.5. Anti-Fatigue and Anti-Sarcopenic/Anti-Cachectic Properties of Fermented Milk Supplemented with Honey
6.3.6. Neuroprotective Properties of Fermented Milk Supplemented with Honey
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1:25(OH)D | 1,25-dihydroxyvitamin D |
| AT1 R | Angiotensin II receptor type 1 |
| AHR | Adjusted hazard risk |
| CHD | Coronary heart disease |
| DMK | Demethylmenaquinone |
| dp-ucMGP | Dephosphorylated-uncarboxylated matrix Gla protein |
| ETP | Endogenous thrombin |
| GGCX | γ-glutamyl carboxylase |
| INR | International Normalized Ratio |
| LAB | Lactic acid bacteria |
| LMWH | Low molecular weight heparin |
| MGP | Matrix Gla protein |
| MK | Menaquinones |
| Mpro | Main protease |
| NAD | Nicotinamide adenine dinucleotide |
| NADP | Phosphorylated NAD |
| NOACs | Non-vitamin K oral anticoagulants |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| NSPs | Non-structural proteins |
| OAT | Oral anticoagulants |
| ORF7 | Open reading frame 7 |
| PABPC4 | Poly(A) binding protein cytoplasmic 4 |
| PIVKAII | Prothrombin induced by vitamin K absence-II |
| PK | Phylloquinone |
| RdRp | RNA-dependent RNA polymerase |
| SERPING1 | Serine/cysteine proteinase inhibitor clade G member 1 |
| S protein | Spike protein |
| TMPRSS2 | Transmembrane protease serine 2 |
| VKA | Vitamin K antagonists |
| VKORC1 | Vitamin K epoxide reductase complex 1 |
| ucFII | Uncarboxylated factor II |
| ucOC | Uncarboxylated osteocalcin |
References
- Walther, B.; Karl, J.P.; Booth, S.L.; Boyaval, P. Menaquinones, bacteria, and the food supply: The relevance of dairy and fermented food products to vitamin K requirements. Adv. Nutr. 2013, 4, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Mereu, M.C.; Aghi, A.; Iervasi, G.; Gallieni, M. Vitamin K and bone. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2017, 14, 200–206. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced Vitamin K Status as a Potentially Modifiable Risk Factor of Severe Coronavirus Disease 2019. Clin. Infect. Dis. 2020, ciaa1258. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Booth, S.L. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients 2016, 8, 8. [Google Scholar] [CrossRef]
- Popa, D.-S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Al-Suhaimi, E.A.; Al-Jafary, M.A. Endocrine roles of vitamin K-dependent- osteocalcin in the relation between bone metabolism and metabolic disorders. Rev. Endocr. Metab. Disord. 2020, 21, 117–125. [Google Scholar] [CrossRef]
- Janssen, R.; Visser, M.P.J.; Dofferhoff, A.S.M.; Vermeer, C.; Janssens, W.; Walk, J. Vitamin K metabolism as the potential missing link between lung damage and thromboembolism in Coronavirus disease 2019. Br. J. Nutr. 2020, 126, 1–8. [Google Scholar] [CrossRef]
- Anastasi, E.; Ialongo, C.; Labriola, R.; Ferraguti, G.; Lucarelli, M.; Angeloni, A. Vitamin K deficiency and COVID-19. Scand. J. Clin. Lab. Investig. 2020, 80, 525–527. [Google Scholar] [CrossRef]
- Schein, J.R.; White, C.M.; Nelson, W.W.; Kluger, J.; Mearns, E.S.; Coleman, C.I. Vitamin K antagonist use: Evidence of the difficulty of achieving and maintaining target INR range and subsequent consequences. Thromb. J. 2016, 14, 14. [Google Scholar] [CrossRef]
- Shea, M.K.; O’Donnell, C.J.; Vermeer, C.; Magdeleyns, E.J.; Crosier, M.D.; Gundberg, C.M.; Ordovas, J.M.; Kritchevsky, S.B.; Booth, S.L. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J. Nutr. 2011, 141, 1529–1534. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. Int. J. Mol. Sci. 2019, 20, 628. [Google Scholar] [CrossRef]
- Theuwissen, E.; Teunissen, K.J.; Spronk, H.M.; Hamulyák, K.; Ten Cate, H.; Shearer, M.J.; Vermeer, C.; Schurgers, L.J. Effect of low-dose supplements of menaquinone-7 (vitamin K2 ) on the stability of oral anticoagulant treatment: Dose-response relationship in healthy volunteers. J. Thromb. Haemost. 2013, 11, 1085–1092. [Google Scholar] [CrossRef]
- Sakamoto, N.; Nishiike, T.; Iguchi, H.; Sakamoto, K. The effect of diet on blood vitamin K status and urinary mineral excretion assessed by a food questionnaire. Nutr. Health 1999, 13, 1–10. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kamao, M.; Suhara, Y.; Tsugawa, N.; Uwano, M.; Yamaguchi, N.; Uenishi, K.; Ishida, H.; Sasaki, S.; Okano, T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J. Nutr. Sci. Vitaminol. 2007, 53, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Magdeleyns, E.J.; Braam, L.A.; Teunissen, K.J.; Knapen, M.H.; Binnekamp, I.A.; van Summeren, M.J.; Vermeer, C. Vitamin K status in healthy volunteers. Food Funct. 2014, 5, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Dismore, M.L.; Haytowitz, D.B.; Gebhardt, S.E.; Peterson, J.W.; Booth, S.L. Vitamin K content of nuts and fruits in the US diet. J. Am. Diet. Assoc. 2003, 103, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Kampmann, F.B.; Israelsen, S.B.; Andersen, L.R.; Jørgensen, H.L.; Sandholt, H.; Jørgensen, N.R.; Thysen, S.M.; Benfield, T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef]
- Walk, J.; Dofferhoff, A.S.M.; van den Ouweland, J.M.W.; van Daal, H.; Janssen, R. Vitamin D—Contrary to vitamin K—Does not associate with clinical outcome in hospitalized COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Desai, A.P.; Dirajlal-Fargo, S.; Durieux, J.C.; Tribout, H.; Labbato, D.; McComsey, G.A. Vitamin K & D Deficiency Are Independently Associated with COVID-19 Disease Severity. Open Forum Infect. Dis. 2021, ofab408. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Goddek, S. Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate. Int. J. Infect. Dis. 2020, 99, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; Cappuccilli, M.; Tondolo, F.; Gasperoni, L.; Zappulo, F.; Barbuto, S.; Iacovella, F.; Conte, D.; Capelli, I.; La Manna, G. Vitamin D Effects on Bone Homeostasis and Cardiovascular System in Patients with Chronic Kidney Disease and Renal Transplant Recipients. Nutrients 2021, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Skeletal muscle damage in COVID-19: A call for action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Hypoproteinemia predicts disease severity and mortality in COVID-19: A call for action. Diagn. Pathol. 2021, 16, 31. [Google Scholar] [CrossRef]
- Cheung, C.L.; Sahni, S.; Cheung, B.M.; Sing, C.W.; Wong, I.C. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin. Nutr. 2015, 34, 235–240. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Approaches to nutritional screening in patients with Coronavirus Disease 2019 (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 2772. [Google Scholar] [CrossRef]
- Tutusaus, A.; Marí, M.; Ortiz-Pérez, J.T.; Nicolaes, G.A.F.; Morales, A.; García de Frutos, P. Role of Vitamin K-Dependent Factors Protein S and GAS6 and TAM Receptors in SARS-CoV-2 Infection and COVID-19-Associated Immunothrombosis. Cells 2020, 9, 2186. [Google Scholar] [CrossRef]
- Singh, J.; Metrani, R.; Shivanagoudra, S.R.; Jayaprakasha, G.K.; Patil, B.S. Review on Bile Acids: Effects of the Gut Microbiome, Interactions with Dietary Fiber, and Alterations in the Bioaccessibility of Bioactive Compounds. J. Agric. Food Chem. 2019, 67, 9124–9138. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Physical frailty/sarcopenia as a key predisposing factor to coronavirus disease 2019 (COVID-19) and its complications in older adults. BioMed 2021, 1, 11–40. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging—A focus on cognitive aging and Alzheimer’s disease: A review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: A review and a case report. Int. J. Environ. Res. Public Health 2020, 17, 9379. [Google Scholar] [CrossRef]
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. The effects of royal jelly acid, 10-hydroxy-trans-2-decenoic acid, on neuroinflammation and oxidative stress in astrocytes stimulated with lipopolysaccharide and hydrogen peroxide. Immuno 2021, 1, 212–222. [Google Scholar] [CrossRef]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.K.; Lui, G.C.Y.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.L.; et al. Alterations in Fecal Fungal Microbiome of Patients with COVID-19 During Time of Hospitalization until Discharge. Gastroenterology 2020, 159, 1302–1310.e5. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Saxena, S.; Sahu, N.; Pradhan, B.; Roychowdhury, A. Systematic Network and Meta-analysis on the Antiviral Mechanisms of Probiotics: A Preventive and Treatment Strategy to Mitigate SARS-CoV-2 Infection. Probiotics Antimicrob. Proteins 2021, 13, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Guss, J.D.; Vasquez-Bolanos, L.S.; Castaneda, M.; Rojas, M.V.; Strong, J.M.; Alabi, D.A.; Dornevil, S.D.; Nixon, J.C.; Taylor, E.A.; et al. Components of the Gut Microbiome That Influence Bone Tissue-Level Strength. J. Bone Miner. Res. 2021, 36, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Seura, T.; Yoshino, Y.; Fukuwatari, T. The Relationship between Habitual Dietary Intake and Gut Microbiota in Young Japanese Women. J. Nutr. Sci. Vitaminol. 2017, 63, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 2019, 20, 208. [Google Scholar] [CrossRef]
- McCann, A.; Jeffery, I.B.; Ouliass, B.; Ferland, G.; Fu, X.; Booth, S.L.; Tran, T.T.T.; O’Toole, P.W.; O’Connor, E.M. Exploratory analysis of covariation of microbiota-derived vitamin K and cognition in older adults. Am. J. Clin. Nutr. 2019, 110, 1404–1415. [Google Scholar] [CrossRef]
- Guss, J.D.; Taylor, E.; Rouse, Z.; Roubert, S.; Higgins, C.H.; Thomas, C.J.; Baker, S.P.; Vashishth, D.; Donnelly, E.; Shea, M.K.; et al. The microbial metagenome and bone tissue composition in mice with microbiome-induced reductions in bone strength. Bone 2019, 127, 146–154. [Google Scholar] [CrossRef]
- Speed, V.; Patel, R.K.; Byrne, R.; Roberts, L.N.; Arya, R. A perfect storm: Root cause analysis of supra-therapeutic anticoagulation with vitamin K antagonists during the COVID-19 pandemic. Thromb. Res. 2020, 192, 73–74. [Google Scholar] [CrossRef]
- Nossent, E.J.; Schuurman, A.R.; Reijnders, T.D.Y.; Saris, A.; Jongerius, I.; Blok, S.G.; de Vries, H.; Duitman, J.; Vonk Noordegraaf, A.; Meijboom, L.J.; et al. Pulmonary Procoagulant and Innate Immune Responses in Critically Ill COVID-19 Patients. Front. Immunol. 2021, 12, 664209. [Google Scholar] [CrossRef]
- Danzi, G.B.; Loffi, M.; Galeazzi, G.; Gherbesi, E. Acute pulmonary embolism and COVID-19 pneumonia: A random association? Eur. Heart J. 2020, 41, 1858. [Google Scholar] [CrossRef]
- Dhainaut, J.F.; Yan, S.B.; Cariou, A.; Mira, J.P. Soluble thrombomodulin, plasma-derived unactivated protein C, and recombinant human activated protein C in sepsis. Crit. Care Med. 2002, 30, S318–S324. [Google Scholar] [CrossRef]
- Mann, H.J. Recombinant human activated protein C in severe sepsis. Am. J. Health Syst. Pharm. 2002, 59 (Suppl. S1), S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, U.; Homoncik, M.; Knöbl, P.; Pernerstorfer, T.; Graggaber, J.; Eichler, H.G.; Handler, S.; Jilma, B. Acenocoumarol decreases tissue factor-dependent coagulation during systemic inflammation in humans. Clin. Pharmacol. Ther. 2002, 71, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, J.; Ozah, D.; Bora, T.; Kalita, J.; Manna, P. Gamma-glutamyl carboxylated Gas6 facilitates the prophylactic effect of vitamin K in inhibiting hyperlipidemia-associated inflammatory pathophysiology via arresting MCP-1/ICAM-1 mediated monocyte-hepatocyte adhesion. J. Nutr. Biochem. 2021, 93, 108635. [Google Scholar] [CrossRef] [PubMed]
- Vilovic, M.; Dogas, Z.; Ticinovic Kurir, T.; Borovac, J.A.; Supe-Domic, D.; Vilovic, T.; Ivkovic, N.; Rusic, D.; Novak, A.; Bozic, J. Bone metabolism parameters and inactive matrix Gla protein in patients with obstructive sleep apnea†. Sleep 2020, 43, zsz243. [Google Scholar] [CrossRef] [PubMed]
- Brnic, D.; Martinovic, D.; Zivkovic, P.M.; Tokic, D.; Vilovic, M.; Rusic, D.; Tadin Hadjina, I.; Libers, C.; Glumac, S.; Supe-Domic, D.; et al. Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 4866–4877. [Google Scholar] [CrossRef]
- Kristensen, J.S.S.; Melholt, L.; Kristensen, K.L.; Dahl, M.; Lindholt, J.S. Vitamin K2 Dependent Matrix Gla Protein Relating to Abdominal Aortic Aneurysm and Overall Mortality: A Combined Case Control and Cohort Study. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 267–274. [Google Scholar] [CrossRef]
- Machado-Fragua, M.D.; Hoogendijk, E.O.; Struijk, E.A.; Rodriguez-Artalejo, F.; Lopez-Garcia, E.; Beulens, J.W.; van Ballegooijen, A.J. High dephospho-uncarboxylated matrix Gla protein concentrations, a plasma biomarker of vitamin K, in relation to frailty: The Longitudinal Aging Study Amsterdam. Eur. J. Nutr. 2020, 59, 1243–1251. [Google Scholar] [CrossRef]
- Pfefferle, S.; Schöpf, J.; Kögl, M.; Friedel, C.C.; Müller, M.A.; Carbajo-Lozoya, J.; Stellberger, T.; von Dall’Armi, E.; Herzog, P.; Kallies, S.; et al. The SARS-coronavirus-host interactome: Identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011, 7, e1002331. [Google Scholar] [CrossRef]
- Holcomb, D.; Alexaki, A.; Hernandez, N.; Hunt, R.; Laurie, K.; Kames, J.; Hamasaki-Katagiri, N.; Komar, A.A.; DiCuccio, M.; Kimchi-Sarfaty, C. Gene variants of coagulation related proteins that interact with SARS-CoV-2. PLoS Comput. Biol. 2021, 17, e1008805. [Google Scholar] [CrossRef]
- Hashemi, S.M.A.; Thijssen, M.; Hosseini, S.Y.; Tabarraei, A.; Pourkarim, M.R.; Sarvari, J. Human gene polymorphisms and their possible impact on the clinical outcome of SARS-CoV-2 infection. Arch. Virol. 2021, 166, 2089–2108. [Google Scholar] [CrossRef]
- Rensi, S.; Altman, R.B.; Liu, T.; Lo, Y.C.; McInnes, G.; Derry, A.; Keys, A. Homology Modeling of TMPRSS2 Yields Candidate Drugs That May Inhibit Entry of SARS-CoV-2 into Human Cells. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (COVID-19): A review of in silico, in vitro, and clinical studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef]
- Baby, K.; Maity, S.; Mehta, C.H.; Suresh, A.; Nayak, U.Y.; Nayak, Y. Targeting SARS-CoV-2 RNA-dependent RNA polymerase: An in silico drug repurposing for COVID-19. F1000Research 2020, 9, 1166. [Google Scholar] [CrossRef]
- Alabboud, M.; Javadmanesh, A. In silico study of various antiviral drugs, vitamins, and natural substances as potential binding compounds with SARS-CoV-2 main protease. DYSONA—Life Sci. 2020, 1, 44–63. [Google Scholar] [CrossRef]
- Shoemark, D.K.; Colenso, C.K.; Toelzer, C.; Gupta, K.; Sessions, R.B.; Davidson, A.D.; Berger, I.; Schaffitzel, C.; Spencer, J.; Mulholland, A.J. Molecular Simulations suggest Vitamins, Retinoids and Steroids as Ligands of the Free Fatty Acid Pocket of the SARS-CoV-2 Spike Protein. Angew. Chem. Int. Ed. 2021, 60, 7098–7110. [Google Scholar] [CrossRef] [PubMed]
- Colantuoni, A.; Martini, R.; Caprari, P.; Ballestri, M.; Capecchi, P.L.; Gnasso, A.; Lo Presti, R.; Marcoccia, A.; Rossi, M.; Caimi, G. COVID-19 Sepsis and Microcirculation Dysfunction. Front. Physiol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Martini, R. The compelling arguments for the need of microvascular investigation in COVID-19 critical patients. Clin. Hemorheol. Microcirc. 2020, 75, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Yao, R.Q.; Ren, D.; Li, J.X.; Li, Y.; Liu, X.Y.; Huang, L.; Liu, Y.; Peng, M.; Yao, Y.; et al. The Clinical Features and Prognostic Assessment of SARS-CoV-2 Infection-Induced Sepsis Among COVID-19 Patients in Shenzhen, China. Front. Med. 2020, 7, 570853. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Sengupta, T.; Majumder, S.; Majumder, R. COVID-19: A probable role of the anticoagulant Protein S in managing COVID-19-associated coagulopathy. Aging 2020, 12, 15954–15961. [Google Scholar] [CrossRef]
- Janssen, R.; Walk, J. Vitamin K epoxide reductase complex subunit 1 (VKORC1) gene polymorphism as determinant of differences in COVID-19-related disease severity. Med. Hypotheses 2020, 144, 110218. [Google Scholar] [CrossRef]
- Özdemir, M.; Köksoy, B.; Ceyhan, D.; Sayın, K.; Erçağ, E.; Bulut, M.; Yalçın, B. Design and in silico study of the novel coumarin derivatives against SARS-CoV-2 main enzymes. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, G.M.; Jeschke, E.; Eichler, U.; Thiele, H.; Alhariri, L.; Reinthaler, M.; Kastrati, A.; Leistner, D.M.; Skurk, C.; Landmesser, U.; et al. Impact of oral anticoagulation on clinical outcomes of COVID-19: A nationwide cohort study of hospitalized patients in Germany. Clin. Res. Cardiol. 2021, 110, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Bottino, R.; D’Andrea, A.; Silverio, A.; Di Maio, M.; Golino, P.; Nigro, G.; Valsecchi, O.; Attena, E.; Canonico, M.E.; et al. Chronic Oral Anticoagulation and Clinical Outcome in Hospitalized COVID-19 Patients. Cardiovasc. Drugs Ther. 2021, 1–8. [Google Scholar] [CrossRef]
- Inama, G.; Dodi, C.; Provini, M.; Bossoni, E.; Inama, L.; Balzarini, L.; Mancini, C.; Ramponi, S.; Marvisi, M. Coronavirus disease 2019 infection in patients with recent cardiac surgery: Does chronic anticoagulant therapy have a protective effect? J. Cardiovasc. Med. 2020, 21, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Grassi, G.; Borghi, C.; Grassi, D.; Mancusi, C.; Muiesan, M.L.; Salvetti, M.; Volpe, M.; Ferri, C. Preexisting Oral Anticoagulant Therapy Ameliorates Prognosis in Hospitalized COVID-19 Patients. Front. Cardiovasc. Med. 2021, 8, 633878. [Google Scholar] [CrossRef] [PubMed]
- Ménager, P.; Brière, O.; Gautier, J.; Riou, J.; Sacco, G.; Brangier, A.; Annweiler, C.; on Behalf of The Geria-COVID Study Group. Regular Use of VKA Prior to COVID-19 Associated with Lower 7-Day Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Cohort Study. Nutrients 2021, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Aslan, B.; Akyüz, A.; Işık, F.; Çap, M.; İnci, Ü.; Kaya, İ.; Karahan, M.Z.; Aktan, A.; Bilge, Ö.; Özbek, M.; et al. The Effect of Chronic DOAC Treatment on Clinical Outcomes of Hospitalized Patients with COVID-19. Int. J. Clin. Pract. 2021, 75, e14467. [Google Scholar] [CrossRef]
- Brouns, S.H.; Brüggemann, R.; Linkens, A.E.M.J.H.; Magdelijns, F.J.; Joosten, H.; Heijnen, R.; ten Cate-Hoek, A.J.; Schols, J.M.G.A.; ten Cate, H.; Spaetgens, B. Mortality and the Use of Antithrombotic Therapies Among Nursing Home Residents with COVID-19. J. Am. Geriatr. Soc. 2020, 68, 1647–1652. [Google Scholar] [CrossRef]
- Huang, H.-K.; Liu, P.P.-S.; Lin, S.-M.; Hsu, J.-Y.; Peng, C.C.-H.; Munir, K.M.; Wu, T.-Y.; Yeh, J.-I.; Loh, C.-H.; Tu, Y.-K. Risk of developing diabetes in patients with atrial fibrillation taking non-vitamin K antagonist oral anticoagulants or warfarin: A nationwide cohort study. Diabetes Obes. Metab. 2021, 23, 499–507. [Google Scholar] [CrossRef]
- Weijs, B.; Blaauw, Y.; Rennenberg, R.J.; Schurgers, L.J.; Timmermans, C.C.; Pison, L.; Nieuwlaat, R.; Hofstra, L.; Kroon, A.A.; Wildberger, J.; et al. Patients using vitamin K antagonists show increased levels of coronary calcification: An observational study in low-risk atrial fibrillation patients. Eur. Heart J. 2011, 32, 2555–2562. [Google Scholar] [CrossRef]
- Kumano, O.; Akatsuchi, K.; Amiral, J. Updates on Anticoagulation and Laboratory Tools for Therapy Monitoring of Heparin, Vitamin K Antagonists and Direct Oral Anticoagulants. Biomedicines 2021, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Lip, G.Y.; Pignatelli, P.; Pastori, D. Interaction Between Dietary Vitamin K Intake and Anticoagulation by Vitamin K Antagonists: Is It Really True?: A Systematic Review. Medicine 2016, 95, e2895. [Google Scholar] [CrossRef]
- Dentali, F.; Crowther, M.; Galli, M.; Pomero, F.; Garcia, D.; Clark, N.; Spadafora, L.; Witt, D.M.; Ageno, W. Effect of Vitamin K Intake on the Stability of Treatment with Vitamin K Antagonists: A Systematic Review of the Literature. Semin. Thromb. Hemost. 2016, 42, 671–681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Testa, S.; Paoletti, O.; Giorgi-Pierfranceschi, M.; Pan, A. Switch from oral anticoagulants to parenteral heparin in SARS-CoV-2 hospitalized patients. Intern. Emerg. Med. 2020, 15, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Busani, S.; Tosi, M.; Mighali, P.; Vandelli, P.; D’Amico, R.; Marietta, M.; Forfori, F.; Donati, A.; Cinnella, G.; De Monte, A.; et al. Multi-centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 724. [Google Scholar] [CrossRef]
- Knapen, M.H.; Braam, L.A.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.; Vermeer, C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.M.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am. J. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Cranenburg, E.C.; Knapen, M.H.; Magdeleyns, E.J.; Teunissen, K.J.; Schurgers, L.J.; Smit, E.; Vermeer, C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br. J. Nutr. 2012, 108, 1652–1657. [Google Scholar] [CrossRef]
- Dahlberg, S.; Schött, U.; Eriksson, E.Ä.; Tahirsylaj, Y.; Schurgers, L.; Kander, T. Intravenous Vitamin K1 for the Correction of Prolonged Prothrombin Times in Non-Bleeding Critically Ill Patients: A Prospective Observational Study. Nutrients 2021, 13, 2580. [Google Scholar] [CrossRef]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Martínez-González, M.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary intake of vitamin K is inversely associated with mortality risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef]
- Shea, M.K.; Barger, K.; Booth, S.L.; Matuszek, G.; Cushman, M.; Benjamin, E.J.; Kritchevsky, S.B.; Weiner, D.E. Vitamin K status, cardiovascular disease, and all-cause mortality: A participant-level meta-analysis of 3 US cohorts. Am. J. Clin. Nutr. 2020, 111, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Zwakenberg, S.R.; den Braver, N.R.; Engelen, A.I.P.; Feskens, E.J.M.; Vermeer, C.; Boer, J.M.A.; Verschuren, W.M.M.; van der Schouw, Y.T.; Beulens, J.W.J. Vitamin K intake and all-cause and cause specific mortality. Clin. Nutr. 2017, 36, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-G.; Sheng, L.-T.; Zhang, Y.-B.; Cao, A.-L.; Lai, Y.-W.; Kunutsor, S.K.; Jiang, L.; Pan, A. Association of vitamin K with cardiovascular events and all-cause mortality: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K status and cardiovascular events or mortality: A meta-analysis. Eur. J. Prev. Cardiol. 2020, 26, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Cao, W.; Liu, Y.; Du, B.; Chen, C.; Liu, Q.; Uddin, M.N.; Jiang, S.; Chen, C.; et al. Identifying novel factors associated with COVID-19 transmission and fatality using the machine learning approach. Sci. Total Environ. 2021, 764, 142810. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Pedersen, J.Z.; Incerpi, S. Computational studies reveal mechanism by which quinone derivatives can inhibit SARS-CoV-2. Study of embelin and two therapeutic compounds of interest, methyl prednisolone and dexamethasone. J. Infect. Public Health 2020, 13, 1868–1877. [Google Scholar] [CrossRef]
- He, Z.; Zhao, W.; Gong, Y.; Gao, X. Molecules inhibit the enzyme activity of 3-chymotrypsin-like cysteine protease of SARS-CoV-2 virus: The experimental and theory studies. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sarkar, A. In Silico Studies on Milk Derived Peptides as Potential Inhibitors against SARS-CoV-2 Spike Protein Receptor Binding Domain; Research Square: Durham, NC, USA, 2021. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef]
- Kim, L.; Brudzynski, K. Identification of menaquinones (vitamin K2 homologues) as novel constituents of honey. Food Chem. 2018, 249, 184–192. [Google Scholar] [CrossRef]
- Brudzynski, K.; Flick, R. Accumulation of soluble menaquinones MK-7 in honey coincides with death of Bacillus spp. present in honey. Food Chem. X 2019, 1, 100008. [Google Scholar] [CrossRef]
- Çakır, B.; Okuyan, B.; Şener, G.; Tunali-Akbay, T. Investigation of beta-lactoglobulin derived bioactive peptides against SARS-CoV-2 (COVID-19): In silico analysis. Eur. J. Pharmacol. 2021, 891, 173781. [Google Scholar] [CrossRef]
- Arenas, A.; Borge, C.; Carbonero, A.; Garcia-Bocanegra, I.; Cano-Terriza, D.; Caballero, J.; Arenas-Montes, A. Bovine Coronavirus Immune Milk Against COVID-19. Front. Immunol. 2021, 12, 637152. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. Can Drinking Microfiltered Raw Immune Milk From Cows Immunized Against SARS-CoV-2 Provide Short-Term Protection Against COVID-19? Front. Immunol. 2020, 11, 1888. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Bertrand, K.; Najera, J.A.; Furst, A.; Honerkamp-Smith, G.; Shandling, A.D.; Chambers, C.D.; Camerini, D.; Campo, J.J. Characterization of SARS-CoV-2 Antibodies in Breast Milk from 21 Women with Confirmed COVID-19 Infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Fox, A.; Marino, J.; Amanat, F.; Oguntuyo, K.; Hahn-Holbrook, J.; Lee, B.; Krammer, F.; Zolla-Pazner, S.; Powell, R.L. The Spike-specific IgA in milk commonly-elicited after SARS-CoV-2 infection is concurrent with a robust secretory antibody response, exhibits neutralization potency strongly correlated with IgA binding, and is highly durable over time. medRxiv 2021. [Google Scholar] [CrossRef]
- Juncker, H.G.; Romijn, M.; Loth, V.N.; Caniels, T.G.; de Groot, C.J.M.; Pajkrt, D.; van Gils, M.J.; van Goudoever, J.B.; van Keulen, B.J. Human Milk Antibodies Against SARS-CoV-2: A Longitudinal Follow-Up Study. J. Hum. Lact. 2021, 37, 485–491. [Google Scholar] [CrossRef]
- Indyk, H.E.; Gill, B.D.; Wei, S.; Harvey, L.; Woollard, D.C. Quantitation of Vitamin K in Milk Products by Pre-column Reduction HPLC–Fluorescence. Food Anal. Methods 2021, 14, 984–988. [Google Scholar] [CrossRef]
- Lim, S.-D.; Kim, K.-S.; Do, J.-R. Physiological Characteristics and Production of Vitamin K2 by Lactobacillus fermentum LC272 Isolated from Raw Milk. Korean J. Food Sci. Anim. Resour. 2011, 31, 513–520. [Google Scholar] [CrossRef]
- Fu, X.; Harshman, S.G.; Shen, X.; Haytowitz, D.B.; Karl, J.P.; Wolfe, B.E.; Booth, S.L. Multiple Vitamin K Forms Exist in Dairy Foods. Curr. Dev. Nutr. 2017, 1, e000638. [Google Scholar] [CrossRef]
- Tarvainen, M.; Fabritius, M.; Yang, B. Determination of vitamin K composition of fermented food. Food Chem. 2019, 275, 515–522. [Google Scholar] [CrossRef]
- Glušac, J.; Stijepić, M.; Đurđević-Milošević, D.; Milanović, S.; Kanurić, K.; Vukić, V. Growth and viability of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in traditional yoghurt enriched by honey and whey protein concentrate. Iran. J. Vet. Res. 2015, 16, 249–254. [Google Scholar] [PubMed]
- Castro, V.M.R.; da Mota Silva, M.; Prudêncio de Souza, E.R.; Guerra, A.F.; Riger, C.J.; Laureano-Melo, R.; Luchese, R.H. Role of milk and honey in the tolerance of lactobacilli to oxidative stress. Braz. J. Microbiol. 2021, 52, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Manoury, E.; Jourdon, K.; Boyaval, P.; Fourcassié, P. Quantitative measurement of vitamin K2 (menaquinones) in various fermented dairy products using a reliable high-performance liquid chromatography method. J. Dairy Sci. 2013, 96, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Bøe, C.A.; Holo, H. Engineering Lactococcus lactis for Increased Vitamin K2 Production. Front. Bioeng. Biotechnol. 2020, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.R.; Dias, G.; Ferreira, C.L.L.F.; Franceschini, S.C.C.; Costa, N.M.B. Growth of preschool children was improved when fed an iron-fortified fermented milk beverage supplemented with Lactobacillus acidophilus. Nutr. Res. 2008, 28, 226–232. [Google Scholar] [CrossRef]
- Levine, E. The Symbolism of milk and honey. ETC Rev. Gen. Semant. 1984, 41, 33–37. [Google Scholar]
- Dai, Y.; Choi, Y.H.; Verpoorte, R. Chapter Fourteen—Honey in traditional Chinese medicine: A guide to future applications of NADES to medicines. In Advances in Botanical Research; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 361–384. [Google Scholar]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Al-Jabri, A. Honey, milk and antibiotics. Afr. J. Biotechnol. 2006, 4, 1580–1587. [Google Scholar]
- Okur, Ö.D. Determination of antioxidant activity and total phenolic contents in yogurt added with black cumin (Nigella sativa L.) honey. Ovidius Univ. Ann. Chem. 2021, 32, 1–5. [Google Scholar] [CrossRef]
- Mohan, A.; Hadi, J.; Gutierrez-Maddox, N.; Li, Y.; Leung, I.K.H.; Gao, Y.; Shu, Q.; Quek, S.-Y. Sensory, Microbiological and Physicochemical Characterisation of Functional Manuka Honey Yogurts Containing Probiotic Lactobacillus reuteri DPC16. Foods 2020, 9, 106. [Google Scholar] [CrossRef]
- Sohrabpour, S.; Rezazadeh Bari, M.; Alizadeh, M.; Amiri, S. Investigation of the rheological, microbial, and physicochemical properties of developed synbiotic yogurt containing Lactobacillus acidophilus LA-5, honey, and cinnamon extract. J. Food Process. Preserv. 2021, 45, e15323. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Czarlewski, W.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; Blain, H.; Vidal, A.; Sheikh, A.; Akdis, C.A.; et al. Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy 2021, 76, 735–750. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Rivas, I.; Romaguera, D.; Quijal, M.; Czarlewski, W.; Vidal, A.; Fonseca, J.A.; Ballester, J.; Anto, J.M.; Basagana, X.; et al. Association between consumption of fermented vegetables and COVID-19 mortality at a country level in Europe. medRxiv 2020. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Antioxidant activity of yogurt made from milk characterized by different casein haplotypes and fortified with chestnut and sulla honeys. J. Dairy Sci. 2014, 97, 6662–6670. [Google Scholar] [CrossRef]
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Development and validation of a LC–MS/MS method to determine sulforaphane in honey. Food Chem. 2015, 181, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, A.; Sterkowicz, P.; Kaszycki, P.; Kołoczek, H. Herb honey containing sulforaphane-aglycone with potential use in cancer prophylaxis. Rocz. Panstw. Zakl. Hig. 2003, 54, 25–32. [Google Scholar]
- Yasuda, S.; Horinaka, M.; Sakai, T. Sulforaphane enhances apoptosis induced by Lactobacillus pentosus strain S-PT84 via the TNFα pathway in human colon cancer cells. Oncol. Lett. 2019, 18, 4253–4261. [Google Scholar] [CrossRef]
- Zhou, J.W.; Wang, M.; Sun, N.X.; Qing, Y.; Yin, T.F.; Li, C.; Wu, D. Sulforaphane-induced epigenetic regulation of Nrf2 expression by DNA methyltransferase in human Caco-2 cells. Oncol. Lett. 2019, 18, 2639–2647. [Google Scholar] [CrossRef]
- Sadeghi, A.R.; Pourahmad, R.; Mokhtare, M. Enrichment of Probiotic Yogurt with Broccoli Sprout Extract and its Effect on Helicobacter pylori. Appl. Food Biotechnol. 2017, 4, 53–57. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef]
- Ali, A.M.; Ali, E.M.; Ahmed, M.S.; Hendawy, A.O. Targeting gut microbiome and the recovery of muscle loss associated with cancer (cachexia): An overview of the possible effect of bee products. Med. Leg. Update 2021, 21, 163–171. [Google Scholar] [CrossRef]
- Darvishi, M.; Jahdi, F.; Hamzegardeshi, Z.; Goodarzi, S.; Vahedi, M. The Comparison of vaginal cream of mixing yogurt, honey and clotrimazole on symptoms of vaginal candidiasis. Glob. J. Health Sci. 2015, 7, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Falces-Romero, I.; Ruiz-Bastián, M.; Díaz-Pollán, B.; Maseda, E.; García-Rodríguez, J. Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish Tertiary Care Hospital. Mycoses 2020, 63, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Sakure, A.; Mandal, S. Impact of Proteolytic Lactobacillus helveticus MTCC5463 on Production of Bioactive Peptides Derived from Honey Based Fermented Milk. Int. J. Pept. Res. Ther. 2017, 23, 297–303. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Screening for sarcopenia (physical frailty) in the COVID-19 era. Int. J. Endocrinol. 2021, 2021, 5563960. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Ali, E.M.; Mousa, A.A.; Ahmed, M.E.; Hendawy, A.O. Bee honey and exercise for improving physical performance, reducing fatigue, and promoting an active lifestyle during COVID-19. Sports Med. Health Sci. 2021, 3, 177–180. [Google Scholar] [CrossRef]
- Makino, S.; Hemmi, J.; Kano, H.; Kashiwagi, M.; Hojo, K.; Asami, Y. Anti-Fatigue Effects of Yogurt Fermented with Lactobacillus delbureckii subsp. bulgaricus OLL1073R-1 in Healthy People Suffering from Summer Heat Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 798. [Google Scholar] [CrossRef]
- Williamson, C.B.; Burns, C.M.; Gossard, C.M.; Pizano, J.M.; Dolan, K.E.; Finley, H.J.; Gasta, M.G.; Parker, E.C.; Lipski, E.A. Probiotics and Disease: A Comprehensive Summary-Part 3, Cardiometabolic Disease and Fatigue Syndromes. Integr. Med. 2017, 16, 30–41. [Google Scholar]
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Kim, D.S.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 2014, 10, 465–474. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Quan, H.; Kang, S.-G.; Huang, K.; Tong, T. Nutraceuticals in the Prevention and Treatment of the Muscle Atrophy. Nutrients 2021, 13, 1914. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Lin, T.S.; Yahaya, M.F. Stingless Bee Honey Reduces Anxiety and Improves Memory of the Metabolic Disease-induced Rats. CNS Neurol. Disord. Drug Targets 2020, 19, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Wang, K.; Wang, Q.; Jiang, H.; Lu, Y.; Chen, X.; Zhang, H.; Su, X.; Du, Y.; Chen, L.; et al. Probiotic Lactobacillus casei Shirota prevents acute liver injury by reshaping the gut microbiota to alleviate excessive inflammation and metabolic disorders. Microb. Biotechnol. 2021, 1–15. [Google Scholar] [CrossRef]
- Ali, A.M.; Hendawy, A.O. Bee Honey as a Potentially Effective Treatment for Depression: A Review of Clinical and Preclinical Findings. JOJ Nurse Health Care 2018, 9, 555764. [Google Scholar] [CrossRef]
- Ali, A.M.; Hendawy, A.O. So, Antidepressant Drugs have Serious Adverse Effects, but what are the Alternatives? Nov. Approaches Drug Des. Dev. 2018, 4, 555636. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, T.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Resveratrol and organic selenium-rich fermented milk reduces D-galactose-induced cognitive dysfunction in mice. Food Funct. 2021, 12, 1318–1326. [Google Scholar] [CrossRef]
- Liu, J.; Yu, C.; Li, R.; Liu, K.; Jin, G.; Ge, R.; Tang, F.; Cui, S. High-altitude Tibetan fermented milk ameliorated cognitive dysfunction by modified gut microbiota in Alzheimer’s disease transgenic mice. Food Funct. 2020, 11, 5308–5319. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s disease: A focus on the effects of propolis and royal jelly. Oxid. Med. Cell Longev. 2020, 2020, 1727142. [Google Scholar] [CrossRef]
- Jaywant, A.; Vanderlind, W.M.; Alexopoulos, G.S.; Fridman, C.B.; Perlis, R.H.; Gunning, F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology 2021, 1–6. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.M.; Kunugi, H.; Abdelmageed, H.A.; Mandour, A.S.; Ahmed, M.E.; Ahmad, S.; Hendawy, A.O. Vitamin K in COVID-19—Potential Anti-COVID-19 Properties of Fermented Milk Fortified with Bee Honey as a Natural Source of Vitamin K and Probiotics. Fermentation 2021, 7, 202. https://doi.org/10.3390/fermentation7040202
Ali AM, Kunugi H, Abdelmageed HA, Mandour AS, Ahmed ME, Ahmad S, Hendawy AO. Vitamin K in COVID-19—Potential Anti-COVID-19 Properties of Fermented Milk Fortified with Bee Honey as a Natural Source of Vitamin K and Probiotics. Fermentation. 2021; 7(4):202. https://doi.org/10.3390/fermentation7040202
Chicago/Turabian StyleAli, Amira Mohammed, Hiroshi Kunugi, Hend A. Abdelmageed, Ahmed S. Mandour, Mostafa Elsayed Ahmed, Saboor Ahmad, and Amin Omar Hendawy. 2021. "Vitamin K in COVID-19—Potential Anti-COVID-19 Properties of Fermented Milk Fortified with Bee Honey as a Natural Source of Vitamin K and Probiotics" Fermentation 7, no. 4: 202. https://doi.org/10.3390/fermentation7040202
APA StyleAli, A. M., Kunugi, H., Abdelmageed, H. A., Mandour, A. S., Ahmed, M. E., Ahmad, S., & Hendawy, A. O. (2021). Vitamin K in COVID-19—Potential Anti-COVID-19 Properties of Fermented Milk Fortified with Bee Honey as a Natural Source of Vitamin K and Probiotics. Fermentation, 7(4), 202. https://doi.org/10.3390/fermentation7040202







