Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement
Abstract
1. Background
2. Non-Conventional Yeasts as Leavening Agents: Gaps, Limitations and Challenges
3. Fermentation of Multiple Simple Sugars Found in Flour Dough: A Key Baking Yeast Trait Is Conserved in Non-Conventional Yeasts
4. Assimilation of Multiple Complex Sugars in Flour Dough: A Desirable Trait in Baking Yeasts Is Patchily Distributed in Non-Conventional Yeasts
5. Robust Stress Tolerance of Non-Conventional Yeasts: A Key Trait Sought for in Alternative Baking Yeasts
5.1. Osmotolerance
5.2. Thermotolerance
5.3. Freezing and Thawing Stress Tolerance
5.4. Ethanol Tolerance
5.5. Oxidative Stress Tolerance
6. Aromatic Diversity of Non-Conventional Yeasts: A Key Trait Sought for in Baking Yeasts
7. Improvement of Non-Conventional Yeasts for Baking
8. Non-GM Strategies
9. GM Strategies
10. Conclusions and Future of Non-Conventional Yeasts in Modern Baking
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donalies, U.E.; Nguyen, H.T.; Stahl, U.; Nevoigt, E. Improvement of Saccharomyces yeast strains used in brewing, wine making and baking. Adv. Biochem. Eng. Biotechnol. 2008, 111, 67–98. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; Echavarri-Erasun, C. Yeast biotechnology. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; pp. 21–44. [Google Scholar]
- Kang, A.; Lee, T.S. Converting sugars to biofuels: Ethanol and beyond. Bioengineering 2015, 2, 184. [Google Scholar] [CrossRef]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. Novel brewing yeast hybrids: Creation and application. Appl. Microbiol. Biotechnol. 2017, 101, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1. [Google Scholar] [CrossRef]
- Zhou, N. Carbon Metabolism in Non-Conventional Yeasts: Biodiversity, Origins of Aerobic Fermentation and Industrial Applications. Ph.D. Thesis, Lund University, Lund, Sweden, 2015. [Google Scholar]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-Conventional Yeast Strains Increase the Aroma Complexity of Bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef]
- Randez-Gil, F.; Sanz, P.; Prieto, J.A. Engineering baker’s yeast: Room for improvement. Trends Biotechnol. 1999, 17, 237–244. [Google Scholar] [CrossRef]
- Struyf, N.; Van der Maelen, E.; Hemdane, S.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Bread dough and baker’s yeast: An uplifting synergy. Compr. Rev. Food Sci. Food Saf. 2017, 16, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Schifferdecker, A.J.; Gamero, A.; Compagno, C.; Boekhout, T.; Piškur, J.; Knecht, W. Kazachstania gamospora and Wickerhamomyces subpelliculosus: Two alternative baker’s yeasts in the modern bakery. Int. J. Food Microbiol. 2017, 250, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shehzad, A.; Khan, M.R.; Shabbir, M.A.; Amjid, M. Yeast, its types and role in fermentation during bread making process—A review. Pak. J. Food Sci. 2012, 22, 170–178. [Google Scholar]
- Gamero, A.; Ingoglia, C.; De Jong, C. Microbread: Use of a micro-scale screening breadbaking platform for high-throughput screening of new ingredients and formulations in baked goods. In Proceedings of the 10th Wartburg Symposium on Current Topics in Flavor Chemistry & Biology, Eisenach, Germany, 16–19 April 2013; pp. 359–362. [Google Scholar]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yin, Y.; Ali, B.; Zhang, Y.; Guo, L.; Xu, X. Isolation of yeast strains from Chinese liquor Daqu and its use in the wheat sourdough bread making. Food Biosci. 2019, 31, 100443. [Google Scholar] [CrossRef]
- Attfield, P.V. Stress tolerance: The key to effective strains of industrial baker’s yeast. Nat. Biotechnol. 1997, 15, 1351–1357. [Google Scholar] [CrossRef]
- Randez-Gil, F.; Córcoles-Sáez, I.; Prieto, J.A. Genetic and phenotypic characteristics of baker’s yeast: Relevance to baking. Annu. Rev. Food Sci. Technol. 2013, 4, 191–214. [Google Scholar] [CrossRef]
- Takagi, H. Construction of baker’s yeast strains with enhanced tolerance to baking-associated stresses. In Biotechnology of Yeasts and Filamentous Fungi; Springer: Berlin/Heidelberg, Germany, 2017; pp. 63–85. [Google Scholar]
- Takagi, H.; Shima, J. Stress Tolerance of Baker’s Yeast During Bread-Making Processes. In Stress Biology of Yeasts and Fungi: Applications for Industrial Brewing and Fermentation; Takagi, H., Kitagaki, H., Eds.; Springer: Japan, Tokyo, 2015; pp. 23–42. [Google Scholar]
- Lahue, C.; Madden, A.; Dunn, R.; Smukowski Heil, C. History and Domestication of Saccharomyces cerevisiae in Bread Baking. Front. Genet. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.L.; Rodriguez, C.; Petit, T.; Gancedo, C. Carbohydrate and energy-yielding metabolism in non-conventional yeasts. FEMS Microbiol. Rev. 2000, 24, 507–529. [Google Scholar] [CrossRef]
- Ostergaard, S.; Olsson, L.; Nielsen, J. Metabolic Engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2000, 64, 34–50. [Google Scholar] [CrossRef]
- Chiva, R.; Celador-Lera, L.; Uña, J.A.; Jiménez-López, A.; Espinosa-Alcantud, M.; Mateos-Horganero, E.; Vega, S.; Santos, M.Á.; Velázquez, E.; Tamame, M.J.M. Yeast Biodiversity in Fermented Doughs and Raw Cereal Matrices and the Study of Technological Traits of Selected Strains Isolated in Spain. Microorganisms 2021, 9, 47. [Google Scholar] [CrossRef]
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Yeasts Markets. Available online: http://www.marketsandmarkets.com/Market-Reports/yeast-industry-268.html (accessed on 1 June 2021).
- Dashko, S.; Zhou, N.; Compagno, C.; Piskur, J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014, 14, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Gamero Lluna, A.; de Jong, C. Novel yeasts, novel flavours. New Food Mag. 2013, 16, 26–28. [Google Scholar]
- Spencer, J.; Ragout de Spencer, A.; Laluce, C. Non-conventional yeasts. Appl. Microbiol. Biotechnol. 2002, 58, 147–156. [Google Scholar] [CrossRef]
- Piskur, J.; Rozpedowska, E.; Polakova, S.; Merico, A.; Compagno, C. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006, 22, 183–186. [Google Scholar] [CrossRef]
- De Deken, R.H. The Crabtree effect: A regulatory system in yeast. J. Gen. Microbiol. 1966, 44, 149–156. [Google Scholar] [CrossRef]
- Pronk, J.T.; Steensma, H.Y.; van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Hagman, A.; Sall, T.; Compagno, C.; Piskur, J. Yeast “make-accumulate-consume” life strategy evolved as a multi-step process that predates the whole genome duplication. PLoS ONE 2013, 8, e68734. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Anaya, M.; Pitarch, B.; Bayarri, P.; De Barber, B.C. Microflora of the sourdoughs of wheat flour bread. X. Interactions between yeasts and lactic acid bacteria in wheat doughs and their effects on bread quality. Cereal Chem. 1990, 67, 85–91. [Google Scholar]
- Meroth, C.B.; Hammes, W.P.; Hertel, C. Identification and Population Dynamics of Yeasts in Sourdough Fermentation Processes by PCR-Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2003, 69, 7453–7461. [Google Scholar] [CrossRef]
- Meroth, C.B.; Walter, J.; Hertel, C.; Brandt, M.J.; Hammes, W.P. Monitoring the bacterial population dynamics in sourdough fermentation processes by using PCR-denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2003, 69, 475–482. [Google Scholar] [CrossRef]
- Lee, M.E.; DeLoache, W.C.; Cervantes, B.; Dueber, J.E. A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth. Biol. 2015, 4, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, M.; Zannini, E.; Arendt, E. Impact of Saccharomyces cerevisiae metabolites produced during fermentation on bread quality parameters: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Iwamoto, F.; Nakamori, S. Isolation of freeze-tolerant laboratory strains of Saccharomyces cerevisiae from proline-analogue-resistant mutants. Appl. Microbiol. Biotechnol. 1997, 47, 405–411. [Google Scholar] [CrossRef]
- Verstrepen, K.; Chambers, P.; Pretorius, I. The Development of Superior Yeast Strains for the Food and Beverage Industries: Challenges, Opportunities and Potential Benefits. In Yeasts in Food and Beverages; Querol, A., Fleet, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 399–444. [Google Scholar]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Barnett, J.A. The utilization of sugars by yeasts. Adv. Carbohydr. Chem. Biochem. 1976, 32, 125–234. [Google Scholar] [PubMed]
- Jeffries, T.W. Engineering yeasts for xylose metabolism. Curr. Opin. Biotechnol. 2006, 17, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.W.; Jin, Y.S. Ethanol and thermotolerance in the bioconversion of xylose by yeasts. Adv. Appl. Microbiol. 2000, 47, 221–268. [Google Scholar]
- Jeffries, T.W. Conversion of xylose to ethanol under aerobic conditions by Candida tropicalis. Biotechnol. Lett. 1981, 3, 213–218. [Google Scholar] [CrossRef]
- Compagno, C.; Porro, D.; Smeraldi, C.; Ranzi, B.M. Fermentation of whey and starch by transformed Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 1995, 43, 822–825. [Google Scholar] [CrossRef]
- Sreekrishna, K.; Dickson, R.C. Construction of strains of Saccharomyces cerevisiae that grow on lactose. Proc. Natl. Acad. Sci. USA 1985, 82, 7909–7913. [Google Scholar] [CrossRef]
- Scrimshaw, N.S.; Murray, E.B. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am. J. Clin. Nutr. 1988, 48, 1142–1159. [Google Scholar] [CrossRef]
- Naumov, G.I.; Naumova, E.S.; Turakainen, H.; Korhola, M. Identification of the alpha-galactosidase MEL genes in some populations of Saccharomyces cerevisiae: A new gene MEL11. Genet. Res. 1996, 67, 101–108. [Google Scholar] [CrossRef]
- Hernandez-Lopez, M.J.; Prieto, J.A.; Randez-Gil, F. Osmotolerance and leavening ability in sweet and frozen sweet dough. Comparative analysis between Torulaspora delbrueckii and Saccharomyces cerevisiae baker’s yeast strains. Antonie Van Leeuwenhoek 2003, 84, 125–134. [Google Scholar] [CrossRef]
- Johansen, P.G.; Owusu-Kwarteng, J.; Parkouda, C.; Padonou, S.W.; Jespersen, L. Occurrence and Importance of Yeasts in Indigenous Fermented Food and Beverages Produced in Sub-Saharan Africa. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Motlhanka, K.; Zhou, N.; Lebani, K. Microbial and Chemical Diversity of Traditional Non-Cereal Based Alcoholic Beverages of Sub-Saharan Africa. Beverages 2018, 4, 36. [Google Scholar] [CrossRef]
- Hittinger, C.T.; Rokas, A.; Bai, F.-Y.; Boekhout, T.; Goncalves, P.; Jeffries, T.W.; Kominek, J.; Lachance, M.-A.; Libkind, D.; Rosa, C.A.; et al. Genomics and the making of yeast biodiversity. Curr. Opin. Genet. Dev. 2015, 35, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. (Eds.) Chapter 1—Definition, Classification and Nomenclature of the Yeasts. In The Yeasts, 5th ed.; Elsevier: London, UK, 2011; pp. 3–5. [Google Scholar]
- Shen, X.-X.; Opulente, D.A.; Kominek, J.; Zhou, X.; Steenwyk, J.L.; Buh, K.V.; Haase, M.A.; Wisecaver, J.H.; Wang, M.; Doering, D.T.; et al. Tempo and mode of genome evolution in the budding yeast subphylum. Cell 2018, 175, 1533–1545.e20. [Google Scholar] [CrossRef] [PubMed]
- Bellora, N.; Moliné, M.; David-Palma, M.; Coelho, M.A.; Hittinger, C.T.; Sampaio, J.P.; Gonçalves, P.; Libkind, D. Comparative genomics provides new insights into the diversity, physiology, and sexuality of the only industrially exploited tremellomycete: Phaffia rhodozyma. BMC Genom. 2016, 17, 901. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Peris, D.; Cubillos, F.A.; Steenwyk, J.L.; Opulente, D.A.; Langdon, Q.K.; Rokas, A.; Hittinger, C.T. Into the wild: New yeast genomes from natural environments and new tools for their analysis. FEMS Yeast Res. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.; Haridas, S.; Wolfe, K.H.; Lopes, M.R.; Hittinger, C.T.; Göker, M.; Salamov, A.A.; Wisecaver, J.H.; Long, T.M.; Calvey, C.H.; et al. Comparative genomics of biotechnologically important yeasts. Proc. Natl. Acad. Sci. USA 2016, 113, 9882–9887. [Google Scholar] [CrossRef] [PubMed]
- Donohoue, P.D.; Barrangou, R.; May, A.P. Advances in industrial biotechnology using CRISPR-Cas systems. Trends Biotechnol. 2018, 36, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Ihmels, J.; Bergmann, S.; Gerami-Nejad, M.; Yanai, I.; McClellan, M.; Berman, J.; Barkai, N. Rewiring of the yeast transcriptional network through the evolution of motif usage. Science 2005, 309, 938–940. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Shields, D.C. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 1997, 387, 708–713. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Knecht, W.; Zameitat, E.; Warneboldt, J.; Coutelis, J.B.; Pynyaha, Y.; Neuveglise, C.; Moller, K.; Loffler, M.; Piskur, J. Horizontal gene transfer promoted evolution of the ability to propagate under anaerobic conditions in yeasts. Mol. Genet. Genom. 2004, 271, 387–393. [Google Scholar] [CrossRef]
- Dujon, B. Yeast evolutionary genomics. Nat. Rev. Genet. 2010, 11, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Lineback, D.R.; Rasper, V.F. Wheat: Chemistry and Technology. In Wheat Carbohydrates; Pomeranz, Y., Ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1988; Volume I, pp. 277–372. [Google Scholar]
- Oura, H.; Suomalainen, H.; Viskari, R. Breadmaking. In Economic Microbiology. Fermented Foods; Rose, A.H., Ed.; Academic Press: London, UK, 1982; Volume 7. [Google Scholar]
- Bell, P.J.L.; Higgins, V.J.; Attfield, P.V. Comparison of fermentative capacities of industrial baking and wild-type yeasts of the species Saccharomyces cerevisiae in different sugar media. Lett. Appl. Microbiol. 2001, 32, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Struyf, N.; Laurent, J.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Saccharomyces cerevisiae and Kluyveromyces marxianus Cocultures Allow Reduction of Fermentable Oligo-, Di-, and Monosaccharides and Polyols Levels in Whole Wheat Bread. J. Agric. Food Chem. 2017, 65, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Beudeker, R.J. Developments in bakers’ yeast production. Yeast Biotechnol. Biocatal. 1990, 103–146. [Google Scholar]
- Oda, Y.; Ouchi, K. Principal-component analysis of the characteristics desirable in baker’s yeasts. Appl. Environ. Microbiol. 1989, 55, 1495–1499. [Google Scholar] [CrossRef]
- Rozpedowska, E.; Hellborg, L.; Ishchuk, O.P.; Orhan, F.; Galafassi, S.; Merico, A.; Woolfit, M.; Compagno, C.; Piskur, J. Parallel evolution of the make-accumulate-consume strategy in Saccharomyces and Dekkera yeasts. Nat. Commun. 2011, 2, 302. [Google Scholar] [CrossRef]
- Sahlström, S.; Park, W.; Shelton, D.R. Factors influencing yeast fermentation and the effect of LMW sugars and yeast fermentation on hearth bread quality. Cereal Chem. 2004, 81, 328–335. [Google Scholar] [CrossRef]
- D’Appolonia, B.; Rayas-Duarte, P. Wheat carbohydrates: Structure and functionality. In Wheat; Springer: Berlin/Heidelberg, Germany, 1994; pp. 107–127. [Google Scholar]
- Randez-Gil, F.; Sanz, P. Construction of industrial baker’s yeast strains able to assimilate maltose under catabolite repression conditions. Appl. Microbiol. Biotechnol. 1994, 42, 581–586. [Google Scholar] [CrossRef]
- Cauvain, S.P.; Young, L.S. Technology of Breadmaking; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hebeda, R. Baked Goods Freshness: Technology, Evaluation, and Inhibition of Staling; CRC Press: Boca Raton, FL, USA, 1996; Volume 75. [Google Scholar]
- Kręgiel, D.; Pawlikowska, E.; Antolak, H. Non-Conventional Yeasts in Fermentation Processes: Potentialities and Limitations; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Tabañag, I.D.F.; Tsai, S.-L. Hemicellulose Degradation and Utilization by a Synthetic Saccharomyces cerevisiae Consortium. bioRxiv 2018. [Google Scholar] [CrossRef]
- Nandal, P.; Sharma, S.; Arora, A. Bioprospecting non-conventional yeasts for ethanol production from rice straw hydrolysate and their inhibitor tolerance. Renew. Energy 2020, 147, 1694–1703. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Radecka, D.; Mukherjee, V.; Mateo, R.Q.; Stojiljkovic, M.; Foulquie-Moreno, M.R.; Thevelein, J.M. Looking beyond Saccharomyces: The potential of non-conventional yeast species for desirable traits in bioethanol fermentation. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef]
- Hernández-López, J.; Vargas-Albores, F. A microplate technique to quantify nutrients (NO2−, NO3−, NH4+ and PO43−) in seawater. Aquac. Res. 2003, 34, 1201–1204. [Google Scholar] [CrossRef]
- Shima, J.; Takagi, H. Stress-tolerance of baker’s-yeast (Saccharomyces cerevisiae) cells: Stress-protective molecules and genes involved in stress tolerance. Biotechnol. Appl. Biochem. 2009, 53, 155–164. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Chapter Two—Microbial Ecology and Process Technology of Sourdough Fermentation. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 100, pp. 49–160. [Google Scholar]
- Alves-Araújo, C.; Almeida, M.J.; Sousa, M.J.; Leão, C. Freeze tolerance of the yeast Torulaspora delbrueckii: Cellular and biochemical basis. FEMS Microbiol. Lett. 2004, 240, 7–14. [Google Scholar] [CrossRef]
- Oda, Y.; Tonomura, K. Selection of a Novel Baking Strain from the Torulaspora Yeasts. Biosci. Biotechnol. Biochem. 1993, 57, 1320–1322. [Google Scholar] [CrossRef][Green Version]
- Lane, M.M.; Morrissey, J.P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 2010, 24, 17–26. [Google Scholar] [CrossRef]
- Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8-1 for high-temperature ethanol production. Braz. J. Microbiol. 2018, 49, 378–391. [Google Scholar] [CrossRef]
- Yuangsaard, N.; Yongmanitchai, W.; Yamada, M.; Limtong, S. Selection and characterization of a newly isolated thermotolerant Pichia kudriavzevii strain for ethanol production at high temperature from cassava starch hydrolysate. Antonie van Leeuwenhoek 2013, 103, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Vaudano, E.; Eleonora, B.; Petrozziello, M. Exploring the possibility of using Kazachstania exigua (ex. Saccharomyces exiguus) in wine production. In Industrial, Medical and Environmental Applications of Microorganisms: Current Status and Trends; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 304–309. [Google Scholar]
- Galafassi, S.; Merico, A.; Pizza, F.; Hellborg, L.; Molinari, F.; Piškur, J.; Compagno, C. Dekkera/Brettanomyces yeasts for ethanol production from renewable sources under oxygen-limited and low-pH conditions. J. Ind. Microbiol. Biotechnol. 2011, 38, 1079–1088. [Google Scholar] [CrossRef]
- Caballero, R.; Olguín, P.; Cruz-Guerrero, A.; Gallardo, F.; García-Garibay, M.; Gómez-Ruiz, L. Evaluation of Kluyveromyces marxianus as baker’s yeast. Food Res. Int. 1995, 28, 37–41. [Google Scholar] [CrossRef]
- Mukherjee, V.; Radecka, D.; Aerts, G.; Verstrepen, K.J.; Lievens, B.; Thevelein, J.M. Phenotypic landscape of non-conventional yeast species for different stress tolerance traits desirable in bioethanol fermentation. Biotechnol. Biofuels 2017, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef]
- Albertin, W.; Chasseriaud, L.; Comte, G.; Panfili, A.; Delcamp, A.; Salin, F.; Marullo, P.; Bely, M. Winemaking and bioprocesses strongly shaped the genetic diversity of the ubiquitous yeast Torulaspora delbrueckii. PLoS ONE 2014, 9, e94246. [Google Scholar] [CrossRef] [PubMed]
- Isono, N.; Hayakawa, H.; Usami, A.; Mishima, T.; Hisamatsu, M. A comparative study of ethanol production by Issatchenkia orientalis strains under stress conditions. J. Biosci. Bioeng. 2012, 113, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Ishchuk, O.P.; Knecht, W.; Compagno, C.; Piškur, J. Acquisition of thermotolerance in Lachancea thermotolerans using a bacterial selection pressure. J. Ind. Microbiol. Biotechnol. 2017, 46, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.-M.; Moons, M.-C.; Huret, S.; Vrancken, G.; De Vuyst, L. Wickerhamomyces anomalus in the sourdough microbial ecosystem. Antonie van Leeuwenhoek 2011, 99, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Z.; Long, F.; Niu, C.; Yuan, Y.; Yue, T. Characterization of osmotolerant yeasts and yeast-like molds from apple orchards and apple juice processing plants in China and investigation of their spoilage potential. J. Food Sci. 2015, 80, M1850–M1860. [Google Scholar] [CrossRef] [PubMed]
- Korcari, D.; Ricci, G.; Capusoni, C.; Fortina, M.G. Physiological performance of Kazachstania unispora in sourdough environments. World J. Microbiol. Biotechnol. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Curtin, C.D.; Pretorius, I.S. Genomic insights into the evolution of industrial yeast species Brettanomyces bruxellensis. FEMS Yeast Res. 2014, 14, 997–1005. [Google Scholar]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Evolutionary adaptation of Kluyveromyces marxianus strain for efficient conversion of whey lactose to bioethanol. Process Biochem. 2017, 62, 69–79. [Google Scholar] [CrossRef]
- Sehnem, N.; Machado, Â.; Matte, C.; de Morais, M., Jr.; Ayub, M. Second-generation ethanol production by Wickerhamomyces anomalus strain adapted to furfural, 5-hydroxymethylfurfural (HMF), and high osmotic pressure. An. Acad. Bras. Ciênc. 2020, 92. [Google Scholar] [CrossRef]
- Prista, C.; Michán, C.; Miranda, I.M.; Ramos, J. The halotolerant Debaryomyces hansenii, the Cinderella of non-conventional yeasts. Yeast 2016, 33, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Mo, W.; Wang, M.; Zhan, R.; Yu, Y.; He, Y.; Lu, H. Kluyveromyces marxianus developing ethanol tolerance during adaptive evolution with significant improvements of multiple pathways. Biotechnol. Biofuels 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Hahn, Y.-S.; Kawai, H. Isolation and Characterization of Freeze-tolerant Yeasts from Nature Available for the Frozen-dough Method. Agric. Biol. Chem. 1990, 54, 829–831. [Google Scholar] [CrossRef]
- Nurcholis, M.; Lertwattanasakul, N.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Integration of comprehensive data and biotechnological tools for industrial applications of Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2020, 104, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Rahmadhani, N.; Astuti, R.; Meryandini, A. Substrate utilization of ethanologenic yeasts co-cultivation of Pichia kudriavzevii and Saccharomyces cerevisiae. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012072. [Google Scholar]
- Liu, T.; Li, Y.; Sadiq, F.A.; Yang, H.; Gu, J.; Yuan, L.; Lee, Y.K.; He, G. Predominant yeasts in Chinese traditional sourdough and their influence on aroma formation in Chinese steamed bread. Food Chem. 2018, 242, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Santos, J.; Chaves, S.; Almeida, J.; Sousa, M. The emerging role of the Torulaspora delbrueckii in bread and wine production: Using genetic manipulation to study molecular basis of physiological responses. In Structure and Function of Food Engineering; IntechOpen: London, UK, 2012. [Google Scholar]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Joseph, C.L.; Albino, E.; Bisson, L.F. Creation and use of a Brettanomyces aroma wheel. Catal. Discov. Pract. 2017, 1, 12–20. [Google Scholar] [CrossRef][Green Version]
- Verstrepen, K.J.; Iserentant, D.; Malcorps, P.; Derdelinckx, G.; Van Dijck, P.; Winderickx, J.; Pretorius, I.S.; Thevelein, J.M.; Delvaux, F.R. Glucose and sucrose: Hazardous fast-food for industrial yeast? Trends Biotechnol. 2004, 22, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, A.; Adler, L. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 1992, 33, 145–212. [Google Scholar]
- Landolfo, S.; Politi, H.; Angelozzi, D.; Mannazzu, I. ROS accumulation and oxidative damage to cell structures in Saccharomyces cerevisiae wine strains during fermentation of high-sugar-containing medium. Biochim. Biophys. Acta BBA Gen. Subj. 2008, 1780, 892–898. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Cell death: Protein misfolding and neurodegenerative diseases. Apoptosis 2009, 14, 455–468. [Google Scholar] [CrossRef]
- Henderson, C.M.; Zeno, W.F.; Lerno, L.A.; Longo, M.L.; Block, D.E. Fermentation temperature modulates phosphatidylethanolamine and phosphatidylinositol levels in the cell membrane of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 5345–5356. [Google Scholar] [CrossRef]
- Nwaefuna, A.E.; Rumbold, K.; Boekhout, T.; Zhou, N. Bioethanolic yeasts from dung beetles: Tapping the potential of extremophilic yeasts for improvement of lignocellulolytic feedstock fermentation. Biotechnol. Biofuels 2021, 14, 86. [Google Scholar] [CrossRef]
- Lehnen, M.; Ebert, B.E.; Blank, L.M. Elevated temperatures do not trigger a conserved metabolic network response among thermotolerant yeasts. BMC Microbiol. 2019, 19, 100. [Google Scholar] [CrossRef]
- Hsu, K.; Rc, H.; Pa, S. Frozen Dough. I: Factors Affecting Stability of Yeasted Doughs. Cereal Chem. 1979, 56, 419–424. [Google Scholar]
- Park, J.-I.; Grant, C.M.; Attfield, P.V.; Dawes, I.W. The freeze-thaw stress response of the yeast Saccharomyces cerevisiae is growth phase specific and is controlled by nutritional state via the RAS-cyclic AMP signal transduction pathway. Appl. Environ. Microbiol. 1997, 63, 3818–3824. [Google Scholar] [CrossRef]
- Shima, J.; Hino, A.; Yamada-Iyo, C.; Suzuki, Y.; Nakajima, R.; Watanabe, H.; Mori, K.; Takano, H. Stress tolerance in doughs of Saccharomyces cerevisiae trehalase mutants derived from commercial Baker’s yeast. Appl. Environ. Microbiol. 1999, 65, 2841–2846. [Google Scholar] [CrossRef]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef]
- Cabiscol, E.; Piulats, E.; Echave, P.; Herrero, E.; Ros, J. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 27393–27398. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Smith, J.M.; Shelton, N.; Smith, J.M.; Smart, K.A. Oxygen as toxin: Oxidative stress and brewing yeast physiology. Cerevisia 2006, 31, 25. [Google Scholar]
- Girotti, A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef]
- Salmon, T.B.; Evert, B.A.; Song, B.; Doetsch, P.W. Biological consequences of oxidative stress-induced DNA damage in Saccharomyces cerevisiae. Nucleic Acids Res. 2004, 32, 3712–3723. [Google Scholar] [CrossRef]
- Barker, M.G.; Brimage, L.J.; Smart, K.A. Effect of Cu,Zn superoxide dismutase disruption mutation on replicative senescence in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1999, 177, 199–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Schieberle, P.; Grosch, W. Potent odorants of the wheat bread crumb Differences to the crust and effect of a longer dough fermentation. Z. Lebensm. Unters. Forsch. 1991, 192, 130–135. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. The aroma profile of wheat bread crumb influenced by yeast concentration and fermentation temperature. LWT Food Sci. Technol. 2013, 50, 480–488. [Google Scholar] [CrossRef]
- Purlis, E. Browning development in bakery products—A review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Frasse, P.; Lambert, S.; Levesque, C.; Melcion, D.; Richard-Molard, D.; Chiron, H. The influence of fermentation on volatile compounds in French bread crumb. Food Sci. Technol. 1992, 25, 66–70. [Google Scholar]
- Carbonetto, B.; Ramsayer, J.; Nidelet, T.; Legrand, J.; Sicard, D. Bakery yeasts, a new model for studies in ecology and evolution. Yeast 2018, 35, 591–603. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of mixed cultures of yeast and lactobacilli on the quality of wheat sourdough bread. Front. Microbiol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Fisher, A.; Koureta, K.; Psarianos, C.; Nigam, P.; Koutinas, A.A. Application of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and L. helveticus for sourdough bread making. Food Chem. 2008, 106, 985–990. [Google Scholar] [CrossRef]
- Poinot, P.; Grua-Priol, J.; Arvisenet, G.; Rannou, C.; Semenou, M.; Le Bail, A.; Prost, C. Optimisation of HS-SPME to study representativeness of partially baked bread odorant extracts. Food Res. Int. 2007, 40, 1170–1184. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. Aroma of wheat bread crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]
- Swamy, K.B.S.; Zhou, N. Experimental evolution: Its principles and applications in developing stress-tolerant yeasts. Appl. Microbiol. Biotechnol. 2019, 103, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.A.; Srikrishnan, S. Introduction and expression of genes for metabolic engineering applications in Saccharomyces cerevisiae. FEMS Yeast Res. 2012, 12, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.S.; Schmidt, T.M. Life history implications of rRNA gene copy number in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 6670–6677. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.; Jin, Y.-S. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biotechnol. 2004, 63, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Deckers, M.; Deforce, D.; Fraiture, M.-A.; Roosens, N.H. Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview. Foods 2020, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Katz, M.; Knecht, W.; Compagno, C.; Piškur, J. Genome dynamics and evolution in yeasts: A long-term yeast-bacteria competition experiment. PLoS ONE 2017, 13, e0194911. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Bottagisi, S.; Katz, M.; Schacherer, J.; Friedrich, A.; Gojkovic, Z.; Swamy, K.B.S.; Knecht, W.; Compagno, C.; Piškur, J. Yeast-bacteria competition induced new metabolic traits through large-scale genomic rearrangements in Lachancea kluyveri. FEMS Yeast Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Harlander, S.; Roller, S.; Harlander, S.K. Genetic Modification in the Food Industry: A Strategy for Food Quality Improvement; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
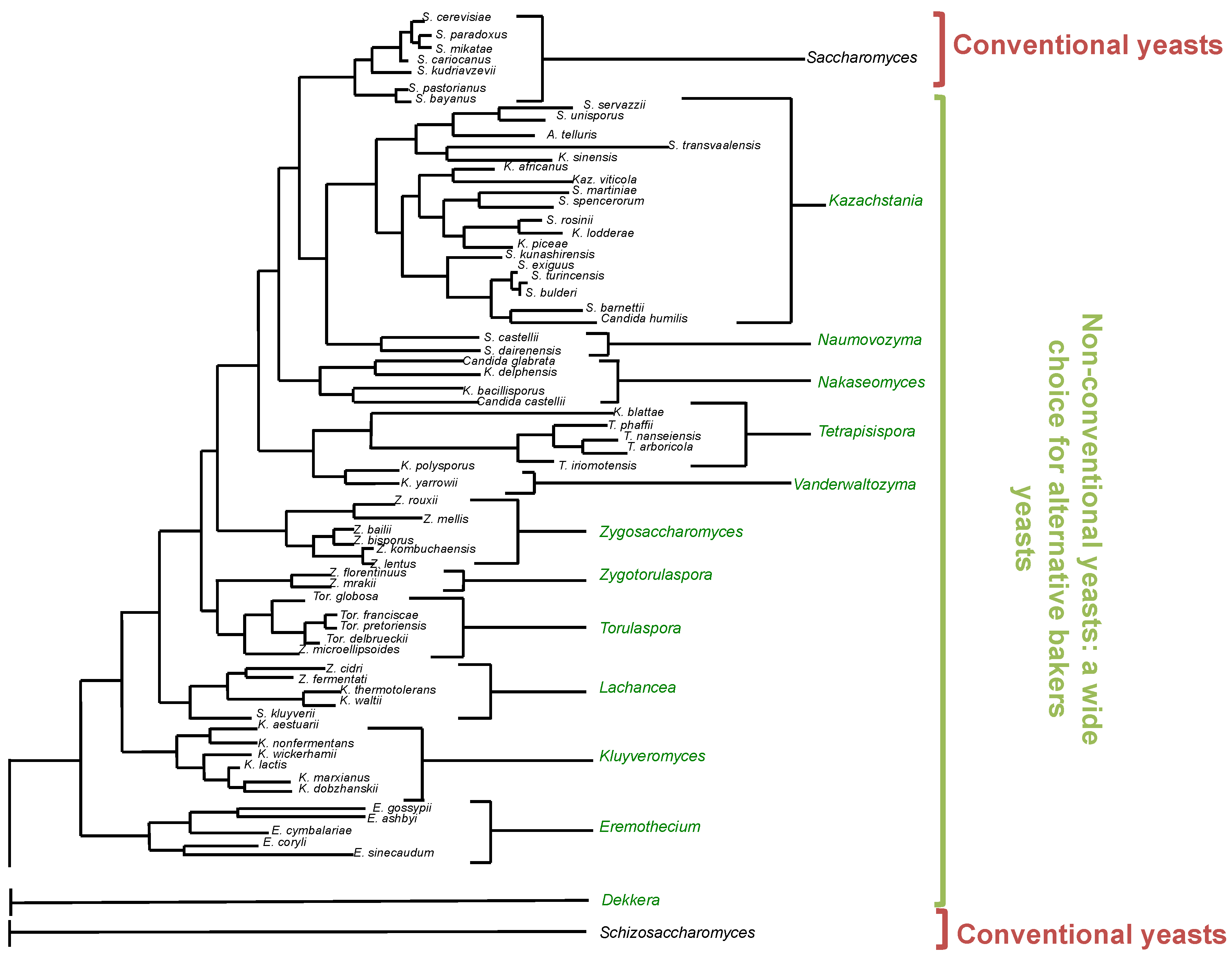
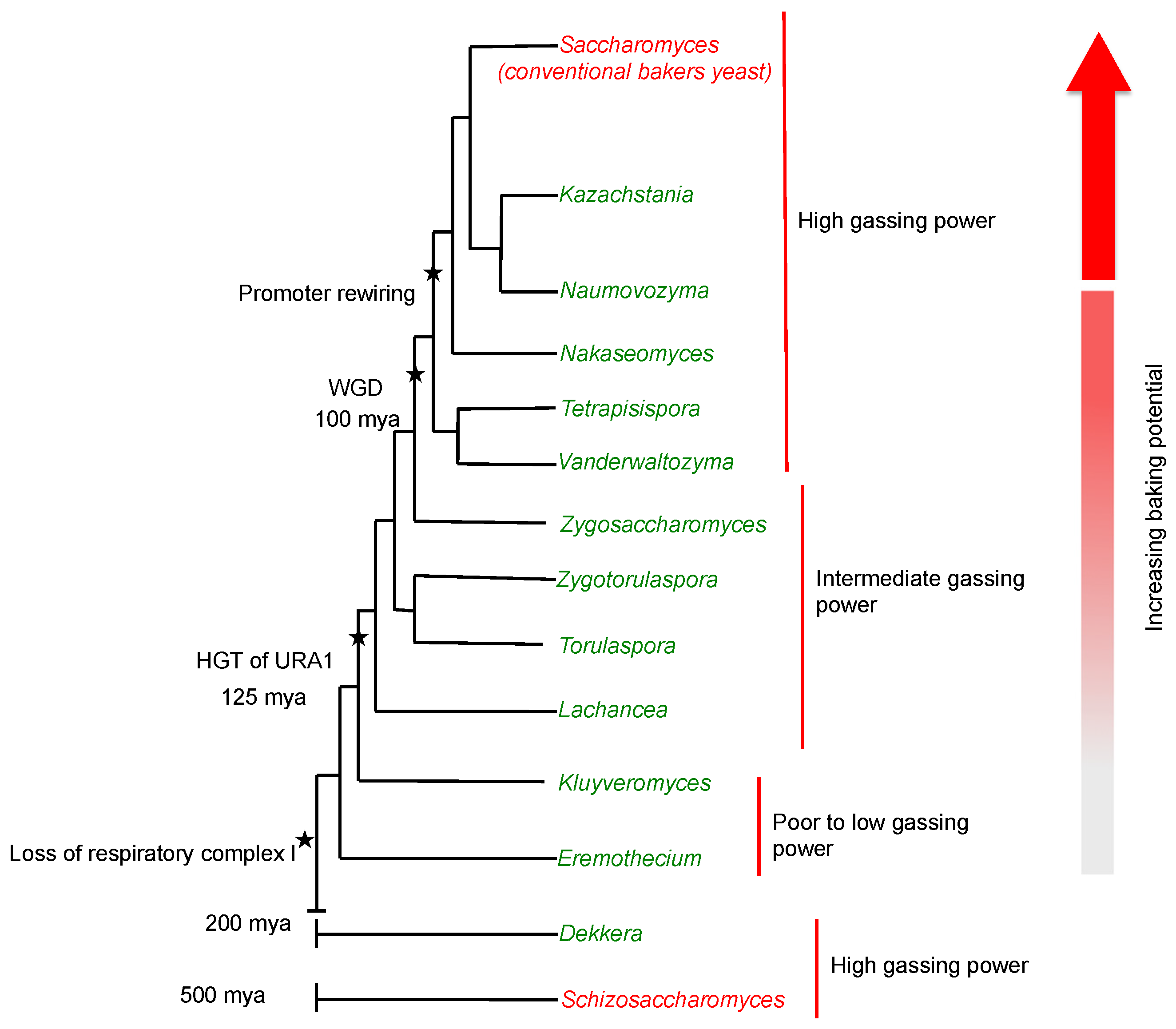
| Trait | Yeast Species |
|---|---|
| Gassing power | Wickerhamomyces anomalus [80,83] Wickerhamomyces subpellicullosus [10] Torulospora delbrueckii [83,84] Torulospora pretoriensis [85] Kluyveromyces marxianus [66,86] Picha kudriavzevii [83,87,88] Kazachstania gamospora [10] Kazakstania humilis [83] Kazachstania exigua [89] Brettanomyces (=Dekkera) bruxellensis [69,80,90] Kluyveromyces marxianus [91] |
| Thermotolerance | Wickerhamomyces anomalus [80] Metschnikowia pulcherima [92] Wickerhamomyces subpellicullosus [10] Kluyveromyces marxianus [86,92,93] Pichia kudriavzevii [88] Torulospora delbrueckii [49,94] Pichia kudriavzevii [87,92,95] Lachancea thermotolerans (Hino et al. 1987) [96] Candida thermophila [95] |
| Osmotolerance | Wickerhamomyces anomalus [97] Wickerhamomyces subpellicullosus [10] Torulospora delbrueckii [84,92] Metschnikowia pulcherima [92] Zygosaccharomyces spp [92] Pichia kudriavzevii [98] Kazachstania gamospora [99] Brettanomyces (=Dekkera) bruxellensis [100] Kluyveromyces marxianus [101] |
| Halotolerance | Wickerhamomyces anomalus [102] Wickerhamomyces subpellicullosus [10] Torulospora delbrueckii [87] Pichia kudriavzevii [87] Brettanomyces (=Dekkera) bruxellensis [100] Kluyveromyces marxianus [101] Debaryomyces hanseni [103] |
| Ethanol tolerance | Wickerhamomyces anomalus [92] Lachancea thermotolerans [92] Saccharomycoides ludwigii [92] Wickerhamomyces subpellicullosus Zygosaccharomyces rouxii [92] Torulospora delbrueckii [104] Pichia kudriavzevii [87,92] Hanseniaspora valbyensis [92] Brettanomyces (=Dekkera) bruxellensis [90] Kluyveromyces marxianus [105] |
| Freezing and thawing stress tolerance | Torulospora delbrueckii [84,106] Zygosaccharomyces rouxii [106] Saccharomyces rosei [106] Kluyveromyces thermotolerans [106] |
| Wide range of sugar utilisation | Kluyveromyces marxianus [80,107] Pichia kudriavzevii [108] |
| Aroma complexity | Wickerhamomyces anomalus [109] Saccharomycopsis fibigulera [109] Wickerhamomyces subpellicullosus [10] Torulospora delbrueckii [110] Pichia kudriavzevii [109] Kazachstania gamospora [10] Kazachstania humilis [109] Kazachstania exigua [89] Kazachstania zonata [111] Brettanomyces (=Dekkera) bruxellensis [112] Kluyveromyces marxianus [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Semumu, T.; Gamero, A. Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement. Fermentation 2021, 7, 102. https://doi.org/10.3390/fermentation7030102
Zhou N, Semumu T, Gamero A. Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement. Fermentation. 2021; 7(3):102. https://doi.org/10.3390/fermentation7030102
Chicago/Turabian StyleZhou, Nerve, Thandiwe Semumu, and Amparo Gamero. 2021. "Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement" Fermentation 7, no. 3: 102. https://doi.org/10.3390/fermentation7030102
APA StyleZhou, N., Semumu, T., & Gamero, A. (2021). Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement. Fermentation, 7(3), 102. https://doi.org/10.3390/fermentation7030102







