Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review
Abstract
1. Introduction
2. Fermentation
3. Fermentation Affects Enzyme Activation to Improve the Nutritional Value of Plant-Based Food
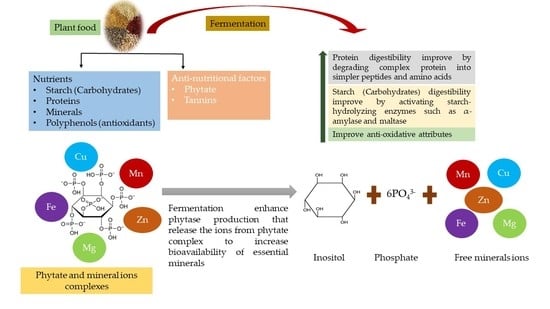
4. Influence of Fermentation on Micronutrients Bioavailability of Plant-Based Foods
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive compounds in legumes: Pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Oladiran, D.A.; Emmambux, N.M. Locally Available African Complementary Foods: Nutritional Limitations and Processing Technologies to Improve Nutritional Quality—A Review. Food Rev. Int. 2020, 1–31. [Google Scholar]
- Skibsted, L.H. Mineral nutrient interaction: Improving bioavailability of calcium and iron. Food Sci. Biotechnol. 2016, 25, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.; Augustine, L.F.; Konapur, A. Food-based interventions to modify diet quality and diversity to address multiple micronutrient deficiency. Front. Public Health 2016, 3, 277. [Google Scholar] [CrossRef]
- Schaffer-Lequart, C.; Lehmann, U.; Ross, A.B.; Roger, O.; Eldridge, A.L.; Ananta, E.; Bietry, M.F.; King, L.R.; Moroni, A.V.; Srichuwong, S.; et al. Whole grain in manufactured foods: Current use, challenges and the way forward. Crit. Rev. Food. Sci. Nutr. 2017, 57, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Raes, K.; Knockaert, D.; Struijs, K.; Van Camp, J. Role of processing on bioaccessibility of minerals: Influence of localization of minerals and anti-nutritional factors in the plant. Trends Food Sci. Technol. 2014, 37, 32–41. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Kruger, J.; Mohamedshah, Z.; Debelo, H.; Taylor, J.R. Insights from in vitro exploration of factors influencing iron, zinc and provitamin A carotenoid bioaccessibility and intestinal absorption from cereals. J. Cereal Sci. 2020, 103126. [Google Scholar] [CrossRef]
- Handa, V.; Kumar, V.; Panghal, A.; Suri, S.; Kaur, J. Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. J. Food Sci. Technol. 2017, 54, 4229–4239. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Kumari, M.; Platel, K. Impact of soaking, germination, fermentation, and thermal processing on the bioaccessibility of trace minerals from food grains. J. Food Process. Preserv. 2020, 44, e14752. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, J.C.; Moon, H.; Yang, J.Y.; Kim, M. Determination of sodium contents in traditional fermented foods in Korea. J. Food Compos. Anal. 2017, 56, 110–114. [Google Scholar] [CrossRef]
- Ansorena, D.; Astiasaran, I. Fermented foods: Composition and health effects. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 649–655. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Keshani. Fermentation of apple juice with a selected yeast strain isolated from the fermented foods of himalayan regions and its organoleptic properties. Front. Microbiol. 2016, 7, 1012. [Google Scholar] [PubMed]
- Narzary, Y.; Brahma, J.; Brahma, C.; Das, S. A study on indigenous fermented foods and beverages of Kokrajhar, Assam, India. J. Ethn. Foods 2016, 3, 284–291. [Google Scholar] [CrossRef]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 503–520. [Google Scholar] [CrossRef]
- Shiferaw Terefe, N.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef] [PubMed]
- Priyodip, P.; Prakash, P.Y.; Balaji, S. Phytases of probiotic bacteria: Characteristics and beneficial aspects. Indian J. Microbiol. 2017, 57, 148–154. [Google Scholar] [CrossRef]
- Greiner, R.; Konietzny, U. Phytase for food application. Food Technol. Biotechnol. 2006, 44. Available online: https://www.ftb.com.hr/images/pdfarticles/2006/April-June/44-125.pdf (accessed on 20 January 2021).
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Afinah, S.; Yazid, A.M.; Anis Shobirin, M.H.; Shuhaimi, M. Phytase: Application in food industry. Int. Food Res. J. 2010, 17, 13–21. Available online: http://www.ifrj.upm.edu.my/17%20(01)%202010/(2)%20IFRJ-2010-13-21%20Anis%20UPM.pdf (accessed on 20 January 2021).
- Song, H.Y.; El Sheikha, A.F.; Hu, D.M. The positive impacts of microbial phytase on its nutritional applications. Trends Food Sci. Technol. 2019, 86, 553–562. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Rollan, G.C.; Gerez, C.L.; LeBlanc, J.G. Lactic fermentation as a strategy to improve the nutritional and functional values of pseudocereals. Front. Nutr. 2019, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Hutkins, R.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 1–13. [Google Scholar]
- El Sheikha, A.F.; Hu, D.M. Molecular techniques reveal more secrets of fermented foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Dhull, S.B.; Punia, S.; Kumar, R.; Kumar, M.; Nain, K.B.; Jangra, K.; Chudamani, C. Solid state fermentation of fenugreek (Trigonella foenum-graecum): Implications on bioactive compounds, mineral content and in vitro bioavailability. J. Food Sci. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Brodmann, T.; Endo, A.; Gueimonde, M.; Vinderola, G.; Kneifel, W.; de Vos, W.M.; Salminen, S.; Gómez-Gallego, C. Safety of novel microbes for human consumption: Practical examples of assessment in the European Union. Front. Microbiol. 2017, 8, 1725. [Google Scholar] [CrossRef]
- Sun, Z.; Yue, Z.; Yang, X.; Hao, X.; Song, M.; Li, L.; Chen, C.; Chu, C.; Li, C. Efficient phytase secretion and phytate degradation by recombinant Bifidobacterium longum JCM 1217. Front. Microbiol. 2019, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Yebra, M.J.; Haros, M.; Monedero, V. Expression of bifidobacterial phytases in Lactobacillus casei and their application in a food model of whole-grain sourdough bread. Int. J. Food Microbiol. 2016, 216, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, E.; Ratisiwi, F.N.; Istiqomah, L.; Sembiring, L.; Febrisiantosa, A. Phytate degrading activities of lactic acid bacteria isolated from traditional fermented food. In AIP Conference Proceedings, Melville, NY, USA, 17 March 2017; AIP Publishing LLC: Melville, NY, USA; p. 020053.
- Saraniya, A.; Jeevaratnam, K. In vitro probiotic evaluation of phytase producing Lactobacillus species isolated from Uttapam batter and their application in soy milk fermentation. J. Food Sci. Technol. 2015, 52, 5631–5640. [Google Scholar] [CrossRef][Green Version]
- Sharma, N.; Kondepudi, K.K.; Gupta, N. Screening of Ethnic Indian Fermented Foods for Effective Phytase Producing Lactic Acid Bacteria for Application in Dephytinization of Phytate Rich Foods. Int. J. Sci. Res. Biol. Sci. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Uslu, F.M.; Kizilkaya, E.G.; Yigittekin, E.S.; Gencoglu, M.; Toroglu, S.; Dincer, S. Phytase characterization and production from Lactobacillus plantarum strain on corn steep liquor. J. Appl. Biol. Sci. 2016, 10, 64–66. Available online: http://www.jabsonline.org/index.php/jabs/article/view/504/507 (accessed on 16 January 2021).
- Sharma, N.; Angural, S.; Rana, M.; Puri, N.; Kondepudi, K.K.; Gupta, N. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 2020, 96, 1–12. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Chaves-López, C.; Serio, A.; Grande-Tovar, C.D.; Cuervo-Mulet, R.; Delgado-Ospina, J.; Paparella, A. Traditional fermented foods and beverages from a microbiological and nutritional perspective: The Colombian heritage. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1031–1048. [Google Scholar] [CrossRef]
- El Hag, M.E.; El Tinay, A.H.; Yousif, N.E. Effect of fermentation and dehulling on starch, total polyphenols, phytic acid content and in vitro protein digestibility of pearl millet. Food Chem. 2002, 77, 193–196. [Google Scholar] [CrossRef]
- Osman, M.A. Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of Lohoh. J. Saudi Soc. Agric. Sci. 2011, 10, 1–6. [Google Scholar] [CrossRef]
- Adejuwon, K.P.; Osundahunsi, O.F.; Akinola, S.A.; Oluwamukomi, M.O.; Mwanza, M. Effect of Fermentation on Nutritional Quality, Growth and Hematological Parameters of Rats Fed Sorghum-Soybean-Orange flesh Sweet Potato Complementary Diet. Food Sci. Nutr. 2021, 9, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Castro-Alba, V.; Lazarte, C.E.; Perez-Rea, D.; Carlsson, N.G.; Almgren, A.; Bergenståhl, B.; Granfeldt, Y. Fermentation of pseudocereals quinoa, canihua, and amaranth to improve mineral accessibility through degradation of phytate. J. Sci. Food Agric. 2019, 99, 5239–5248. [Google Scholar] [CrossRef]
- Mohapatra, D.; Patel, A.S.; Kar, A.; Deshpande, S.S.; Tripathi, M.K. Effect of different processing conditions on proximate composition, anti-oxidants, antinutrients and amino acid profile of grain sorghum. Food Chem. 2019, 271, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Ogawa, Y. Evaluation of protein digestibility of fermented soybeans and changes in biochemical characteristics of digested fractions. J. Funct. Foods. 2019, 52, 640–647. [Google Scholar] [CrossRef]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of fermentation on the protein digestibility and levels of non-nutritive compounds of pea protein concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [CrossRef]
- Pranoto, Y.; Anggrahini, S.; Efendi, Z. Effect of natural and Lactobacillus plantarum fermentation on in-vitro protein and starch digestibilities of sorghum flour. Food Biosci. 2013, 2, 46–52. [Google Scholar] [CrossRef]
- Untersmayr, E.; Jensen-Jarolim, E. The role of protein digestibility and antacids on food allergy outcomes. J. Allergy Clin. Immunol. 2008, 121, 1301–1308. [Google Scholar] [CrossRef]
- Hassan, G.F.; Yusuf, L.; Adebolu, T.T.; Onifade, A.K. Effect of fermentation on mineral and anti-nutritional composition of cocoyam (Colocasia esculenta linn). Sky J. Food Sci. 2015, 4, 42–49. Available online: http://www.skyjournals.org/sjfs/pdf/2015/Jun/Hassan%20et%20al%20pdf.pdf (accessed on 20 January 2021).
- Ahmed, M.I.; Xu, X.; Sulieman, A.A.; Na, Y.; Mahdi, A.A. The effect of fermentation time on in vitro bioavailability of iron, zinc, and calcium of kisra bread produced from koreeb (Dactyloctenium aegyptium) seeds flour. Microchem. J. 2020, 154, 104644. [Google Scholar] [CrossRef]
- Day, C.N.; Morawicki, R.O. Effects of fermentation by yeast and amylolytic lactic acid bacteria on grain sorghum protein content and digestibility. J. Food Qual. 2016, 2018, 1–8. [Google Scholar] [CrossRef]
- Ogu, G.I.; Orjiakor, P.I. Microbiological and nutritional qualities of fermented melon seed shells. Int. J. Life Sci. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Mottaghitalab, M.; Hashemi, M. Effects of microbial fermented sesame meal and enzyme supplementation on the intestinal morphology, microbiota, pH, tibia bone and blood parameters of broiler chicks. Ital. J. Anim. Sci. 2020, 19, 457–467. [Google Scholar] [CrossRef]
- Liang, J.; Han, B.Z.; Nout, M.R.; Hamer, R.J. Effects of soaking, germination and fermentation on phytic acid, total and in vitro soluble zinc in brown rice. Food Chem. 2008, 110, 821–828. [Google Scholar] [CrossRef]
- Towo, E.; Matuschek, E.; Svanberg, U. Fermentation and enzyme treatment of tannin sorghum gruels: Effects on phenolic compounds, phytate and in vitro accessible iron. Food Chem. 2006, 94, 369–376. [Google Scholar] [CrossRef]
- Sarvani, B.H.; Suvarna, V.C.; Kumar, K.H.; Ranadev, P.; Girisha, H.C. Effect of Processing and Fermentation on Functional Properties and on Anti-nutritional Factors in Horse Gram (Macrotyloma uniflorum). Curr. J. Appl. Sci. Technol. 2020, 38–45. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Z.; Gao, Y.; Huang, X.; Zou, Y.; Yang, T. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. J. Food Sci. 2015, 80, H1111–H1119. [Google Scholar] [CrossRef] [PubMed]
- Lara, M.V.; Bonghi, C.; Famiani, F.; Vizzotto, G.; Walker, R.P.; Drincovich, M.F. Stone fruit as biofactories of phytochemicals with potential roles in human nutrition and health. Front. Plant. Sci. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, S.J.; Shyu, Y.T. Antioxidant properties of certain cereals as affected by food-grade bacteria fermentation. J. Biosci. Bioeng. 2014, 117, 449–456. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, 37. [Google Scholar] [CrossRef]
- Mutshinyani, M.; Mashau, M.E.; Jideani, A.I.O. Bioactive compounds, antioxidant activity and consumer acceptability of porridges of finger millet (Eleusine coracana) flours: Effects of spontaneous fermentation. Int. J. Food Prop. 2020, 23, 1692–1710. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, 13145. [Google Scholar] [CrossRef] [PubMed]
- Ihediohanma, N.C. Determination of the glycemic indices of three different cassava granules (Garri) and the effect of fermentation period on their glycemic responses. Pak. J. Nutr. 2011, 10, 6–9. [Google Scholar] [CrossRef]
- Ihekoronye, A.I.; Ngoody, P.O. Tropical Roots and Tubers Crops in Integrated Food Science and Technology for the Tropics; Macmillan: London, UK, 1985; pp. 266–282. [Google Scholar]
- Uchechi, O.N.C.; Esther, B.P.T.; Doobue, M.H. In vitro digestibilities, predicted glycemic index and sensory evaluation of biscuits produced from composite flours of wheat and processed tiger nut. GSC Biol. Pharm. Sci. 2020, 10, 164–172. [Google Scholar]
- Mlotha, V.; Mwangwela, A.M.; Kasapila, W.; Siyame, E.W.; Masamba, K. Glycemic responses to maize flour stiff porridges prepared using local recipes in Malawi. Food Sci. Nutr. 2016, 4, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Chun Ng, C.W.; Ismail, A.F.; Zaini Makhtar, M.M.; Fikri Jamaluddin, M.N.; Tajarudin, H.A. Conversion of food waste via two-stage fermentation to controllable chicken Feed Nutrients by local isolated microorganism. Int. J. Recycl. Org. Waste Agric. 2020, 9, 33–47. [Google Scholar]
- Östman, E.; Granfeldt, Y.; Persson, L.; Björck, I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur. J. Clin. Nutr. 2005, 59, 983–988. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Östman, E.M.; Nilsson, M.; Elmståhl, H.L.; Molin, G.; Björck, I.M.E. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough bread: Starch digestibility and postprandial glycemic response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Chileshe, J.; Talsma, E.F.; Schoustra, S.E.; Borgonjen-Van den Berg, K.J.; Handema, R.; Zwaan, B.J.; Brouwer, I.D. Potential contribution of cereal and milk based fermented foods to dietary nutrient intake of 1-5 years old children in Central province in Zambia. PLoS ONE 2020, 15, e0232824. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Gómez-Gallego, C.; Korhonen, J.; Palo-oja, O.M.; El-Nezami, H.; Kolehmainen, M. Harnessing Microbes for Sustainable Development: Food Fermentation as a Tool for Improving the Nutritional Quality of Alternative Protein Sources. Nutrients 2020, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Bahaciu, G.V.; Nicolae, C.G.; Șuler, A.D.; Segal, R. Germinated and Lactic Fermented Soybean Seeds, a Natural Alternative for Healthy Bones. A Scientific Approach. Bull. UASVM Food Sci. Technol. 2018, 75. [Google Scholar] [CrossRef]
- Makokha, A.O.; Oniang’o, R.K.; Njoroge, S.M.; Kamar, O.K. Effect of traditional fermentation and malting on phytic acid and mineral availability from sorghum (Sorghum bicolor) and finger millet (Eleusine coracana) grain varieties grown in Kenya. Food Nutr. Bull. 2002, 23, 241–245. [Google Scholar] [CrossRef]
- Valencia, S.; Ulf, S.; Ann-Sofie, S.; Ruales, J. Processing of quinoa (Chenopodium quinoa, Willd): Effects on in vitro iron availability and phytate hydrolysis. Int. J. Food Sci. Nutr. 1999, 50, 203–211. [Google Scholar] [CrossRef]
- Castro-Alba, V.; Lazarte, C.E.; Perez-Rea, D.; Sandberg, A.S.; Carlsson, N.G.; Almgren, A.; Bergenståhl, B.; Granfeldt, Y. Effect of fermentation and dry roasting on the nutritional quality and sensory attributes of quinoa. Food Sci. Nutr. 2019, 7, 3902–3911. [Google Scholar] [CrossRef]
- Lopez, H.W.; Duclos, V.; Coudray, C.; Krespine, V.; Feillet-Coudray, C.; Messager, A.; Demigné, C.; Rémésy, C. Making bread with sourdough improves mineral bioavailability from reconstituted whole wheat flour in rats. Nutrition 2003, 19, 524–530. [Google Scholar] [CrossRef]
- Chiş, M.S.; Păucean, A.; Man, S.M.; Bonta, V.; Pop, A.; Stan, L.; Pop, C.R.; Mureşan, V.; Muste, S. Effect of rice flour fermentation with Lactobacillus spicheri DSM 15429 on the nutritional features of gluten-free muffins. Foods 2020, 9, 822. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Bartkiene, E.; Damasius, J.; Paskevicius, A. Phytase activity of lactic acid bacteria and their impact on the solubility of minerals from wholemeal wheat bread. Int. J. Food Sci. Nutr. 2015, 66, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, M.; Pepe, O.; Cirillo, T.; Palomba, S.; Blaiotta, G.; Villani, F. Selection and use of phytate-degrading LAB to improve cereal-based products by mineral solubilization during dough fermentation. J. Food Sci. 2010, 75, M28–M35. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, S.W.; Andlid, T.; Sandberg, A.S. Lactic acid fermentation stimulated iron absorption by Caco-2 cells is associated with increased soluble iron content in carrot juice. Br. J. Nutr. 2006, 96, 705–711. [Google Scholar]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a potential probiotic, Lactobacillus plantarum L7, for the preparation of a rice-based fermented beverage. Front. Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Lazarte, C.E.; Vargas, M.; Granfeldt, Y. Zinc bioavailability in rats fed a plant-based diet: A study of fermentation and zinc supplementation. Food Nutr. Res. 2015, 59, 27796. [Google Scholar]
- Scheers, N.; Rossander-Hulthen, L.; Torsdottir, I.; Sandberg, A.S. Increased iron bioavailability from lactic-fermented vegetables is likely an effect of promoting the formation of ferric iron (Fe3+). Eur. J. Nutr. 2016, 55, 373–382. [Google Scholar] [CrossRef]
- Amritha, G.K.; Dharmaraj, U.; Halami, P.M.; Venkateswaran, G. Dephytinization of seed coat matter of finger millet (Eleusine coracana) by Lactobacillus pentosus CFR3 to improve zinc bioavailability. LWT 2018, 87, 562–566. [Google Scholar] [CrossRef]
- Chawla, P.; Bhandari, L.; Sadh, P.K.; Kaushik, R. Impact of Solid-State Fermentation (Aspergillus oryzae) on Functional Properties and Mineral Bioavailability of Black-Eyed Pea (Vigna unguiculata) Seed Flour. Cereal Chem. 2017, 94, 437–442. [Google Scholar] [CrossRef]
- Khodaii, Z.; Zadeh, M.N.; Kamali, J.; Natanzi, M.M. Enhanced iron absorption from lactic acid fermented bread (an in vivo/ex vivo study). Gene Rep. 2019, 15, 100389. [Google Scholar] [CrossRef]
- Kaur, K.D.; Jha, A.; Sabikhi, L.; Singh, A.K. Significance of coarse cereals in health and nutrition: A review. J. Food Sci. Technol. 2014, 51, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, S.L.; de LeBlanc, A.D.M.; LeBlanc, J.G.; Rollán, G.C. Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res. Int. 2020, 127, 108735. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.M.; G Biliaderis, C. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Panikuttira, B.; O’Shea, N.; Tobin, J.T.; Tiwari, B.K.; O’Donnell, C.P. Process analytical technology for cheese manufacture. Int. J. Food Sci. Technol. 2018, 53, 1803–1815. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant-based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef] [PubMed]
| Plant Food | Phytate Content (%), Dry Weight Basis |
|---|---|
| Almonds | 0.35–9.42 |
| Beans | 0.61–2.38 |
| Brazil nuts | 0.29–6.34 |
| Cashew nuts | 0.19–4.98 |
| Chickpeas | 0.28–1.60 |
| Corn | 0.72–6.39 |
| Eggplant seed | 1.42 |
| Kiwi fruits | 1.34 |
| Lentils | 0.27–1.51 |
| Peas | 0.22–1.22 |
| Rapeseed | 2.50–7.50 |
| Rice | 0.06–8.70 |
| Sesame seed | 1.44–5.36 |
| Soybean | 1.00–10.7 |
| Tomato seed | 1.66 |
| Walnuts | 0.20–6.69 |
| Types of Food | Fermentation Organisms | Results | References |
|---|---|---|---|
| Soymilk | Lactobacillus acidophilus B4496, Lactobacillus bulgaricus CFR2028, Lactobacillus casei B1922, Lactobacillus plantarum B4495 and Lactobacillus fermentum B4655 | Increased calcium and magnesium availability | [79] |
| Soybeans | Lactobacilli | Increased calcium (53.4%), iron (59.56%), magnesium (43.41%) and Zinc (40.87%) levels | [80] |
| Sorghum and finger millet | Natural fermentation | Improved solubility of Fe, Ca, Mg and Mn. | [81] |
| Quinoa seeds | L. plantarum | Iron solubility increased | [82] |
| Quinoa seeds | L. plantarum | Reduced phytic acid content and improved Zn, Fe and Ca availability | [83] |
| Wheat flour | Saccharomyces cerevisiae and natural fermentation | Increased the apparent absorption of Fe and Zn | [84] |
| Rice | Lactobacillus Spicheri DSM 15429 | Improved levels of calcium (2.3-fold), magnesium (1.94-fold), potassium (1.98-fold), copper (2.55-fold), zinc (1.92-fold), and manganese (1.68-fold). | [85] |
| Wheat | Pediococcus pentosaceus strains KTU05-8 and KTU05-9 | Increased solubility of iron, zinc, manganese and calcium and phosphorus (average of 30%). | [86] |
| Carrot | Lactobacillus pentosus FSC1 and Leuconostoc mesenteroides FSC2 | Improved mineral solubility: manganese (2.2–2.5-fold); iron (1.5–1.7-fold); Zinc (1.2-fold); Copper (1-fold). Cellular (Caco-2) uptake of ferrous iron in LAB fermented juice improved by 6 to 7-fold. | [88] |
| Rice | L. plantarum L7 | Increased in the levels of free minerals like sodium, calcium, magnesium, manganese and iron | [89] |
| Cassava | Spontaneous fermentation | Increased bioavailability of zinc | [90] |
| Vegetable mix (carrots, turnips, white cabbage, parsnip, celery and onion) | L. plantarum | Bioavailability of iron increased | [91] |
| Finger millet seed | L. pentosus CFR3 | Increased in the bioavailability and the in vitro bioaccessibility of zinc. | [92] |
| Black-eyed pea | Aspergillus oryzae | In vitro bioaccessibility of iron and zinc after 96 h of fermentation increased from 17.2% to 30.2% and 14.3% to 29.6%, respectively. Uptake of iron and zinc by Caco-2 cells similarly improved from 22% to 32% and 18% to 28%, respectively after fermentation. | [93] |
| Whole meal flour (bread) | L. acidophilus | Increased iron absorption; ferritin formation increased significantly compared to controls in the intestinal cells (in vitro) | [94] |
| Quinoa pasta | L. plantarum CRL 2017 and L. plantarum CRL 1964 | Improved the nutritional status, improve calcium, iron and magnesium levels in the blood | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samtiya, M.; Aluko, R.E.; Puniya, A.K.; Dhewa, T. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation 2021, 7, 63. https://doi.org/10.3390/fermentation7020063
Samtiya M, Aluko RE, Puniya AK, Dhewa T. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation. 2021; 7(2):63. https://doi.org/10.3390/fermentation7020063
Chicago/Turabian StyleSamtiya, Mrinal, Rotimi E. Aluko, Anil Kumar Puniya, and Tejpal Dhewa. 2021. "Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review" Fermentation 7, no. 2: 63. https://doi.org/10.3390/fermentation7020063
APA StyleSamtiya, M., Aluko, R. E., Puniya, A. K., & Dhewa, T. (2021). Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation, 7(2), 63. https://doi.org/10.3390/fermentation7020063