Effect of the Addition of Non-Saccharomyces at First Alcoholic Fermentation on the Enological Characteristics of Cava Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Winemaking Process
2.2. Enological Analysis
2.3. Protein Analysis
2.3.1. Total Protein Concentration Determined by UV Spectrophotometric Method
2.3.2. Wine Protein Composition Evaluated by SDS-PAGE
2.4. Foamability
2.5. GC-MS Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Enological Analysis
3.2. Protein Analysis
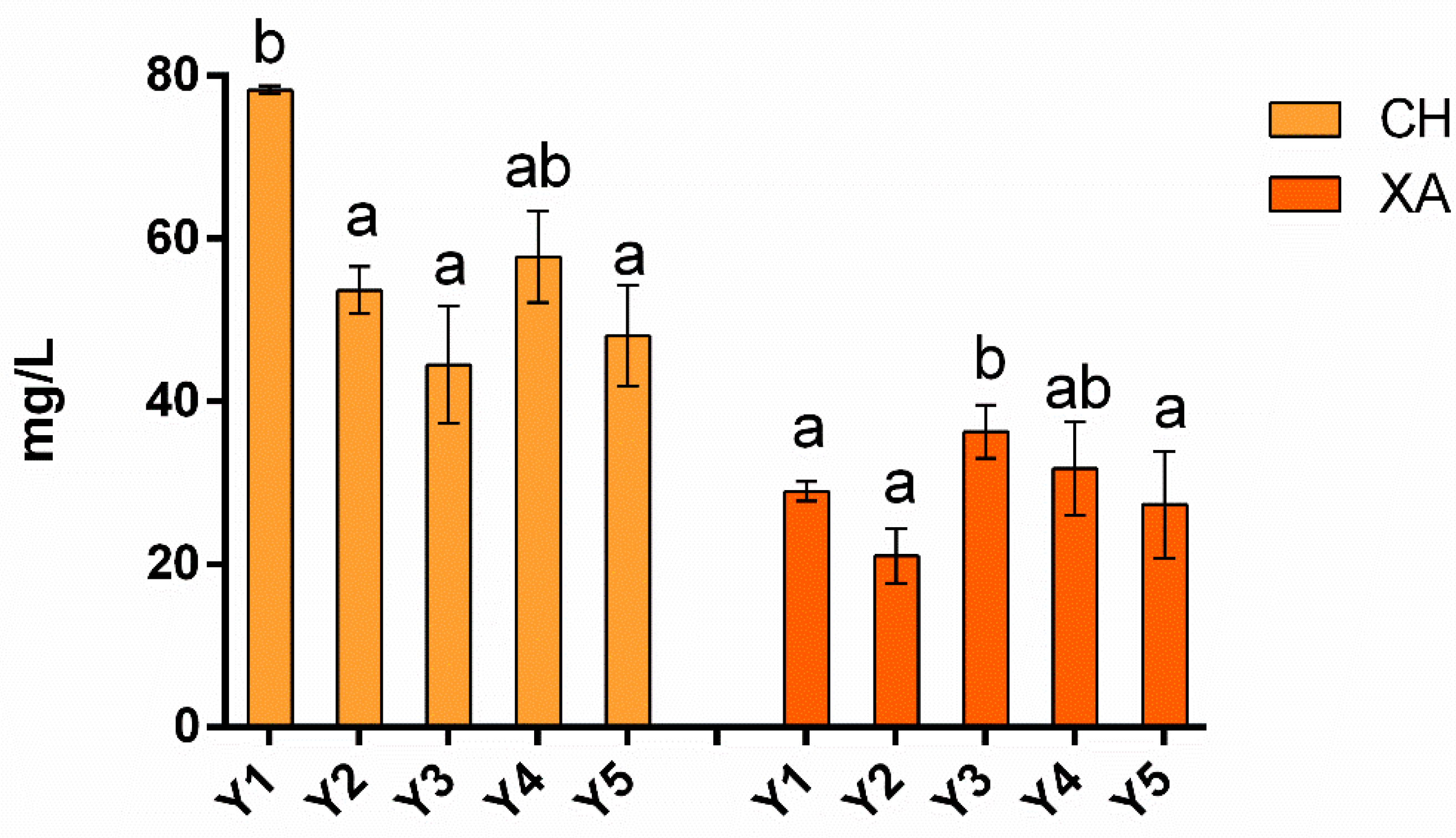
3.2.1. Total Protein Concentration Determined by UV-Visible Spectrophotometry
3.2.2. Wine Protein Composition Evaluated by SDS-PAGE
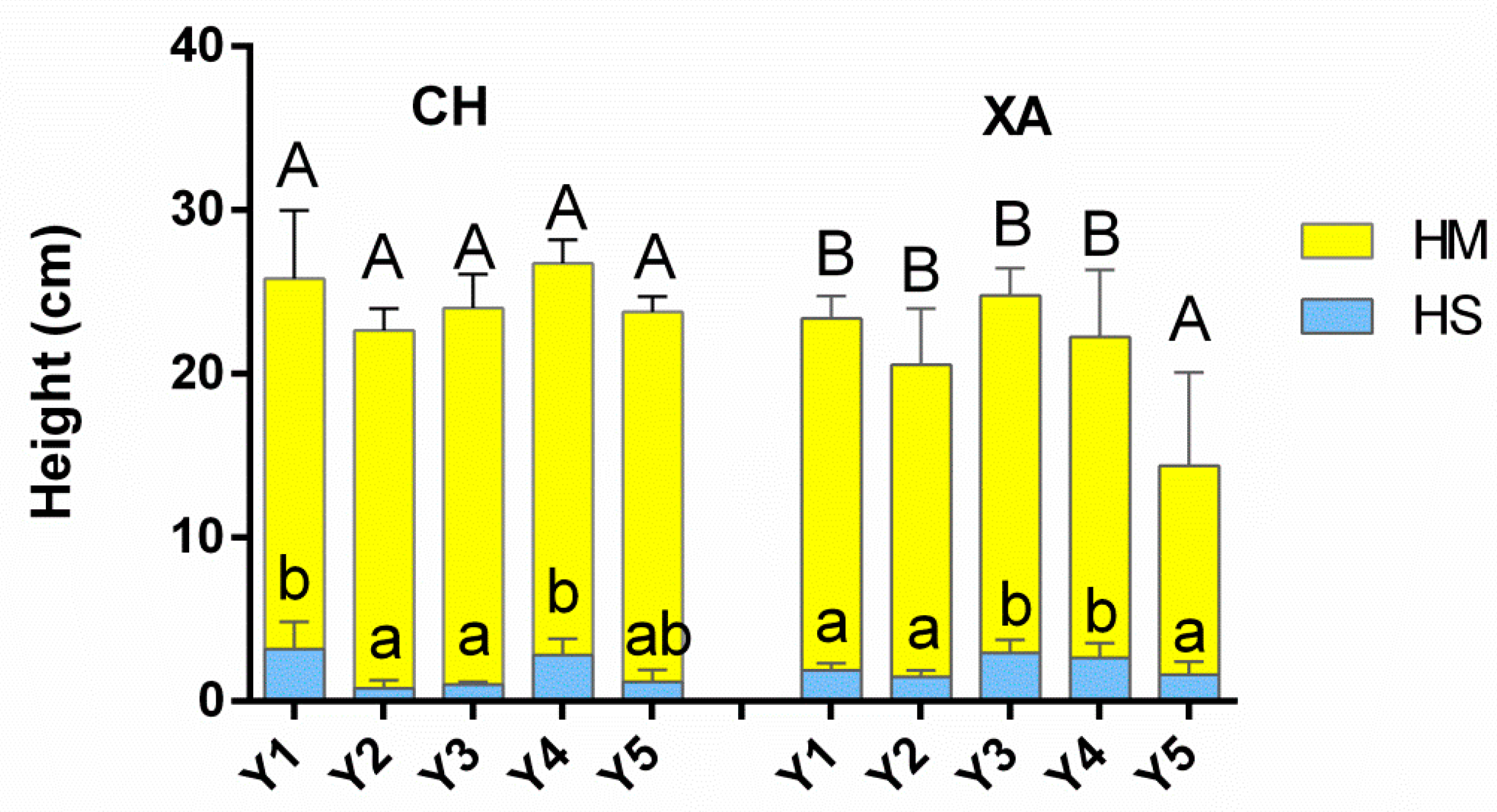
3.3. Foamability
3.4. Volatile Composition
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ivit, N.N.; Kemp, B. The Impact of Non-Saccharomyces Yeast on Traditional Method Sparkling Wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Schizosaccharomyces on winemaking. Appl. Microbiol. Biotechnol. 2019, 103, 4291–4312. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of non-saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef]
- Comitini, F.; Capece, A.; Ciani, M.; Romano, P. New insights on the use of wine yeasts. Curr. Opin. Food Sci. 2017, 13, 44–49. [Google Scholar] [CrossRef]
- Benito, Á.; Jeffares, D.; Palomero, F.; Calderón, F.; Bai, F.-Y.; Bähler, J.; Benito, S. Selected Schizosaccharomyces pombe Strains Have Characteristics That Are Beneficial for Winemaking. PLoS ONE 2016, 11, e0151102. [Google Scholar] [CrossRef]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J. Making natural sparkling wines with non-Saccharomyces yeasts. Eur. Food Res. Technol. 2018, 244. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; Gonzalez, C.; Calderón, F.; Suárez-Lepe, J. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for secondary fermentation in sparkling wine production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.-M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2014, 240. [Google Scholar] [CrossRef]
- Medina-Trujillo, L.; González-Royo, E.; Sieczkowski, N.; Heras, J.; Canals, J.M.; Zamora, F. Effect of sequential inoculation (Torulaspora delbrueckii/Saccharomyces cerevisiae) in the first fermentation on the foaming properties of sparkling wine. Eur. Food Res. Technol. 2017, 243, 681–688. [Google Scholar] [CrossRef]
- OIV. International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis. In Paris, France. 2020. Volumes 1 and 2. Available online: http://www.oiv.int (accessed on 7 May 2020).
- Silvestri, A.C.; Sabatier, J.; Ducruet, J. A new method of protein extraction from red wines. J. Int. Sci. Vigne Vin. 2013, 47, 213–220. [Google Scholar] [CrossRef]
- Maujean, A.; Poinsaut, P.; Dantan, H.; Brissonnet, F.; Cossiez, E. Etude de la tenue et de la qualité de mousse des vins effervescents. II: Mise au point d’une technique de mesure de la moussabilité de la tenue et de la stabilité de la mousse des vins effervescents. Bull. OIV 1990, 63, 405–427. [Google Scholar]
- Torrens, J.; Riu-Aumatell, M.; López-Tamames, E.; Buxaderas, S. Volatile Compounds of Red and White Wines by Headspace-Solid-Phase Microextraction Using Different Fibers. J. Chromatogr. Sci. 2004, 42, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Mislata, A.M.; Puxeu, M.; Tomás, E.; Nart, E.; Ferrer-Gallego, R. Influence of the oxidation in the aromatic composition and sensory profile of Rioja red aged wines. Eur. Food Res. Technol. 2020, 246, 1167–1181. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Cilindre, C.; Liger-Belair, G.; Villaume, S.; Jeandet, P.; Marchal, R. Foaming properties of various Champagne wines depending on several parameters: Grape variety, aging, protein and CO2 content. Anal. Chim. Acta 2010, 660, 164–170. [Google Scholar] [CrossRef]
- Dambrouck, T.; Marchal, R.; Marchal-Delahaut, L.; Parmentier, M.; Maujean, A.; Jeandet, P. Immunodetection of proteins from grapes and yeast in a white wine. J. Agric. Food Chem. 2003, 51, 2727–2732. [Google Scholar] [CrossRef]
- Mostert, T.T.; Divol, B. Investigating the proteins released by yeasts in synthetic wine fermentations. Int. J. Food Microbiol. 2014, 171, 108–118. [Google Scholar] [CrossRef]
- Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Buxaderas, S.; de la Torre-Boronat, M.d.C. Influence of Variety and Aging on Foaming Properties of Cava (Sparkling Wine). 2. J. Agric. Food Chem. 1997, 45, 2520–2525. [Google Scholar] [CrossRef]
- Vanrell, G.; Canals, R.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of the use of bentonite as a riddling agent on foam quality and protein fraction of sparkling wines (Cava). Food Chem. 2007, 104, 148–155. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Carrascosa, A.; Barcenilla, J.; Pozo-Bayón, M.; Polo, M. Autolytic capacity and foam analysis as additional criteria for the selection of yeast strains for sparkling wine production. Food Microbiol. 2001, 18, 183–191. [Google Scholar] [CrossRef]
- Condé, B.C.; Bouchard, E.; Culbert, J.A.; Wilkinson, K.L.; Fuentes, S.; Howell, K.S. Soluble Protein and Amino Acid Content Affects the Foam Quality of Sparkling Wine. J. Agric. Food Chem. 2017, 65, 9110–9119. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; González-Marco, A.; Jiḿenez-Moreno, N. Evolution of esters in aged Chardonnay wines obtained with different vinification methods. J. Sci. Food Agric. 2009, 89, 2446–2451. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Tao, Y.-S. Active volatiles of cabernet sauvignon wine from Changli County. Health 2009, 01, 176–182. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Lambrechts, M.; Pretorius, I. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol Vitic 2000, 21. [Google Scholar] [CrossRef]

| CHARDONNAY | ||||||
| Alcoholic Strength | Volatile Acidity | Abs. 420 nm | Abs. 520 nm | Abs. 620 nm | Color Intensity | |
| BW | ||||||
| Y1 | 10.1 a ± 0.0 | 0.40 c ± 0.00 | 0.099 a ± 0.005 | 0.036 ab ± 0.002 | 0.011 ab ± 0.003 | 0.15 a ± 0.01 |
| Y2 | 9.9 a ± 0.3 | 0.38 bc ± 0.00 | 0.179 a ± 0.098 | 0.047 b ± 0.002 | 0.013 b ± 0.001 | 0.24 a ± 0.10 |
| Y3 | 10.1 a ± 0.0 | 0.52 d ± 0.01 | 0.090 a ± 0.012 | 0.023 a ± 0.006 | 0.006 a ± 0.002 | 0.12 a ± 0.02 |
| Y4 | 10.5 a ± 0.6 | 0.33 a ± 0.01 | 0.118 a ± 0.011 | 0.035 ab ± 0.006 | 0.012 ab ± 0.000 | 0.17 a ± 0.02 |
| Y5 | 9.7 a ± 0.0 | 0.37 b ± 0.01 | 0.122 a ± 0.023 | 0.038 ab ± 0.008 | 0.013 b ± 0.001 | 0.17 a ± 0.03 |
| 18M | ||||||
| Y1 | 11.3 c ± 0.0 | 0.54 b ± 0.02 | 0.146 e ± 0.001 | 0.065 c ± 0.007 | 0.029 c ± 0.001 | 0.24 c ± 0.01 |
| Y2 | 11.2 b ± 0.0 | 0.48 ab ± 0.04 | 0.123 d ± 0.001 | 0.036 b ± 0.001 | 0.019 abc ± 0.001 | 0.18 b ± 0.00 |
| Y3 | 11.5 e ± 0.0 | 0.72 c ± 0.01 | 0.110 b ± 0.000 | 0.023 a ± 0.001 | 0.007 a ± 0.001 | 0.14 a ± 0.01 |
| Y4 | 11.1 a ± 0.0 | 0.44 a ± 0.01 | 0.106 a ± 0.001 | 0.037 b ± 0.001 | 0.022 bc ± 0.001 | 0.16 b ± 0.01 |
| Y5 | 11.3 c ± 0.0 | 0.41 a ± 0.01 | 0.119 c ± 0.001 | 0.030 ab ± 0.001 | 0.015 ab ± 0.007 | 0.16 b ± 0.01 |
| XAREL.LO | ||||||
| Alcoholic Strength | Volatile Acidity | Abs. 420 nm | Abs. 520 nm | Abs. 620 nm | Color Intensity | |
| BW | ||||||
| Y1 | 9.9 a ± 0.0 | 0.26 a ± 0.02 | 0.075 a ± 0.013 | 0.016 ab ± 0.001 | 0.004 a ± 0.001 | 0.09 a ± 0.01 |
| Y2 | 9.9 a ± 0.0 | 0.26 a ± 0.00 | 0.064 a ± 0.006 | 0.015 a ± 0.002 | 0.005 a ± 0.002 | 0.08 a ± 0.01 |
| Y3 | 11.7 d ± 0.0 | 0.31 b ± 0.00 | 0.065 a ± 0.001 | 0.023 b ± 0.001 | 0.006 a ± 0.001 | 0.09 a ± 0.00 |
| Y4 | 10.0 b ± 0.0 | 0.25 a ± 0.00 | 0.067 a ± 0.005 | 0.017 ab ±0.004 | 0.005 a ± 0.001 | 0.09 a ± 0.01 |
| Y5 | 10.1 c ± 0.0 | 0.29 ab ± 0.01 | 0.081 a ± 0.001 | 0.019 ab ± 0.001 | 0.004 a ± 0.001 | 0.10 a ± 0.00 |
| 18M | ||||||
| Y1 | 11.1 b ± 0.0 | 0.30 b ± 0.01 | 0.075 bc ± 0.000 | 0.017 b ± 0.001 | 0.008 c ± 0.000 | 0.10 c ± 0.01 |
| Y2 | 11.4 e ± 0.0 | 0.29 b ± 0.01 | 0.074 b ± 0.001 | 0.018 b ± 0.001 | 0.003 a ± 0.001 | 0.09 b ± 0.00 |
| Y3 | 11.3 d ± 0.0 | 0.35 c ± 0.01 | 0.065 a ± 0.001 | 0.010 a ± 0.000 | 0.002 a ± 0.000 | 0.08 a ± 0.1 |
| Y4 | 11.0 a ± 0.0 | 0.24 a ± 0.00 | 0.078 c ± 0.001 | 0.016 b ± 0.001 | 0.005 b ± 0.000 | 0.10 c ± 0.1 |
| Y5 | 11.2 c ± 0.0 | 0.30 b ± 0.01 | 0.087 d ± 0.001 | 0.027 c ± 0.000 | 0.015 d ± 0.001 | 0.13 d ± 0.01 |
| CH Y1 (Bands) | V12 | V13 | V14 | V15 | V16 | V17 | ||
| MW (Kda) | 96.1 | 59.4 | 28.6 | 26.1 | 22.8 | 19.4 | ||
| % | 12.8 | 2.7 | 9.1 | 7.4 | 63 | 5.1 | ||
| CH Y2 (Bands) | V30 | V19 | V20 | V21 | V22 | V23 | ||
| MW (Kda) | 97.7 | 64.3 | 27.2 | 24.9 | 22.1 | 18.6 | ||
| % | 10.1 | 2.7 | 11.6 | 25.2 | 48.4 | 2 | ||
| CH Y3 (Bands) | V19 | V20 | V21 | V22 | V23 | V24 | V25 | V26 |
| MW (Kda) | 66.7 | 57.4 | 47.2 | 40.4 | 32.7 | 26.4 | 21 | 18 |
| % | 14.6 | 12.8 | 5.7 | 5.7 | 7.2 | 7.2 | 32.2 | 2.2 |
| CH Y4 (Bands) | V11 | V12 | V13 | V14 | V15 | V16 | V17 | V18 |
| MW (Kda) | 74.8 | 53.9 | 36.3 | 28.2 | 23.4 | 20.2 | 18.7 | 15.9 |
| % | 28.8 | 4.5 | 2.4 | 12.2 | 42.9 | 3.7 | 4.3 | 1.2 |
| CH Y5 (Bands) | V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 |
| MW (Kda) | 68.5 | 48.7 | 35.9 | 26.8 | 23.1 | 20.3 | 18 | 16.2 |
| % | 20.2 | 2.9 | 5.7 | 25.1 | 30 | 3.7 | 3.2 | 9.3 |
| XA Y1 (Bands) | V29 | V30 | V31 | V32 | ||||
| MW (Kda) | 68.4 | 26.9 | 21.4 | 18.2 | ||||
| % | 27.6 | 33.9 | 26.5 | 12.1 | ||||
| XA Y2 (Bands) | V22 | V23 | V24 | V25 | ||||
| MW (Kda) | 73.2 | 29.5 | 27.6 | 25.5 | ||||
| % | 43.9 | 26.8 | 19.5 | 9.8 | ||||
| XA Y3 (Bands) | V11 | V12 | V13 | V14 | V15 | |||
| MW (Kda) | 92.1 | 32.9 | 28.9 | 25.3 | 22.9 | |||
| % | 10.5 | 14.4 | 3.2 | 70.4 | 1.5 | |||
| XA Y4 (Bands) | V1 | V2 | V3 | V4 | V5 | |||
| MW (Kda) | 91.6 | 31.7 | 29.5 | 27.6 | 23.9 | |||
| % | 24.9 | 3.3 | 38.2 | 15.4 | 18.3 | |||
| XA Y5 (Bands) | V25 | V26 | V27 | V28 | V29 | |||
| MW (Kda) | 92.2 | 31.5 | 28.7 | 25 | 20.8 | |||
| % | 28.1 | 50.2 | 11.3 | 6.1 | 4.3 | |||
| 10% < | < 80% |
| CHARDONNAY | XAREL.LO | |||||||||
| Sample BW | Y1 | Y2 | Y3 | Y4 | Y5 | Y1 | Y2 | Y3 | Y4 | Y5 |
| ESTERS | 26,557 b ± 387 | 29,560 b ± 311 | 17,351 a ± 209 | 29,375 b ± 357 | 29,256 b ± 825 | 30,854 ± 1266 | 32,710 b ± 1173 | 21,101 a ± 101 | 29,373 ab ± 2103 | 30,109 b ± 3834 |
| Ethyl butyrate | 164 b ± 2 | 208 b ± 8 | 88 a ± 11 | 210 b ± 17 | 201 b ± 16 | 599 ab ± 94 | 669 b ± 127 | 329 a ± 2 | 391 ab ± 26 | 411 ab ± 81 |
| Ethyl isovalerate | 267 b ± 4 | 233 b ± 0 | 111 a ± 19 | 265 b ± 35 | 241 b ± 8 | 279 b ± 13 | 278 ab ± 15 | 161 a ± 1 | 179 ab ± 19 | 270 ab ± 60 |
| Ethyl hexanoate | 5143 b ± 251 | 5272 b ± 134 | 2182 a ± 217 | 5326 b ± 704 | 5265 b ± 160 | 7149 b ± 244 | 7988 b ± 100 | 3755 a ± 14 | 6730 ab ± 336 | 7171 b ± 1283 |
| Ethyl octanoate | 14,015 b ± 172 | 15,199 b ± 56 | 8683 a ± 1498 | 15,457 b ± 41 | 15,060 b ± 192 | 14,242 b ± 464 | 15,093 b ± 738 | 9426 a ± 15 | 13,970 b ± 815 | 13,765 b ± 1901 |
| Ethyl decanoate | 6825 ab ± 286 | 8481 b ± 222 | 6027 a ± 399 | 7903 ab ± 1034 | 8321 b ± 463 | 7939 a ± 412 | 8065 a ± 634 | 7003 a ± 67 | 7602 a ± 78 | 7895 a ± 477 |
| Ethyl dodecanoate | 123 a ± 16 | 152 ab ± 5 | 243 b ± 47 | 198 ab ± 36 | 153 ab ± 4 | 621 a ± 42 | 595 a ± 79 | 413 a ± 3 | 468 a ± 119 | 556 a ± 35 |
| Diethyl succinate | 16 a ± 1 | 14 a ± 2 | 18 a ± 1 | 14 a ± 2 | 14 a ± 1 | 24 ab ± 3 | 21 a ± 4 | 13 a ± 0 | 34 b ± 4 | 36 b ± 3834 |
| ACETATES | 13,319 b ± 229 | 13,029 b ± 274 | 9219 a ± 319 | 14,189 b ± 1565 | 12,988 b ± 426 | 17,177 a ± 1237 | 16,816 a ± 1096 | 14,721 a ± 685 | 13,728 a ± 1091 | 16,104 a ± 783 |
| Ethyl acetate | 2809 a ± 45 | 3060 a ± 107 | 2902 a ± 67 | 2928 a ± 151 | 3016 a ± 150 | 4819 b ± 273 | 4492 b ± 49 | 4801 b ± 67 | 3195.3 a ± 250 | 4234 b ± 254 |
| Isoamyl acetate | 10,270 b ± 185 | 9741 b ± 158 | 6190 a ± 249 | 11,043 b ± 1412 | 9745 b ± 264 | 12,137 b ± 934 | 12,063 b ± 101 | 9724 a ± 621 | 10,342.3 ab ± 826 | 11,603 b ± 517 |
| Hexyl acetate | 81 bc ± 0 | 88 c ± 2 | 43 a ± 0 | 71 b ± 1 | 75 b ± 5 | 62 b ± 9 | 65 b ± 3 | 41 a ± 0 | 53.4 ab ± 4 | 58 ab ± 3 |
| 2-phenylethyl acetate | 159 c ± 1 | 140 b ± 7 | 83 a ± 2 | 146 bc ± 1 | 151 bc ± 7 | 159 a ± 20 | 195 a ± 31 | 154 a ± 3 | 137 a ± 11 | 208 a ± 10 |
| ALCOHOLS | 3703 ab ± 59 | 3217 a ± 150 | 3823 ab ± 9 | 3944 b ± 132 | 3682 ab ± 339 | 7611 a ± 4 | 7671 a ± 147 | 8166 a ± 189 | 8282 a ± 711 | 7965 a ± 341 |
| Isoamyl alcohol | 3147 ab ± 54 | 2705 a ± 139 | 3291 ab ± 9 | 3408 b ± 120 | 3125 ab ± 306 | 6609 a ± 55 | 6592 a ± 137 | 7014 b ± 176 | 7146 b ± 623 | 6914 ab ± 280 |
| Isobutanol | 117 b ± 1 | 105 a ± 2 | 103 a ± 2 | 95 a ± 1 | 101 a ± 5 | 78 ab ± 1 | 81 ab ± 8 | 64 a ± 1 | 100 c ± 1 | 85 bc ± 5 |
| Benzyl alcohol | 2 a ± 0 | 2 cd ± 0 | 2 b ± 0 | 2 c ± 0 | 3 d ± 0 | 5 a ± 0 | 6 ab ± 0 | 5 a ± 0 | 6 ab ± 0 | 7 b ± 0 |
| 2-phenylethyl alcohol | 437 a ± 6 | 405 a ± 16 | 426 a ± 1 | 439 a ± 11 | 452 a ± 29 | 919 a ± 50 | 991 a ± 19 | 1082 b ± 14 | 1030 b ± 86 | 959 a ± 56 |
| FATTY ACIDS | 924 b ± 19 | 1016 b ± 18 | 543 a ± 3 | 963 b ± 12 | 1032 b ± 55 | 1362 b ± 31 | 1626 b ± 131 | 819 a ± 4 | 1353 b ± 110 | 1640 b ± 102 |
| Hexanoic acid | 169 b ± 10 | 168 b ± 2 | 90 a ± 0 | 151 b ± 1 | 172 b ± 5 | 223 b ± 2 | 260 bc ± 8 | 117 a ± 1 | 223 b ± 21 | 282 c ± 22 |
| Octanoic acid | 511 b ± 3 | 550 b ± 12 | 273 a ± 2 | 516 b ± 15 | 561 b ± 29 | 786 b ± 17 | 930 b ± 69 | 461 a ± 4 | 790 b ± 62 | 944 b ± 56 |
| Decanoic acid | 243 b ± 6 | 299 c ± 3 | 180 a ± 1 | 296 c ± 4 | 299 c ± 19 | 353 ab ± 16 | 435 b ± 54 | 242 a ± 0 | 339 ab ± 37 | 414 b ± 24 |
| TOTAL AROMAS | 44,503 b ± 538 | 46,824 b ± 138 | 30,937 a ± 2410 | 48,471 b ± 1327 | 46,957 b ± 5 | 57,004 ab ± 2538 | 58,823 b ± 2547 | 44,808 a ± 601 | 52,737 ab ± 4015 | 55,819 ab ± 5061 |
| CHARDONNAY | XAREL.LO | |||||||||
| Sample 18M | Y1 | Y2 | Y3 | Y4 | Y5 | Y1 | Y2 | Y3 | Y4 | Y5 |
| ESTERS | 11,917 a ± 140 | 15,320 c ± 13 | 10,801 a ± 107 | 11,867 a ± 438 | 13,842 b ± 487 | 13,274 d ± 118 | 13,837 d ± 192 | 9072 a ± 297 | 10,390 b ± 134 | 11,931 c ± 12 |
| Ethyl butyrate | 305 b ± 3 | 437 d ± 6 | 258 a ± 14 | 283 ab ± 3 | 370 c ± 2. | 417 d ± 0 | 423 d ± 4 | 237 a ± 0 | 328 b ± 1 | 387 c ± 7 |
| Ethyl isovalerate | 64 a ± 1 | 113 bc ± 2 | 71 a ± 0 | 110 b ± 4 | 126 c ± 7 | 67 b ± 0 | 89 c ± 2 | 51 a ± 1 | 64 b ± 1 | 120 d ± 3 |
| Ethyl hexanoate | 3452 a ± 89 | 4261 c ± 39 | 3240 a ± 71 | 3271 a ± 42 | 3792 b ± 47 | 4110 cd ± 49 | 4353 d ± 65 | 2505 a ± 103 | 3625 b ± 66 | 3875 bc ± 45 |
| Ethyl octanoate | 6105 ab ± 19 | 7805 c ± 35 | 5351 a ± 34 | 6044 a ± 347 | 7013 bc ± 390 | 6761 d ± 11 | 7549 e ± 238 | 4562 a ± 120 | 5370 b ± 29 | 6121 c ± 48 |
| Ethyl decanoate | 1938 a ± 27 | 2649 d ± 13 | 1824 a ± 13 | 2112 b ± 41 | 2475 c ± 40 | 1854 c ± 154 | 1353 b ± 20 | 1660 bc ± 70 | 943 a ± 35 | 1369 b ± 4 |
| Ethyl dodecanoate | 19 b ± 0 | 18 a ± 0 | 20 c ± 0 | 25 d ± 0 | 53 e ± 0 | 38 b ± 1 | 37 b ± 0 | 20 a ± 0 | 37 b ± 2 | 37 b ± 0 |
| Diethyl succinate | 33 c ± 1 | 36 d ± 0 | 37 d ± 1 | 21 b ± 0 | 12 a ± 0 | 25 a ± 0 | 33 b ± 1 | 36 b ± 2 | 24 a ± 1 | 23 a ± 0 |
| ACETATES | 9613 b ± 2 | 12,472 d ± 28 | 13,010 e ± 268 | 9086 a ± 90 | 10,955 c ± 701 | 9724 b ± 85 | 10,018 bc ± 172 | 8491 a ± 96 | 8132 a ± 38 | 10,387 c ± 31 |
| Ethyl acetate | 5715 b ± 68 | 7168 b ± 23 | 8496 c ± 170 | 4554 a ± 15 | 5853 b± 24 | 5703 b ± 6 | 5490 b ± 75 | 5432 b ± 35 | 4500 a ± 16 | 5402 b ± 37 |
| Isoamyl acetate | 3776 a ± 67 | 5136 c ± 42 | 4399 b ± 92 | 4430 b ± 73 | 4961 c ± 37 | 3931 c ± 78 | 4405 d ± 95 | 2978 a ± 61 | 3535 b ± 21 | 4828 e ± 5 |
| Hexyl acetate | 104 bc ± 2 | 143 d ± 2 | 95 b ± 5 | 67 a ± 1 | 117 c ± 9 | 51 b ± 1 | 67 c ± 1 | 28 a ± 0 | 51 b ± 0 | 97 d ± 1 |
| 2-phenylethyl acetate | 17 a ± 1 | 24 ab ± 6 | 20 a ± 0 | 34 b ± 0 | 23 ab ± 0 | 39 a ± 0 | 56 d ± 1 | 52 c ± 0 | 46 b ± 0 | 59 e ± 0 |
| ALCOHOLS | 5437 b ± 88 | 6353 c ± 7 | 7507 d ± 180 | 4964 a ± 24 | 5621 b ± 39 | 6153 b ± 16 | 6063 b ± 105 | 6175 b ± 78 | 6066 b ± 14 | 5657 a ± 25 |
| Isoamyl alcohol | 4879 b ± 77 | 5780 c ± 9 | 4806 b ± 15 | 4465 a ± 21 | 5110 b ± 18 | 5402 c ± 16 | 5241 bc ± 94 | 4960 a ± 45 | 5089 ab ± 7 | 4960 a ± 24 |
| Isobutanol | 114 ab ± 5 | 146 cd ± 2 | 168 d ± 13 | 97 a ± 0 | 134 bc ± 1 | 135 bc ± 0 | 132 b ± 1 | 99 a ± 5 | 147 cd ± 4 | 149 d ± 2 |
| Benzyl alcohol | 2 a ± 0 | 4 c ± 0 | 6 e ± 0 | 5 d ± 0 | 3 b ± 0 | 4 b ± 0 | 4 c ± 0 | 4 a ± 0 | 5 d ± 0 | 4 a ± 0 |
| 2-phenylethyl alcohol | 441 b ± 6 | 424 ab ± 0 | 527 c ± 15 | 396 ab ± 3 | 374 a ± 3 | 611 b ± 0 | 686 c ± 10 | 1111 e ± 30 | 826 d ± 19 | 544 a ± 1 |
| FATTY ACIDS | 845 b ± 3 | 988 d ± 2 | 625 a ± 14 | 877 c ± 4 | 853 bc ± 0 | 875 b ± 5 | 1068 d ± 4 | 709 a ± 10 | 945 c ± 14 | 971 c ± 9 |
| Hexanoic acid | 214 c ± 1 | 243 d ± 1 | 150 a ± 0 | 201 b ± 3 | 210 c ± 0 | 223 b ± 3 | 259 c ± 2 | 144 a ± 3 | 231 b ± 7 | 236 b ± 4 |
| Octanoic acid | 497 b ± 3 | 589 d ± 0 | 352 a ± 7 | 525 c ± 1 | 509 b ± 0 | 511 b ± 0 | 628 e ± 11 | 404 a ± 2 | 554 c ± 2 | 584 d ± 0 |
| Decanoic acid | 133 a ± 1 | 156 b ± 1 | 122 a ± 6 | 151 b ± 1 | 133 a ± 0 | 141 a ± 2 | 181 c ± 5 | 160 b ± 5 | 160 b ± 5 | 151 ab ± 5 |
| TOTAL AROMAS | 27,811 a ± 47 | 35,134 c ± 9 | 31,943 b ± 569 | 26,794 a ± 556 | 31,271 b ± 596 | 30,026 d ± 225 | 30,987 d ± 89 | 24,446 a ± 481 | 25,534 b ± 200 | 28,946 c ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mislata, A.M.; Puxeu, M.; Andorrà, I.; Espligares, N.; de Lamo, S.; Mestres, M.; Ferrer-Gallego, R. Effect of the Addition of Non-Saccharomyces at First Alcoholic Fermentation on the Enological Characteristics of Cava Wines. Fermentation 2021, 7, 64. https://doi.org/10.3390/fermentation7020064
Mislata AM, Puxeu M, Andorrà I, Espligares N, de Lamo S, Mestres M, Ferrer-Gallego R. Effect of the Addition of Non-Saccharomyces at First Alcoholic Fermentation on the Enological Characteristics of Cava Wines. Fermentation. 2021; 7(2):64. https://doi.org/10.3390/fermentation7020064
Chicago/Turabian StyleMislata, Ana María, Miquel Puxeu, Immaculada Andorrà, Noelia Espligares, Sergi de Lamo, Montserrat Mestres, and Raúl Ferrer-Gallego. 2021. "Effect of the Addition of Non-Saccharomyces at First Alcoholic Fermentation on the Enological Characteristics of Cava Wines" Fermentation 7, no. 2: 64. https://doi.org/10.3390/fermentation7020064
APA StyleMislata, A. M., Puxeu, M., Andorrà, I., Espligares, N., de Lamo, S., Mestres, M., & Ferrer-Gallego, R. (2021). Effect of the Addition of Non-Saccharomyces at First Alcoholic Fermentation on the Enological Characteristics of Cava Wines. Fermentation, 7(2), 64. https://doi.org/10.3390/fermentation7020064







