Utilizing Coffee Pulp and Mucilage for Producing Alcohol-Based Beverage
Abstract
1. Introduction
2. Materials and Methods
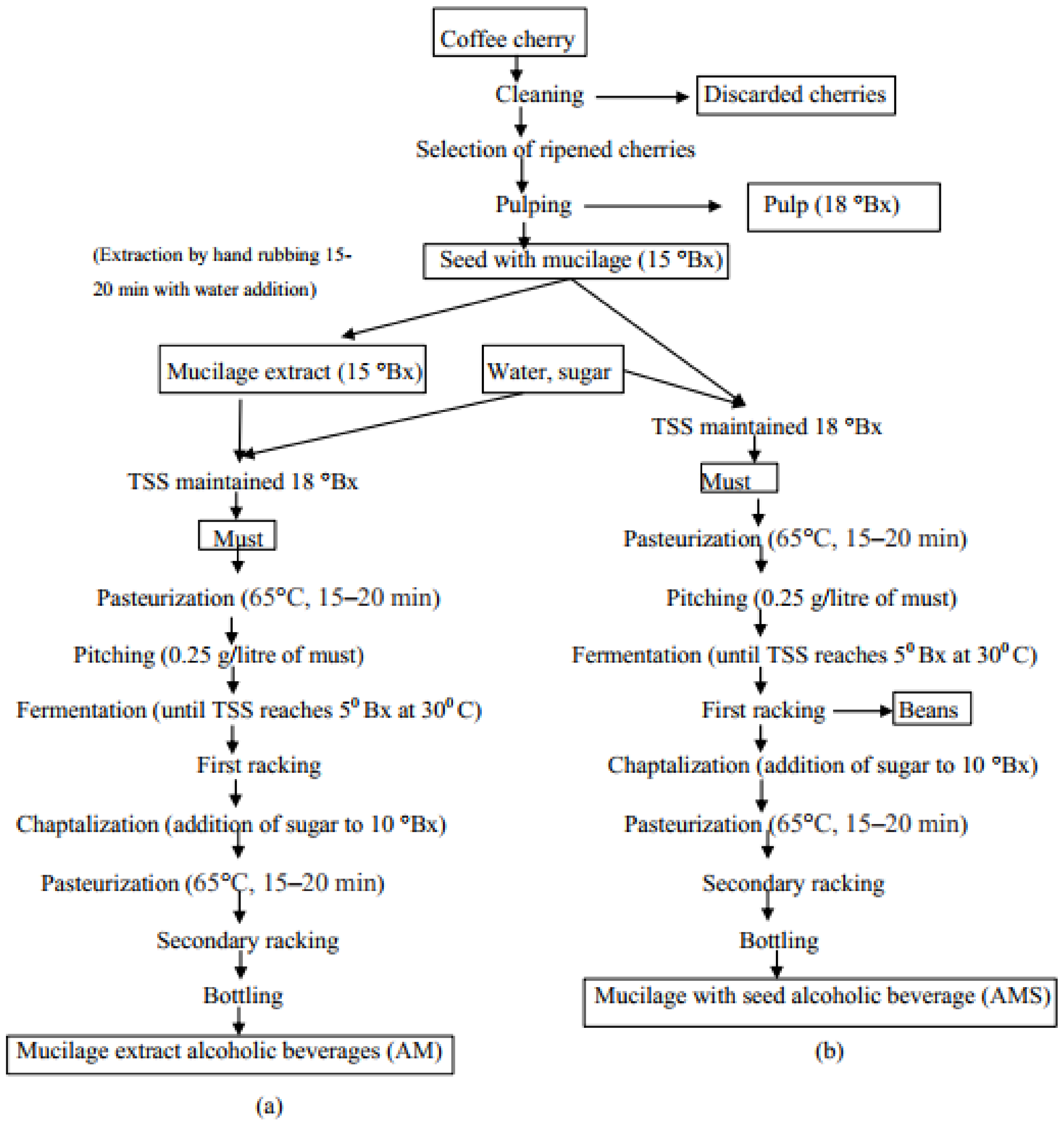
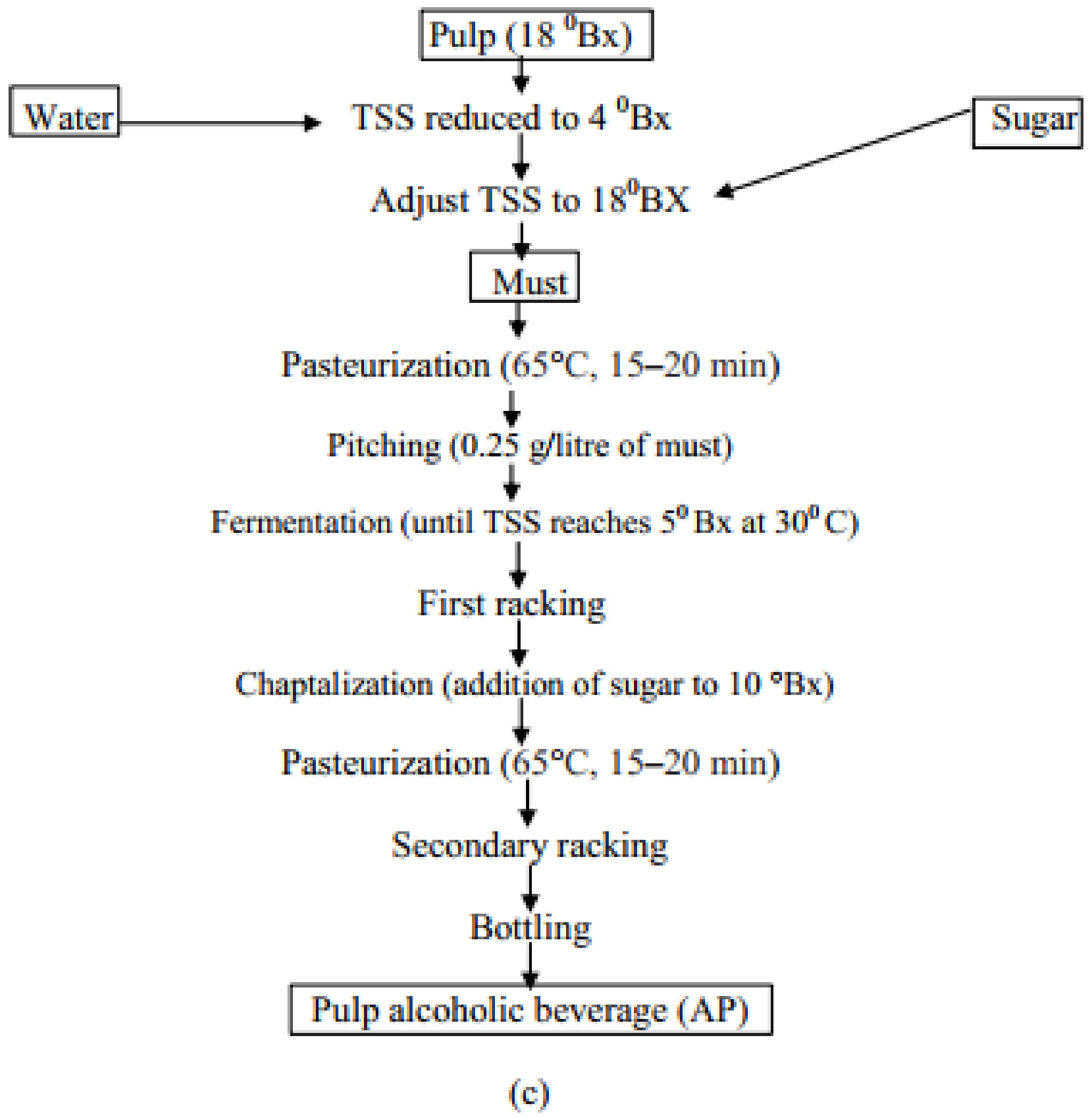
2.1. Raw Material Collection
2.2. Preparation for Fermentation
2.3. Extraction of Phytochemicals
2.4. Analytical Methods
2.4.1. Alcohol Content and Total Dry Extract
2.4.2. Methanol Content
2.4.3. Ester Content
2.4.4. Aldehyde Content
2.4.5. Total Acidity and Volatile Acidity
2.4.6. Caffeine Content
2.4.7. Total Phenolic Content
2.4.8. Total Flavonoid Content
2.4.9. Color Measurement
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of Coffee Pulp and Mucilage
3.2. Fermentation Kinetics with Respect to pH and TSS
3.3. Chemical Analysis
3.4. Colour of Fermented Alcoholic Beverages
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reidy, S. Global Ethanol Production Could Drop to 2013 Levels. World Grain. Available online: https://www.world-grain.com/articles/13635-global-ethanol-production-could-drop-to-2013-levels (accessed on 1 May 2020).
- IEA. Transport Biofuels—Renewables 2020—Analysis; IEA: Paris, France, 2020. [Google Scholar]
- Yadira, P.-S.B.; Sergio, S.-T.; Fernando, S.; Sebastian, P.; Eapen, D. Bioethanol Production from Coffee Mucilage. Energy Procedia 2014, 57, 950–956. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Chacón-Perez, Y.; Alzate, C.A.C. The biorefinery concept for the industrial valorization of coffee processing by-products. In Handbook of Coffee Processing By-Products: Sustainable Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 63–92. [Google Scholar]
- Braham, J.E.; Bressani, R. Coffee Pulp: Composition, Technology, and Utilization; IDRC: Ottawa, ON, Canada, 1979; ISBN 0889361908. [Google Scholar]
- NTCDB. Coffee Production Technology (कफी उत्पादन प्रविधि); National Tea and Coffee Development Board: Kirtipur, Nepal, 2018. [Google Scholar]
- Roussos, S.; Aquiáhuatl, M.D.L.A.; Trejo-Hernández, M.D.R.; Perraud, I.G.; Favela, E.; Ramakrishna, M.; Raimbault, M.; Viniegra-González, G. Biotechnological management of coffee pulp—Isolation, screening, characterization, selection of caffeine-degrading fungi and natural microflora present in coffee pulp and husk. Appl. Microbiol. Biotechnol. 1995, 42, 756–762. [Google Scholar] [CrossRef]
- Blinová, L.; Sirotiak, M.; Bartošová, A.; Soldán, M. Review: Utilization of Waste from Coffee Production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2017, 25, 91–101. [Google Scholar] [CrossRef]
- Antier, P.; Minjares, A.; Roussos, S.; Raimbault, M.; Viniegra-Gonzalez, G. Pectinase-hyperproducing mutants of Aspergillus niger C28B25 for solid-state fermentation of coffee pulp. Enzyme Microb. Technol. 1993, 15, 254–260. [Google Scholar] [CrossRef]
- Silva, C.F.; Vilela, D.M.; Cordeiro, C.D.S.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Evaluation of a potential starter culture for enhance quality of coffee fermentation. World J. Microbiol. Biotechnol. 2013, 29, 235–247. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S. An Overview of the Potential Uses for Coffee Husks. In Coffee in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 283–291. ISBN 9780124167162. [Google Scholar]
- Navia, P.D.P.; Velasco, M.R.d.J.; Hoyos, C.J.L. Production and evaluation of ethanol from coffee processing by-products. Vitae 2011, 18, 287–294. [Google Scholar]
- Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production. Energies 2018, 11, 786. [Google Scholar] [CrossRef]
- Kefale, A. Bioethanol Production and Optimization test from Agricultural Waste: The case of wet coffee processing waste (pulp). Int. J. Renew. Energy Res. 2012, 3, 445–450. [Google Scholar]
- Shenoy, D.; Pai, A.; Vikas, R.; Neeraja, H.; Deeksha, J.; Nayak, C.; Rao, C.V. A study on bioethanol production from cashew apple pulp and coffee pulp waste. Biomass Bioenergy 2011, 35, 4107–4111. [Google Scholar] [CrossRef]
- Torres-Mancera, M.T.; Córdova-López, J.; Rodríguez-Serrano, G.; Roussos, S.; Ramírez-Coronel, M.A.; Favela-Torres, E.; Saucedo-Castañeda, G. Enzymatic Extraction of Hydroxycinnamic Acids from Coffee Pulp. Food Technol. Biotechnol. 2011, 49, 369–373. [Google Scholar]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Hirwa, O.; Nyagahungu, I.; Bitwayiki, C. Ethanol prodution from mucilage and pulp of processed coffee. Ukr. Food J. 2016, 5, 523–530. [Google Scholar] [CrossRef]
- Clarke, R.J. Technology. In Coffee; Springer: Dordrecht, The Netherlands, 1987; Volume 2. [Google Scholar]
- Breisha, G.Z. Production of 16% ethanol from 35% sucrose. Biomass Bioenergy 2010, 34, 1243–1249. [Google Scholar] [CrossRef]
- Miyawaki, O.; Gunathilake, M.; Omote, C.; Koyanagi, T.; Sasaki, T.; Take, H.; Matsuda, A.; Ishisaki, K.; Miwa, S.; Kitano, S. Progressive freeze-concentration of apple juice and its application to produce a new type apple wine. J. Food Eng. 2016, 171, 153–158. [Google Scholar] [CrossRef]
- Geremu, M.; Tola, Y.B.; Sualeh, A. Extraction and determination of total polyphenols and antioxidant capacity of red coffee (Coffea arabica L.) pulp of wet processing plants. Chem. Biol. Technol. Agric. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- FSSAI. Manual of Methods of Analysis of Foods (Alcoholic Beverages); Food Safety and Standards Authority of India: New Delhi, India, 2019; pp. 2–64. [Google Scholar]
- Belay, A.; Ture, K.; Redi, M.; Asfaw, A. Measurement of caffeine in coffee beans with UV/vis spectrometer. Food Chem. 2008, 108, 310–315. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Baljak, A. Determination of Caffeine Content in Tea and Maté Tea by using Different Methods. Czech J. Food Sci. 2009, 27, S213–S216. [Google Scholar] [CrossRef]
- Jaradat, N.; Hussen, F.; Al Ali, A. Preliminary Phytochemical Screening, Quantitative Estimation of Total Flavonoids, Total Phenols and Antioxidant Activity of Ephedra alata Decne. J. Mater. Environ. Sci. 2015, 6, 1771–1778. [Google Scholar]
- Barek, M.; Hasmadi, M.; Zaleha, A.Z.; Fadzelly, A.B.M. Effect of different drying methods on phytochemicals and antioxidant properties of unfermented and fermented teas from Sabah Snake Grass (Clinacanthus nutans Lind.) leaves. Int. Food Res. J. 2015, 22, 661–670. [Google Scholar]
- Lawson-Wood, K.; Robertson, I. Determination of Wine Color and Total Phenol Content Using the LAMBDA PDA UV/Vis Spectrophotometers; PerkinElmer, Inc.: Waltham, MA, USA, 2015. [Google Scholar]
- Fessler, H.J. Guidelines to Practical Wine making; Oregon Speciality Colins: Portland, OR, USA, 1971. [Google Scholar]
- Haile, M.; Kang, W.H. The Harvest and Post-Harvest Management Practices’ Impact on Coffee Quality. Coffee Prod. Res. 2020. [Google Scholar] [CrossRef]
- Gathuo, B.; Rantala, P.; Määttä, R. Coffee Industry Wastes. Water Sci. Technol. 1991, 24, 53–60. [Google Scholar] [CrossRef]
- Bonilla-Hermosa, V.A.; Duarte, W.F.; Schwan, R.F. Utilization of coffee by-products obtained from semi-washed process for production of value-added compounds. Bioresour. Technol. 2014, 166, 142–150. [Google Scholar] [CrossRef]
- Setyobudi, R.H.; Wahono, S.K.; Adinurani, P.G.; Wahyudi, A.; Widodo, W.; Mel, M.; Nugroho, Y.A.; Prabowo, B.; Liwang, T. Characterisation of Arabica Coffee Pulp—Hay from Kintamani—Bali as Prospective Biogas Feedstocks. MATEC Web Conf. 2018, 164, 01039. [Google Scholar] [CrossRef][Green Version]
- Ameca, G.M.; Cerrilla, M.E.O.; Córdoba, P.Z.; Cruz, A.D.; Hernández, M.S.; Haro, J.H. Chemical composition and antioxidant capacity of coffee pulp. Ciência e Agrotecnologia 2018, 42, 307–313. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Coffee, Tea, Cocoa. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2008; pp. 938–970. [Google Scholar]
- Brand, D.; Pandey, A.; Roussos, S.; Brand, I.; Soccol, C.R. Microbial degradation of caffeine and tannins from coffee husk. In Coffee Biotechnology and Quality; Sera, T., Soccol, C.R., Pandey, A., Roussos, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 393–400. [Google Scholar]
- Akin, H.; Brandam, C.; Meyer, X.-M.; Strehaiano, P. A model for pH determination during alcoholic fermentation of a grape must by Saccharomyces cerevisiae. Chem. Eng. Process. Process. Intensif. 2008, 47, 1986–1993. [Google Scholar] [CrossRef]
- Coote, N.; Kirsop, B.H. Factors responsible for the decrease in pH during beer fermentations. J. Inst. Brew. 1976, 82, 149–153. [Google Scholar] [CrossRef]
- Akubor, P.I.; Obio, S.O.; Nwadomere, K.A.; Obiomah, E. Production and quality evaluation of banana wine. Plant Foods Hum. Nutr. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Wall, M.M.; Nishijima, K.A.; Sarnoski, P.; Keith, L.; Chang, L.C.; Wei, Y. Postharvest Ripening of Noni Fruit (Morinda citrifolia) and the Microbial and Chemical Properties of Its Fermented Juice. J. Herbs Spices Med. Plants 2014, 21, 294–307. [Google Scholar] [CrossRef]
- García, R.; Arriola, D.; Rolz, O. Characterization of coffee pectin. Food Sci. Technol. Leb. Technol. 1991, 24, 125–129. [Google Scholar]
- Hodson, G.; Wilkes, E.; Azevedo, S.; Battaglene, T. Methanol in wine. BIO Web Conf. 2017, 9, 02028. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Lin, Y.-S.; Wang, Y.; Jiang, C.-M.; Lin, K.; Wu, M.-C. Addition of Phenolic Acids on the Reduction of Methanol Content in Wine. J. Food Sci. 2008, 73, C432–C437. [Google Scholar] [CrossRef] [PubMed]
- Postel, W.; Adam, L.; Drawert, F. Gas chromatographic determination of volatile components of fermented beverages. III. Volatile components of wines (Ger.). Chem. Mikrobiol. Technol. Lebensm. 1972, 1, 224–235. [Google Scholar]
- Martínez, P.; Valcárcel, M.J.; Piérez, L.; Benítez, T. Metabolism of Saccharomyces cerevisiae flor yeasts during fermentation and biological aging of Fino sherry: By-products and aroma compounds. Am. J. Enol. Vitic. 1998, 49, 240–250. [Google Scholar]
- The Australian Wine Research Institute. Wine Flavours, Faults and Taints. Available online: https://www.awri.com.au/industry_support/winemaking_resources/sensory_assessment/recognition-of-wine-faults-and-taints/wine_faults/ (accessed on 30 March 2021).
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2007, 74, 454–461. [Google Scholar] [CrossRef]
- Osborne, J.; Morneau, A.D.; De Orduña, R.M. Degradation of free and sulfur-dioxide-bound acetaldehyde by malolactic lactic acid bacteria in white wine. J. Appl. Microbiol. 2006, 101, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Asfew, Z.; Dekebo, A. Determination of caffeine content in Wollega Zones, Ethiopian coffee bean, pulp and leaves by high performance liquid chromatography. Trends Phytochem. Res. Trends Phytochem. Res 2019, 3, 261–274. [Google Scholar]
- Horman, I.; Viani, R. The nature and conformation of the caffeine-chlorogenate complex of coffee. J. Food Sci. 1972, 37, 925–927. [Google Scholar] [CrossRef]
- Murthy, P.S.; Manjunatha, M.R.; Sulochannama, G.; Madhava Naidu, M. Extraction, Characterization and Bioactivity of Coffee Anthocyanins. Eur. J. Biol. Sci. 2012, 4, 13–19. [Google Scholar]
- Maroniac, A.; Mihali, C. Comparative color analysis of red, rosé and white wines by spectrophotometric method. Carpathian J. Food Sci. Technol. 2014, 6, 137–144. [Google Scholar]
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2020, 128, 108773. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.R.; De La Fuente, G.; Batten, M.A.; Bressani, R. Decaffeination. Process to detoxify coffe pulp. J. Agric. Food Chem. 1974, 22, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J. The Oxford Companion to Wine, 3rd ed.; Oxford University Press: Oxford, UK, 2006; ISBN 9780198609902. [Google Scholar]
- Ashok, P.K.; Upadhyaya, K. Tannins are Astringent. J. Pharmacogn. Phytochem. 2012, 1, 45–50. [Google Scholar]


| Particulars | Coffee Pulp | Mucilage |
|---|---|---|
| Moisture (%) | 75.7 ± 0.2 | 85.3 ± 0.6 |
| Dry matter (%) | 24.3 ± 0.2 | 14.7 ± 0.6 |
| Crude Protein (%) | 8.1 ± 0.36 | 7.2 ± 0.3 |
| Fat (%) | 1.53 ± 0.05 | 0.7 ± 0.00 |
| Ash (%) | 6.4 ± 0.05 | 1.1 ± 0.1 |
| Crude fiber (%) | 6.3 ± 0.2 | 1.5 ± 0.22 |
| Total sugar (%) | 12.06 ± 0.41 | 4.3 ± 0.4 |
| Reducing sugar (%) | 10.9 ± 0.36 | - |
| Caffeine (%) | 1.11 ± 0.11 | 1.05 ± 0.05 |
| TSS (°Bx) | 18 ± 0.5 | 15 ± 0.5 |
| pH | 4.3 ± 0.15 | 3.7± 0.1 |
| Polyphenols (mg GAE/g dry extract) | 1862.62 ± 4.42 | 1618.32 ± 3.2 |
| Tannins (mg GAE/g dry extract) | 412 ± 4.7 | 370 ± 3.6 |
| Flavonoid (mg QE/g dry extract) | 697.3 ± 2.1 | 531.54 ± 2.7 |
| Particulars | AP | AM | AMS |
|---|---|---|---|
| Methanol content (mg/L) | 335 ± 1.21 c | 298.9 ± 0.28 a | 313.2 ± 1.81 b |
| Esters content (ppm) | 70.58 ± 1.45 b | 38.21 ± 6.09 a | 33.86 ± 3.29 a |
| Aldehydes (ppm) | 9.15 ± 0.877 a | 22 ± 0.4 b | 42.94 ± 1.5 c |
| Alcohol (ABV%) | 8.867 ± 0.067 b | 8.707 ± 0.092 b | 8.25 ± 0.026 a |
| Total acidity (%) | 0.411 ± 0.02 ab | 0.393 ± 0.005 a | 0.443 ± 0.011 b |
| Volatile acidity (%) | 0.013 ± 0.00 c | 0.007 ± 0.00 a | 0.0094 ± 0.00 b |
| Caffeine content (ppm) | 30.94 ± 0.674 b | 21.29 ± 0.643 a | 42.44 ± 0.737 c |
| Polyphenols (mg GAE/g dry extract) | 845.7 ± 14.36 c | 554 ± 7.93 a | 709.7± 4.5 b |
| Tannin (mg GAE/g dry extract) | 305 ± 4 c | 235 ± 4 a | 268.3 ± 3.5 b |
| Flavonoid (mg QE/g dry extract) | 440.7 ± 5.03 c | 349.3 ± 4.5 a | 395 ± 3 b |
| Chromatic structure | 0.41 ± 0.00 c | 0.27 ± 0.00 a | 0.28 ± 0.00 b |
| Hue | 1.71 ± 0.00 c | 1.64 ± 0.00 b | 1.51 ± 0.00 a |
| Particulars | Appearance | Aroma | Taste | Aftertaste | Overall Acceptability |
|---|---|---|---|---|---|
| AM | 9.03 ± 2.53 a | 18.89 ± 4.9 a | 21.21 ± 3.73 a | 10.32 ± 2.32 a | 6.35 ± 1.25 a |
| AMS | 9.07 ± 2 a | 20.11 ± 5.07 ab | 21.96 ± 4.17 a | 11.11 ± 3.17 ab | 6.32 ± 1.56 a |
| AP | 11.07 ± 2.19 b | 21.86 ± 3.71 b | 23 ± 2.34 a | 12.21 ± 1.67 b | 8.25 ± 0.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
KC, Y.; Subba, R.; Shiwakoti, L.D.; Dhungana, P.K.; Bajagain, R.; Chaudhary, D.K.; Pant, B.R.; Bajgai, T.R.; Lamichhane, J.; Timilsina, S.; et al. Utilizing Coffee Pulp and Mucilage for Producing Alcohol-Based Beverage. Fermentation 2021, 7, 53. https://doi.org/10.3390/fermentation7020053
KC Y, Subba R, Shiwakoti LD, Dhungana PK, Bajagain R, Chaudhary DK, Pant BR, Bajgai TR, Lamichhane J, Timilsina S, et al. Utilizing Coffee Pulp and Mucilage for Producing Alcohol-Based Beverage. Fermentation. 2021; 7(2):53. https://doi.org/10.3390/fermentation7020053
Chicago/Turabian StyleKC, Yadav, Raju Subba, Lila Devi Shiwakoti, Pramesh Kumar Dhungana, Rishikesh Bajagain, Dhiraj Kumar Chaudhary, Bhoj Raj Pant, Tirtha Raj Bajgai, Janardan Lamichhane, Sampada Timilsina, and et al. 2021. "Utilizing Coffee Pulp and Mucilage for Producing Alcohol-Based Beverage" Fermentation 7, no. 2: 53. https://doi.org/10.3390/fermentation7020053
APA StyleKC, Y., Subba, R., Shiwakoti, L. D., Dhungana, P. K., Bajagain, R., Chaudhary, D. K., Pant, B. R., Bajgai, T. R., Lamichhane, J., Timilsina, S., Upadhyaya, J., & Dahal, R. H. (2021). Utilizing Coffee Pulp and Mucilage for Producing Alcohol-Based Beverage. Fermentation, 7(2), 53. https://doi.org/10.3390/fermentation7020053











