Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety
Abstract
1. LAB in Oenology: Introductory Aspects and Biodiversity
2. Impact of Wine Environment on LAB Metabolisms
3. Plus Effects: Influence of MLF on Wine Organoleptic Properties, Bioprotection and the Removal of Undesired Compounds
3.1. Esters
3.2. Carbonyl Compounds
3.3. Thiols
3.4. Monoterpenes
3.5. Degradation of Toxic Compounds
3.6. Bioprotection
4. Minus Effects: Production of Off-Flavours and Other Undesired Compounds
4.1. Production of Off-Flavours by Lactic Acid Bacteria
4.2. Production of By-products Harmful to Consumer Health (i.e., BAs, EC)
5. Novel Inoculation Approaches to Enhance LAB Impact on Wine Quality
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic Acid Bacteria in Wine: Technological Advances and Evaluation of Their Functional Role. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Lactic Acid Bacteria in the Quality Improvement and Depreciation of Wine. Antonie Van Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G.; Grieco, F. Influence of Autochthonous Saccharomyces cerevisiae Strains on Volatile Profile of Negroamaro Wines. LWT Food Sci. Technol. 2014, 58, 35–48. [Google Scholar] [CrossRef]
- Berbegal, C.; Khomenko, I.; Russo, P.; Spano, G.; Fragasso, M.; Biasioli, F.; Capozzi, V. PTR-ToF-MS for the Online Monitoring of Alcoholic Fermentation in Wine: Assessment of VOCs Variability Associated with Different Combinations of Saccharomyces/Non-Saccharomyces as a Case-Study. Fermentation 2020, 6, 55. [Google Scholar] [CrossRef]
- Bartowsky, E.J. Wines. Malolactic Fermentation. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 800–804. ISBN 978-0-12-384733-1. [Google Scholar]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter Cultures for Sparkling Wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and Technological Potential of Lactobacillus plantarum Bacteria Suitable for Wine Malolactic Fermentation and Grape Aroma Release. LWT 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Takase, H.; Sasaki, K.; Kiyomichi, D.; Kobayashi, H.; Matsuo, H.; Takata, R. Impact of Lactobacillus plantarum on Thiol Precursor Biotransformation Leading to Production of 3-Sulfanylhexan-1-Ol. Food Chem. 2018, 259, 99–104. [Google Scholar] [CrossRef]
- Lytra, G.; Miot-Sertier, C.; Moine, V.; Coulon, J.; Barbe, J.-C. Influence of Must Yeast-Assimilable Nitrogen Content on Fruity Aroma Variation during Malolactic Fermentation in Red Wine. Food Res. Int. 2020, 135, 109294. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The Oenological Potential of Hanseniaspora uvarum in Simultaneous and Sequential Co-Fermentation with Saccharomyces cerevisiae for Industrial Wine Production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V. A Metagenomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int. J. Mol. Sci. 2019, 20, 3980. [Google Scholar] [CrossRef]
- Kioroglou, D.; LLeixá, J.; Mas, A.; Portillo, M.D.C. Massive Sequencing: A New Tool for the Control of Alcoholic Fermentation in Wine? Fermentation 2018, 4, 7. [Google Scholar] [CrossRef]
- Abdo, H.; Catacchio, C.R.; Ventura, M.; D’Addabbo, P.; Alexandre, H.; Guilloux-Bénatier, M.; Rousseaux, S. The Establishment of a Fungal Consortium in a New Winery. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of New Research and Technologies for Malolactic Fermentation in Wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132. [Google Scholar] [CrossRef] [PubMed]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Selection and Characterisation of Oenococcus oeni and Lactobacillus plantarum South African Wine Isolates for Use as Malolactic Fermentation Starter Cultures. South Afr. J. Enol. Vitic. 2011, 32, 280–295. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Endo, A. Taxonomic Status of Lactic Acid Bacteria in Wine and Key Characteristics to Differentiate Species. South Afr. J. Enol. Vitic. 2009, 30, 72–90. [Google Scholar] [CrossRef][Green Version]
- Mesas, J.M.; Rodríguez, M.C.; Alegre, M.T. Characterization of Lactic Acid Bacteria from Musts and Wines of Three Consecutive Vintages of Ribeira Sacra. Lett. Appl. Microbiol. 2011, 52, 258–268. [Google Scholar] [CrossRef]
- Rodas, A.M.; Ferrer, S.; Pardo, I. Polyphasic Study of Wine Lactobacillus Strains: Taxonomic Implications. Int. J. Syst. Evol. Microbiol. 2005, 55, 197–207. [Google Scholar] [CrossRef]
- König, H.; Fröhlich, J. Lactic Acid Bacteria. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–41. ISBN 978-3-319-60021-5. [Google Scholar]
- Franquès, J.; Araque, I.; Palahí, E.; Portillo, M.d.C.; Reguant, C.; Bordons, A. Presence of Oenococcus oeni and Other Lactic Acid Bacteria in Grapes and Wines from Priorat (Catalonia, Spain). LWT Food Sci. Technol. 2017, 81, 326–334. [Google Scholar] [CrossRef]
- Miranda-Castilleja, D.E.; Martínez-Peniche, R.Á.; Aldrete-Tapia, J.A.; Soto-Muñoz, L.; Iturriaga, M.H.; Pacheco-Aguilar, J.R.; Arvizu-Medrano, S.M. Distribution of Native Lactic Acid Bacteria in Wineries of Queretaro, Mexico and Their Resistance to Wine-Like Conditions. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus Plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook. Beverages 2020, 6, 23. [Google Scholar] [CrossRef]
- Lorentzen, M.P.G.; Lucas, P.M. Distribution of Oenococcus oeni Populations in Natural Habitats. Appl. Microbiol. Biotechnol. 2019, 103, 2937–2945. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Beneduce, L.; Weidmann, S.; Grieco, F.; Guzzo, J.; Spano, G. Technological Properties of Oenococcus oeni Strains Isolated from Typical Southern Italian Wines. Lett. Appl. Microbiol. 2010, 50, 327–334. [Google Scholar] [CrossRef]
- Guzzo, J. Stress Responses of Oenococcus oeni. In Stress Responses of Lactic Acid Bacteria; Tsakalidou, E., Papadimitriou, K., Eds.; Springer: Boston, MA, USA, 2011; pp. 349–365. ISBN 978-0-387-92771-8. [Google Scholar]
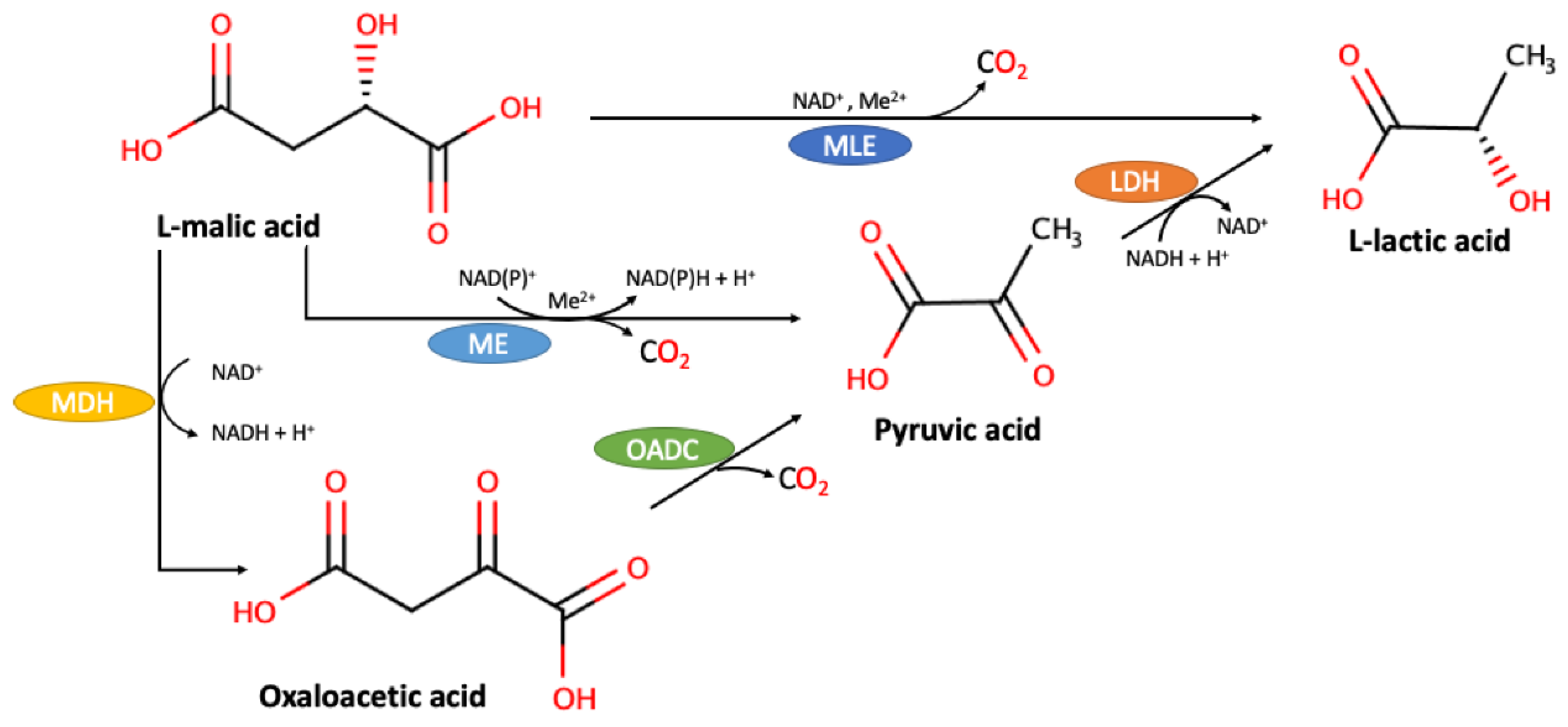
- Acevedo, W.; Cañón, P.; Gómez-Alvear, F.; Huerta, J.; Aguayo, D.; Agosin, E. L-Malate (−2) Protonation State Is Required for Efficient Decarboxylation to l-Lactate by the Malolactic Enzyme of Oenococcus oeni. Molecules 2020, 25, 3431. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures—An Overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Berbegal, C.; Peña, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological Properties of Lactobacillus plantarum Strains Isolated from Grape Must Fermentation. Food Microbiol. 2016, 57, 187–194. [Google Scholar] [CrossRef]
- Mtshali, P.S.; Divol, B.; Van Rensburg, P.; Du Toit, M. Genetic Screening of Wine-Related Enzymes in Lactobacillus Species Isolated from South African Wines. J. Appl. Microbiol. 2010, 108, 1389–1397. [Google Scholar] [CrossRef]
- Sereni, A.; Phan, Q.; Osborne, J.; Tomasino, E. Impact of the Timing and Temperature of Malolactic Fermentation on the Aroma Composition and Mouthfeel Properties of Chardonnay Wine. Foods 2020, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Chira, K.; Teissedre, P.-L. Comparison between Malolactic Fermentation Container and Barrel Toasting Effects on Phenolic, Volatile, and Sensory Profiles of Red Wines. J. Agric. Food Chem. 2017, 65, 3320–3329. [Google Scholar] [CrossRef]
- Wade, M.E.; Strickland, M.T.; Osborne, J.P.; Edwards, C.G. Role of Pediococcus in Winemaking. Aust. J. Grape Wine Res. 2019, 25, 7–24. [Google Scholar] [CrossRef]
- Juega, M.; Costantini, A.; Bonello, F.; Cravero, M.-C.; Martinez-Rodriguez, A.J.; Carrascosa, A.V.; Garcia-Moruno, E. Effect of Malolactic Fermentation by Pediococcus damnosus on the Composition and Sensory Profile of Albariño and Caiño White Wines. J. Appl. Microbiol. 2014, 116, 586–595. [Google Scholar] [CrossRef]
- Mills, D.A.; Rawsthorne, H.; Parker, C.; Tamir, D.; Makarova, K. Genomic Analysis of Oenococcus oeni PSU-1 and Its Relevance to Winemaking. FEMS Microbiol. Rev. 2005, 29, 465–475. [Google Scholar] [CrossRef][Green Version]
- Borneman, A.R.; McCarthy, J.M.; Chambers, P.J.; Bartowsky, E.J. Comparative Analysis of the Oenococcus oeni Pan Genome Reveals Genetic Diversity in Industrially-Relevant Pathways. BMC Genom. 2012, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Lamontanara, A.; Orrù, L.; Cattivelli, L.; Russo, P.; Spano, G.; Capozzi, V. Genome Sequence of Oenococcus oeni OM27, the First Fully Assembled Genome of a Strain Isolated from an Italian Wine. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Russo, P.; Lamontanara, A.; Orrù, L.; Cattivelli, L.; Spano, G. Genome Sequences of Five Oenococcus oeni Strains Isolated from Nero Di Troia Wine from the Same Terroir in Apulia, Southern Italy. Genome Announc. 2014, 2. [Google Scholar] [CrossRef]
- Campbell-Sills, H.; Khoury, M.E.; Gammacurta, M.; Miot-Sertier, C.; Dutilh, L.; Vestner, J.; Capozzi, V.; Sherman, D.; Hubert, C.; Claisse, O.; et al. Two Different Oenococcus oeni Lineages Are Associated to Either Red or White Wines in Burgundy: Genomics and Metabolomics Insights. OENO One 2017, 51, 309. [Google Scholar] [CrossRef]
- Campbell-Sills, H.; El Khoury, M.; Favier, M.; Romano, A.; Biasioli, F.; Spano, G.; Sherman, D.J.; Bouchez, O.; Coton, E.; Coton, M.; et al. Phylogenomic Analysis of Oenococcus oeni Reveals Specific Domestication of Strains to Cider and Wines. Genome Biol. Evol. 2015, 7, 1506–1518. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Saavedra, L.; Raya, R.R. Draft Genome Sequence of Oenococcus oeni Strain X2L (CRL1947), Isolated from Red Wine of Northwest Argentina. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Sternes, P.R.; Borneman, A.R. Consensus Pan-Genome Assembly of the Specialised Wine Bacterium Oenococcus oeni. BMC Genom. 2016, 17, 308. [Google Scholar] [CrossRef]
- Iglesias, N.G.; Valdés La Hens, D.; Olguin, N.T.; Bravo-Ferrada, B.M.; Brizuela, N.S.; Tymczyszyn, E.E.; Bibiloni, H.; Caballero, A.C.; Delfederico, L.; Semorile, L. Genome Sequence of Oenococcus oeni UNQOe19, the First Fully Assembled Genome Sequence of a Patagonian Psychrotrophic Oenological Strain. Microbiol. Resour. Announc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Lamontanara, A.; Caggianiello, G.; Orrù, L.; Capozzi, V.; Michelotti, V.; Bayjanov, J.R.; Renckens, B.; van Hijum, S.A.F.T.; Cattivelli, L.; Spano, G. Draft Genome Sequence of Lactobacillus plantarum Lp90 Isolated from Wine. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; He, L.; Tian, Y. Draft Genome Sequence of Lactobacillus plantarum XJ25 Isolated from Chinese Red Wine. Genome Announc. 2016, 4. [Google Scholar] [CrossRef]
- Iglesias, N.G.; Brizuela, N.S.; Tymczyszyn, E.E.; Hollmann, A.; Hens, D.V.L.; Semorile, L.; Bravo-Ferrada, B.M. Complete Genome Sequencing of Lactobacillus plantarum UNQLp 11 Isolated from a Patagonian Pinot Noir Wine. South Afr. J. Enol. Vitic. 2020, 41, 197–209. [Google Scholar] [CrossRef]
- Betteridge, A.; Grbin, P.; Jiranek, V. Improving Oenococcus oeni to Overcome Challenges of Wine Malolactic Fermentation. Trends Biotechnol. 2015, 33, 547–553. [Google Scholar] [CrossRef]
- Quirós, C.; Herrero, M.; García, L.A.; Díaz, M. Effects of SO2 on Lactic Acid Bacteria Physiology When Used as a Preservative Compound in Malolactic Fermentation. J. Inst. Brew. 2012, 118, 89–96. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; du Toit, M. Influence of pH and Ethanol on Malolactic Fermentation and Volatile Aroma Compound Composition in White Wines. LWT Food Sci. Technol. 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Yuan, L.; Hu, K.; Peng, S.; Li, H.; Wang, H. Mechanism Analysis of Combined Acid-and-Ethanol Shock on Oenococcus oeni Using RNA-Seq. Eur. Food Res. Technol. 2020, 246, 1637–1646. [Google Scholar] [CrossRef]
- Contreras, A.; Ribbeck, M.; Gutiérrez, G.D.; Cañon, P.M.; Mendoza, S.N.; Agosin, E. Mapping the Physiological Response of Oenococcus oeni to Ethanol Stress Using an Extended Genome-Scale Metabolic Model. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Liu, L. Microbial Response to Acid Stress: Mechanisms and Applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Dicks, L.M.T. Control of Malolactic Fermentation in Wine. A Review. South Afr. J. Enol. Vitic. 2004, 25, 74–88. [Google Scholar] [CrossRef]
- Herrero, M.; García, L.A.; Díaz, M. Volatile Compounds in Cider: Inoculation Time and Fermentation Temperature Effects. J. Inst. Brew. 2006, 112, 210–214. [Google Scholar] [CrossRef]
- Guzzon, R.; Roman, T.; Larcher, R. Impact of Different Temperature Profiles on Simultaneous Yeast and Bacteria Fermentation. Ann. Microbiol. 2020, 70, 44. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging Trends in the Application of Malolactic Fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking Wine Lactic Acid Bacteria Diversity with Wine Aroma and Flavour. Int. J. Food Microbiol. 2017, 243, 16–27. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; De Revel, G. Characterization of Fruity Aroma Modifications in Red Wines during Malolactic Fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef]
- Sumby, K.M.; Jiranek, V.; Grbin, P.R. Ester Synthesis and Hydrolysis in an Aqueous Environment, and Strain Specific Changes during Malolactic Fermentation in Wine with Oenococcus oeni. Food Chem. 2013, 141, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Gammacurta, M.; Lytra, G.; Marchal, A.; Marchand, S.; Christophe Barbe, J.; Moine, V.; de Revel, G. Influence of Lactic Acid Bacteria Strains on Ester Concentrations in Red Wines: Specific Impact on Branched Hydroxylated Compounds. Food Chem. 2018, 239, 252–259. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Characterization of EstCOo8 and EstC34, Intracellular Esterases, from the Wine-Associated Lactic Acid Bacteria Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2013, 114, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Lerm, E.; Engelbrecht, L.; Du Toit, M. Malolactic Fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar] [CrossRef]
- Pérez-Martín, F.; Seseña, S.; Izquierdo, P.M.; Palop, M.L. Esterase Activity of Lactic Acid Bacteria Isolated from Malolactic Fermentation of Red Wines. Int. J. Food Microbiol. 2013, 163, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Costello, P.J.; Siebert, T.E.; Solomon, M.R.; Bartowsky, E.J. Synthesis of Fruity Ethyl Esters by Acyl Coenzyme A: Alcohol Acyltransferase and Reverse Esterase Activities in Oenococcus oeni and Lactobacillus plantarum. J. Appl. Microbiol. 2013, 114, 797–806. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Reverón, I.; Mancheño, J.M.; de las Rivas, B.; Muñoz, R. Characterization of a Feruloyl Esterase from Lactobacillus plantarum. Appl. Environ. Microbiol. 2013, 79, 5130–5136. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.P.; Dubé Morneau, A.; Mira de Orduña, R. Degradation of Free and Sulfur-Dioxide-Bound Acetaldehyde by Malolactic Lactic Acid Bacteria in White Wine. J. Appl. Microbiol. 2006, 101, 474–479. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Pretorius, I.S. Microbial Formation and Modification of Flavor and Off-Flavor Compounds in Wine. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Cham, Switzerland, 2009; pp. 209–231. ISBN 978-3-540-85463-0. [Google Scholar]
- Lasik, M. The Application of Malolactic Fermentation Process to Create Good-Quality Grape Wine Produced in Cool-Climate Countries: A Review. Eur. Food Res. Technol. 2013, 237, 843–850. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. South Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in Varietal Thiol (3-SH and 4-MSP) Release in Wine Sequential Fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Baltenweck-Guyot, R.; Gachons, C.P.D.; Dubourdieu, D. Contribution of Volatile Thiols to the Aromas of White Wines Made from Several Vitis vinifera Grape Varieties. Am. J. Enol. Vitic. 2000, 51, 178–181. [Google Scholar]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of Volatile Sulfur Compounds and Metabolism of Methionine and Other Sulfur Compounds in Fermented Food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and Their Role in Wine Flavour: Recent Advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Palmeri, R.; Spagna, G. β-Glucosidase in Cellular and Acellular Form for Winemaking Application. Enzyme Microb. Technol. 2007, 40, 382–389. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Spagna, G.; Palmeri, R.; Torriani, S. Assessment of β-Glucosidase Activity in Selected Wild Strains of Oenococcus oeni for Malolactic Fermentation. Enzyme Microb. Technol. 2004, 34, 292–296. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase Activities of Lactic Acid Bacteria: Mechanisms, Impact on Fermented Food and Human Health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Nauer, S.; Brandes, W.; Schümann, C.; Kulbe, K.D.; del Hierro, A.M.; Eder, R. Release of Wine Monoterpenes from Natural Precursors by Glycosidases from Oenococcus oeni. Food Chem. 2012, 135–334, 80–87. [Google Scholar] [CrossRef]
- Sestelo, A.B.F.; Poza, M.; Villa, T.G. β-Glucosidase Activity in a Lactobacillus plantarum Wine Strain. World J. Microbiol. Biotechnol. 2004, 20, 633. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. The Influence of Malolactic Fermentation and Oenococcus oeni Strain on Glycosidic Aroma Precursors and Related Volatile Compounds of Red Wine. J. Sci. Food Agric. 2006, 86, 2468–2476. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Spano, G.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Metabolites of Microbial Origin with an Impact on Health: Ochratoxin A and Biogenic Amines. Front. Microbiol. 2016, 7, 482. [Google Scholar] [CrossRef]
- Tamura, M.; Takahashi, A.; Uyama, A.; Mochizuki, N. A Method for Multiple Mycotoxin Analysis in Wines by Solid Phase Extraction and Multifunctional Cartridge Purification, and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Toxins 2012, 4, 476–486. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Wine Contamination with Ochratoxins: A Review. Beverages 2018, 4, 6. [Google Scholar] [CrossRef]
- Grazioli, B.; Fumi, M.D.; Silva, A. The Role of Processing on Ochratoxin A Content in Italian Must and Wine: A Study on Naturally Contaminated Grapes. Int. J. Food Microbiol. 2006, 111, S93–S96. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Saari, N.; Meor Hussin, A.S. Review on the Biological Detoxification of Mycotoxins Using Lactic Acid Bacteria to Enhance the Sustainability of Foods Supply. Molecules 2020, 25, 2655. [Google Scholar] [CrossRef]
- Petruzzi, L.; Bevilacqua, A.; Corbo, M.R.; Speranza, B.; Capozzi, V.; Sinigaglia, M. A Focus on Quality and Safety Traits of Saccharomyces cerevisiae Isolated from Uva di Troia Grape Variety. J. Food Sci. 2017, 82, 124–133. [Google Scholar] [CrossRef]
- Del Prete, V.; Rodriguez, H.; Carrascosa, A.V.; de las Rivas, B.; Garcia-Moruno, E.; Muñoz, R. In Vitro Removal of Ochratoxin A by Wine Lactic Acid Bacteria. J. Food Prot. 2007, 70, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Medina, Á.; Mateo, F.; Valle-Algarra, F.M.; Pardo, I.; Jiménez, M. Ochratoxin A Removal in Synthetic Media by Living and Heat-Inactivated Cells of Oenococcus oeni Isolated from Wines. Food Control 2010, 21, 23–28. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Inês, A.; Rodrigues, A.I.; Guimarães, A.; Pereira, V.L.; Parpot, P.; Mendes-Faia, A.; Venâncio, A. Biodegradation of Ochratoxin A by Pediococcus parvulus Isolated from Douro Wines. Int. J. Food Microbiol. 2014, 188, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Beneduce, L.; Romano, A.; Capozzi, V.; Lucas, P.; Barnavon, L.; Bach, B.; Vuchot, P.; Grieco, F.; Spano, G. Biogenic Amine in Wines. Ann. Microbiol. 2010, 60, 573–578. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic Amines Degradation by Lactobacillus plantarum: Toward a Potential Application in Wine. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Identification of a Novel Enzymatic Activity from Lactic Acid Bacteria Able to Degrade Biogenic Amines in Wine. Appl. Microbiol. Biotechnol. 2014, 98, 185–198. [Google Scholar] [CrossRef]
- Niu, T.; Li, X.; Guo, Y.; Ma, Y. Identification of a Lactic Acid Bacteria to Degrade Biogenic Amines in Chinese Rice Wine and Its Enzymatic Mechanism. Foods 2019, 8, 312. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, D.; Fan, M.; Li, H.; Jin, C.; Liu, W. Selection of a Versatile Lactobacillus plantarum for Wine Production and Identification and Preliminary Characterisation of a Novel Histamine-Degrading Enzyme. Int. J. Food Sci. Technol. 2020, 55, 2608–2618. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Nardi, T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms 2020, 8, 507. [Google Scholar] [CrossRef]
- Russo, P.; Berbegal, C.; De Ceglie, C.; Grieco, F.; Spano, G.; Capozzi, V. Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations. Fermentation 2019, 5, 23. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Fares, C.; Longo, A.; Spano, G.; Capozzi, V. Lactobacillus plantarum with Broad Antifungal Activity as a Protective Starter Culture for Bread Production. Foods 2017, 6, 110. [Google Scholar] [CrossRef]
- Voidarou, C.; Alexopoulos, A.; Tsinas, A.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. Effectiveness of Bacteriocin-Producing Lactic Acid Bacteria and Bifidobacterium Isolated from Honeycombs against Spoilage Microorganisms and Pathogens Isolated from Fruits and Vegetables. Appl. Sci. 2020, 10, 7309. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and Probiosis: Antimicrobial Activity of Lactic Acid Bacteria with a Focus on Their Antiviral and Antifungal Properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. Exploration of the Microbial Biodiversity Associated with North Apulian Sourdoughs and the Effect of the Increasing Number of Inoculated Lactic Acid Bacteria Strains on the Biocontrol against Fungal Spoilage. Fermentation 2019, 5, 97. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- De Senna, A.; Lathrop, A. Antifungal Screening of Bioprotective Isolates against Botrytis cinerea, Fusarium pallidoroseum and Fusarium moniliforme. Fermentation 2017, 3, 53. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Badosa, E.; Montesinos, E. Bioprotection of Golden Delicious Apples and Iceberg Lettuce against Foodborne Bacterial Pathogens by Lactic Acid Bacteria. Int. J. Food Microbiol. 2008, 123, 50–60. [Google Scholar] [CrossRef] [PubMed]
- López-Seijas, J.; García-Fraga, B.; da Silva, A.F.; Sieiro, C. Wine Lactic Acid Bacteria with Antimicrobial Activity as Potential Biocontrol Agents against Fusarium oxysporum f. sp. lycopersici. Agronomy 2020, 10, 31. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Fragasso, M.; Grieco, F.; Russo, P.; Capozzi, V. Starter Cultures as Biocontrol Strategy to Prevent Brettanomyces bruxellensis Proliferation in Wine. Appl. Microbiol. Biotechnol. 2018, 102, 569–576. [Google Scholar] [CrossRef]
- Di Toro, M.R.; Capozzi, V.; Beneduce, L.; Alexandre, H.; Tristezza, M.; Durante, M.; Tufariello, M.; Grieco, F.; Spano, G. Intraspecific Biodiversity and ‘Spoilage Potential’ of Brettanomyces bruxellensis in Apulian Wines. LWT Food Sci. Technol. 2015, 60, 102–108. [Google Scholar] [CrossRef]
- Berbegal, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of Autochthonous Yeasts and Bacteria in Order to Control Brettanomyces bruxellensis in Wine. Fermentation 2017, 3, 65. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Gonzalo-Diago, A.; Iribarren, M.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. Bioprotection as a Tool to Free Additives Winemaking: Effect on Sensorial, Anthocyanic and Aromatic Profile of Young Red Wines. LWT 2018, 98, 458–464. [Google Scholar] [CrossRef]
- Miyagusuku-Cruzado, G.; García-Cano, I.; Rocha-Mendoza, D.; Jiménez-Flores, R.; Giusti, M.M. Monitoring Hydroxycinnamic Acid Decarboxylation by Lactic Acid Bacteria Using High-Throughput UV-Vis Spectroscopy. Molecules 2020, 25, 3142. [Google Scholar] [CrossRef]
- Couto, J.A.; Campos, F.M.; Figueiredo, A.R.; Hogg, T.A. Ability of Lactic Acid Bacteria to Produce Volatile Phenols. Am. J. Enol. Vitic. 2006, 57, 166–171. [Google Scholar]
- Santamaría, L.; Reverón, I.; de Felipe, F.L.; de Las Rivas, B.; Muñoz, R. Ethylphenol Formation by Lactobacillus plantarum: Identification of the Enzyme Involved in the Reduction of Vinylphenols. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Costello, P.J.; Lee, T.H.; Henschke, P. Ability of Lactic Acid Bacteria to Produce N-Heterocycles Causing Mousy off-Flavour in Wine. Aust. J. Grape Wine Res. 2001, 7, 160–167. [Google Scholar] [CrossRef]
- Costello, P.J.; Henschke, P.A. Mousy Off-Flavor of Wine: Precursors and Biosynthesis of the Causative N-Heterocycles 2-Ethyltetrahydropyridine, 2-Acetyltetrahydropyridine, and 2-Acetyl-1-Pyrroline by Lactobacillus Hilgardii DSM 20176. J. Agric. Food Chem. 2002, 50, 7079–7087. [Google Scholar] [CrossRef]
- Pripis-Nicolau, L.; de Revel, G.; Bertrand, A.; Lonvaud-Funel, A. Methionine Catabolism and Production of Volatile Sulphur Compounds by Oenococcus oeni. J. Appl. Microbiol. 2004, 96, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Vallet, A.; Lucas, P.; Lonvaud-Funel, A.; Revel, G.D. Pathways That Produce Volatile Sulphur Compounds from Methionine in Oenococcus oeni. J. Appl. Microbiol. 2008, 104, 1833–1840. [Google Scholar] [CrossRef]
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl Carbamate in Fermented Beverages: Presence, Analytical Chemistry, Formation Mechanism, and Mitigation Proposals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 611–626. [Google Scholar] [CrossRef]
- Russo, P.; Fragasso, M.; Berbegal, C.; Grieco, F.; Spano, G.; Capozzi, V. Chapter 2: Microorganisms Able to Produce Biogenic Amines and Factors Affecting Their Activity. In Biogenic Amines in Food; The Royal Society of Chemistry: London, UK, 2019; pp. 18–40. [Google Scholar]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Ladero, V.; Beneduce, L.; Fernández, M.; Alvarez, M.A.; Benoit, B.; Laurent, B.; Grieco, F.; Spano, G. Isolation and Characterization of Tyramine-Producing Enterococcus faecium Strains from Red Wine. Food Microbiol. 2011, 28, 434–439. [Google Scholar] [CrossRef]
- Moreno-Arribas, V.; Lonvaud-Funel, A. Tyrosine Decarboxylase Activity of Lactobacillus brevis IOEB 9809 Isolated from Wine and L. brevis ATCC 367. FEMS Microbiol. Lett. 1999, 180, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, V.; Torlois, S.; Joyeux, A.; Bertrand, A.; Lonvaud-Funel, A. Isolation, Properties and Behaviour of Tyramine-Producing Lactic Acid Bacteria from Wine. J. Appl. Microbiol. 2000, 88, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Cersosimo, M.; Del Prete, V.; Garcia-Moruno, E. Production of Biogenic Amines by Lactic Acid Bacteria: Screening by PCR, Thin-Layer Chromatography, and High-Performance Liquid Chromatography of Strains Isolated from Wine and Must. J. Food Prot. 2006, 69, 391–396. [Google Scholar] [CrossRef]
- Arena, M.P.; Romano, A.; Capozzi, V.; Beneduce, L.; Ghariani, M.; Grieco, F.; Lucas, P.; Spano, G. Expression of Lactobacillus brevis IOEB 9809 Tyrosine Decarboxylase and Agmatine Deiminase Genes in Wine Correlates with Substrate Availability. Lett. Appl. Microbiol. 2011, 53, 395–402. [Google Scholar] [CrossRef]
- Mazzoli, R.; Lamberti, C.; Coisson, J.D.; Purrotti, M.; Arlorio, M.; Giuffrida, M.G.; Giunta, C.; Pessione, E. Influence of Ethanol, Malate and Arginine on Histamine Production of Lactobacillus hilgardii Isolated from an Italian Red Wine. Amino Acids 2009, 36, 81–89. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Amárita, F.; Lavilla, M.; Rainieri, S. Ecology of Indigenous Lactic Acid Bacteria from Rioja Alavesa Red Wines, Focusing on Biogenic Amine Production Ability. LWT 2019, 116, 108544. [Google Scholar] [CrossRef]
- Bonnin-Jusserand, M.; Grandvalet, C.; Rieu, A.; Weidmann, S.; Alexandre, H. Tyrosine-Containing Peptides Are Precursors of Tyramine Produced by Lactobacillus plantarum Strain IR BL0076 Isolated from Wine. BMC Microbiol. 2012, 12, 199. [Google Scholar] [CrossRef]
- Henríquez-Aedo, K.; Durán, D.; Garcia, A.; Hengst, M.B.; Aranda, M. Identification of Biogenic Amines-Producing Lactic Acid Bacteria Isolated from Spontaneous Malolactic Fermentation of Chilean Red Wines. LWT Food Sci. Technol. 2016, 68, 183–189. [Google Scholar] [CrossRef]
- Marcobal, A.; de Las Rivas, B.; Moreno-Arribas, M.V.; Muñoz, R. Identification of the Ornithine Decarboxylase Gene in the Putrescine-Producer Oenococcus oeni BIFI-83. FEMS Microbiol. Lett. 2004, 239, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.M.; Claisse, O.; Lonvaud-Funel, A. High Frequency of Histamine-Producing Bacteria in the Enological Environment and Instability of the Histidine Decarboxylase Production Phenotype. Appl. Environ. Microbiol. 2008, 74, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Ferrer, S.; Pardo, I. Which Lactic Acid Bacteria Are Responsible for Histamine Production in Wine? J. Appl. Microbiol. 2005, 99, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Carafa, T.; Garcia-Moruno, E. An Overview on Biogenic Amines in Wine. Beverages 2019, 5, 19. [Google Scholar] [CrossRef]
- Arena, M.P.; Russo, P.; Capozzi, V.; Beneduce, L.; Spano, G. Effect of Abiotic Stress Conditions on Expression of the Lactobacillus brevis IOEB 9809 Tyrosine Decarboxylase and Agmatine Deiminase Genes. Ann. Microbiol. 2011, 61, 179–183. [Google Scholar] [CrossRef]
- Garcia-Moruno, E.; Muñoz, R. Does Oenococcus oeni Produce Histamine? Int. J. Food Microbiol. 2012, 157, 121–129. [Google Scholar] [CrossRef]
- Izquierdo Cañas, P.M.; Gómez Alonso, S.; Ruiz Pérez, P.; Seseña Prieto, S.; García Romero, E.; Palop Herreros, M.L.L. Biogenic Amine Production by Oenococcus oeni Isolates from Malolactic Fermentation of Tempranillo Wine. J. Food Prot. 2009, 72, 907–910. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. Occurrence of Lactic Acid Bacteria and Biogenic Amines in Biologically Aged Wines. Food Microbiol. 2008, 25, 875–881. [Google Scholar] [CrossRef]
- Sebastian, P.; Herr, P.; Fischer, U.; König, H. Molecular Identification of Lactic Acid Bacteria Occurring in Must and Wine. South Afr. J. Enol. Vitic. 2011, 32, 300–309. [Google Scholar] [CrossRef]
- Battistelli, N.; Perpetuini, G.; Perla, C.; Arfelli, G.; Zulli, C.; Rossetti, A.P.; Tofalo, R. Characterization of Natural Oenococcus oeni Strains for Montepulciano d’Abruzzo Organic Wine Production. Eur. Food Res. Technol. 2020, 246, 1031–1039. [Google Scholar] [CrossRef]
- Tristezza, M.; Vetrano, C.; Bleve, G.; Spano, G.; Capozzi, V.; Logrieco, A.; Mita, G.; Grieco, F. Biodiversity and Safety Aspects of Yeast Strains Characterized from Vineyards and Spontaneous Fermentations in the Apulia Region, Italy. Food Microbiol. 2013, 36, 335–342. [Google Scholar] [CrossRef]
- Sciancalepore, A.G.; Mele, E.; Arcadio, V.; Reddavide, F.; Grieco, F.; Spano, G.; Lucas, P.; Mita, G.; Pisignano, D. Microdroplet-Based Multiplex PCR on Chip to Detect Foodborne Bacteria Producing Biogenic Amines. Food Microbiol. 2013, 35, 10–14. [Google Scholar] [CrossRef]
- Vincenzini, M.; Guerrini, S.; Mangani, S.; Granchi, L. Amino Acid Metabolisms and Production of Biogenic Amines and Ethyl Carbamate. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 231–253. ISBN 978-3-319-60021-5. [Google Scholar]
- Uthurry, C.A.; Lepe, J.A.S.; Lombardero, J.; García Del Hierro, J.R. Ethyl Carbamate Production by Selected Yeasts and Lactic Acid Bacteria in Red Wine. Food Chem. 2006, 94, 262–270. [Google Scholar] [CrossRef]
- Mira De Orduña, R.; Patchett, M.L.; Liu, S.Q.; Pilone, G.J. Growth and Arginine Metabolism of the Wine Lactic Acid Bacteria Lactobacillus buchneri and Oenococcus oeni at Different pH Values and Arginine Concentrations. Appl. Environ. Microbiol. 2001, 67, 1657–1662. [Google Scholar] [CrossRef]
- Tonon, T.; Lonvaud-Funel, A. Arginine Metabolism by Wine Lactobacilli Isolated from Wine. Food Microbiol. 2002, 19, 451–461. [Google Scholar] [CrossRef]
- Cañas, P.M.I.; Pérez-Martín, F.; Romero, E.G.; Prieto, S.S.; de los Llanos Palop Herreros, M. Influence of Inoculation Time of an Autochthonous Selected Malolactic Bacterium on Volatile and Sensory Profile of Tempranillo and Merlot Wines. Int. J. Food Microbiol. 2012, 156, 245–254. [Google Scholar] [CrossRef]
- Zapparoli, G.; Tosi, E.; Azzolini, M.; Vagnoli, P.; Krieger, S. Bacterial Inoculation Strategies for the Achievement of Malolactic Fermentation in High-Alcohol Wines. South Afr. J. Enol. Vitic. 2009, 30, 49–55. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.C.; de Revel, G. Co-Inoculation with Yeast and LAB Under Winery Conditions: Modification of the Aromatic Profile of Merlot Wines. South Afr. J. Enol. Vitic. 2013, 34, 223–232. [Google Scholar] [CrossRef]
- Guzzon, R.; Moser, S.; Davide, S.; Villegas, T.R.; Malacarne, M.; Larcher, R.; Nardi, T.; Vagnoli, P.; Krieger-Weber, S. Exploitation of Simultaneous Alcoholic and Malolactic Fermentation of Incrocio Manzoni, a Traditional Italian White Wine. South Afr. J. Enol. Vitic. 2016, 37, 124–131. [Google Scholar] [CrossRef][Green Version]
- Bartle, L.; Sumby, K.; Sundstrom, J.; Jiranek, V. The Microbial Challenge of Winemaking: Yeast-Bacteria Compatibility. FEMS Yeast Res. 2019, 19. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, J.M.; Simó, G.; Pérez-Magariño, S.; Cano-Mozo, E.; Fernández-Fernández, E.; Ruipérez, V.; Vila-Crespo, J. Evaluating the Influence of Simultaneous Inoculation of SiO2-Alginate Encapsulated Bacteria and Yeasts on Volatiles, Amino Acids, Biogenic Amines and Sensory Profile of Red Wine with Lysozyme Addition. Food Chem. 2020, 327, 126920. [Google Scholar] [CrossRef]
- Smit, A.Y.; Engelbrecht, L.; du Toit, M. Managing Your Wine Fermentation to Reduce the Risk of Biogenic Amine Formation. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; La Hens, D.V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E.E.; Semorile, L. Comparative Vinification Assays with Selected Patagonian Strains of Oenococcus oeni and Lactobacillus plantarum. LWT 2017, 77, 348–355. [Google Scholar] [CrossRef]
- Russo, P.; Englezos, V.; Capozzi, V.; Pollon, M.; Río Segade, S.; Rantsiou, K.; Spano, G.; Cocolin, L. Effect of Mixed Fermentations with Starmerella bacillaris and Saccharomyces cerevisiae on Management of Malolactic Fermentation. Food Res. Int. 2020, 134, 109246. [Google Scholar] [CrossRef] [PubMed]
- Nardi, T.; Panero, L.; Petrozziello, M.; Guaita, M.; Tsolakis, C.; Cassino, C.; Vagnoli, P.; Bosso, A. Managing Wine Quality Using Torulaspora delbrueckii and Oenococcus oeni Starters in Mixed Fermentations of a Red Barbera Wine. Eur. Food Res. Technol. 2019, 245, 293–307. [Google Scholar] [CrossRef]
- Capozzi, V.; Berbegal, C.; Tufariello, M.; Grieco, F.; Spano, G.; Grieco, F. Impact of Co-Inoculation of Saccharomyces cerevisiae, Hanseniaspora uvarum and Oenococcus oeni Autochthonous Strains in Controlled Multi Starter Grape Must Fermentations. LWT 2019, 109, 241–249. [Google Scholar] [CrossRef]
- Tempère, S.; Marchal, A.; Barbe, J.-C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The Complexity of Wine: Clarifying the Role of Microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef] [PubMed]
- Sipsas, V.; Kolokythas, G.; Kourkoutas, Y.; Plessas, S.; Nedovic, V.A.; Kanellaki, M. Comparative Study of Batch and Continuous Multi-Stage Fixed-Bed Tower (MFBT) Bioreactor during Wine-Making Using Freeze-Dried Immobilized Cells. J. Food Eng. 2009, 90, 495–503. [Google Scholar] [CrossRef]
- Bleve, G.; Lezzi, C.; Chiriatti, M.A.; D’Ostuni, I.; Tristezza, M.; Venere, D.D.; Sergio, L.; Mita, G.; Grieco, F. Selection of Non-Conventional Yeasts and Their Use in Immobilized Form for the Bioremediation of Olive Oil Mill Wastewaters. Bioresour. Technol. 2011, 102, 982–989. [Google Scholar] [CrossRef]
- Simó, G.; Vila-Crespo, J.; Fernández-Fernández, E.; Ruipérez, V.; Rodríguez-Nogales, J.M. Highly Efficient Malolactic Fermentation of Red Wine Using Encapsulated Bacteria in a Robust Biocomposite of Silica-Alginate. J. Agric. Food Chem. 2017, 65, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Genisheva, Z.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Malolactic Fermentation of Wines with Immobilised Lactic Acid Bacteria—Influence of Concentration, Type of Support Material and Storage Conditions. Food Chem. 2013, 138, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Tufariello, M.; Vetrano, C.; Mita, G.; Grieco, F. Simultaneous Alcoholic and Malolactic Fermentations by Saccharomyces cerevisiae and Oenococcus oeni Cells Co-Immobilized in Alginate Beads. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Izquierdo, P.M.; Seseña, S.; Palop, M.L. Selection of Autochthonous Oenococcus oeni Strains According to Their Oenological Properties and Vinification Results. Int. J. Food Microbiol. 2010, 137, 230–235. [Google Scholar] [CrossRef] [PubMed]
| Grape and Harvest | Must and AF | Wine and MLF |
|---|---|---|
| Oenococcus oeni (0–10%) Limosilactobacillus alvi Levilactobacillus brevis Limosilactobacillus frumenti Liquorilactobacillus mali Apilactobacillus kunkeei Fructilactobacillus lindneri Fructilactobacillus sanfranciscensis Lentilactobacillus kefiri Lactococcus lactis Enterococcus faecium Enterococcus avium Enterococcus durans Enterococcus hermanniensis Leuconostoc mesenteroides Pediococcus damnosus Pediococcus parvalus Weissella paramesenteroides | Oenococcus oeni (80–100%) Lactiplantibacillus plantarum Lentilactobacillus hilgardii Lentilactobacillus buchneri Lentilactobacillus diolivorans Lacticaseibacillus casei Latilactobacillus curvatus Limosilactobacillus alvi Levilactobacillus brevis Limosilactobacillus frumenti Secundilactobacillus collinoides Lacticaseibacillus paracasei Lactiplantibacillus pentosus Liquorilactobacillus mali Fructilactobacillus lindneri Fructilactobacillus fructivorans Lactobacillus delbrueckii Lactococcus lactis Leuconostoc citreum Leuconostoc fructosum Leuconostoc mesenteroides Enterococcus faecium Pediococcus damnosus Pediococcus parvalus Weissella paramesenteroides | Oenococcus oeni Lactiplantibacillus plantarum Lentilactobacillus hilgardii Levilactobacillus brevis Fructilactobacillus lindneri Limosilactobacillus frumenti Lactococcus sp. Pediococcus parvalus |
| Strains | Place of isolation | Reference |
|---|---|---|
| Oenococcus oeni PSU-1 | United States of America | [38] |
| 11 O. oeni strains (6 commercial and 5 environmental isolates) | Commercial starter cultures and Australia | [39] |
| Oenococcus oeni OM27 | Italy | [40] |
| 5 O. oeni strains isolated from the same terroir | Italy | [41] |
| 14 O. oeni strains isolated from different wines | France | [42] |
| 28 O. oeni strains isolated from different wines | Several countries (mainly from France and Australia) | [43] |
| Oenococcus oeni X2L | Argentina | [44] |
| About 135 O. oeni wine strains | Several countries (mainly from France and Australia) | [45] |
| Oenococcus oeni UNQOe19 | Patagonia | [46] |
| Lactiplantibacillus plantarum Lp90 | Italy | [47] |
| Lactiplantibacillus plantarum XJ25 | China | [48] |
| Lactiplantibacillus plantarum UNQLp 11 | Patagonia | [49] |
| Microbial Species | Produced BAs | References |
|---|---|---|
| Enterococcus faecium | tyramine | [128] |
| Levilactobacillus brevis | tyramine, putrescine, phenylethylamine | [129,130,131,132] |
| Lentilactobacillus hilgardii | histamine, tyramine, putrescine, phenylethylamine | [130,131,133,134] |
| Lactiplantibacillus plantarum | tyramine | [135] |
| Lacticaseibacillus rhamnosus | histamine | [136] |
| Oenococcus oeni | histamine, putrescine | [137,138] |
| Pediococcus parvulus | histamine | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. https://doi.org/10.3390/fermentation7010024
Capozzi V, Tufariello M, De Simone N, Fragasso M, Grieco F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation. 2021; 7(1):24. https://doi.org/10.3390/fermentation7010024
Chicago/Turabian StyleCapozzi, Vittorio, Maria Tufariello, Nicola De Simone, Mariagiovanna Fragasso, and Francesco Grieco. 2021. "Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety" Fermentation 7, no. 1: 24. https://doi.org/10.3390/fermentation7010024
APA StyleCapozzi, V., Tufariello, M., De Simone, N., Fragasso, M., & Grieco, F. (2021). Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation, 7(1), 24. https://doi.org/10.3390/fermentation7010024







