Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits
Abstract
1. Introduction
2. EPS Classification
2.1. Homopolysaccharides
2.2. Heteropolysaccharides
3. EPS Production
3.1. Effect of the Substrate Composition
3.2. Effect of Bacterial Strain and Incubation Parameters on EPS Production
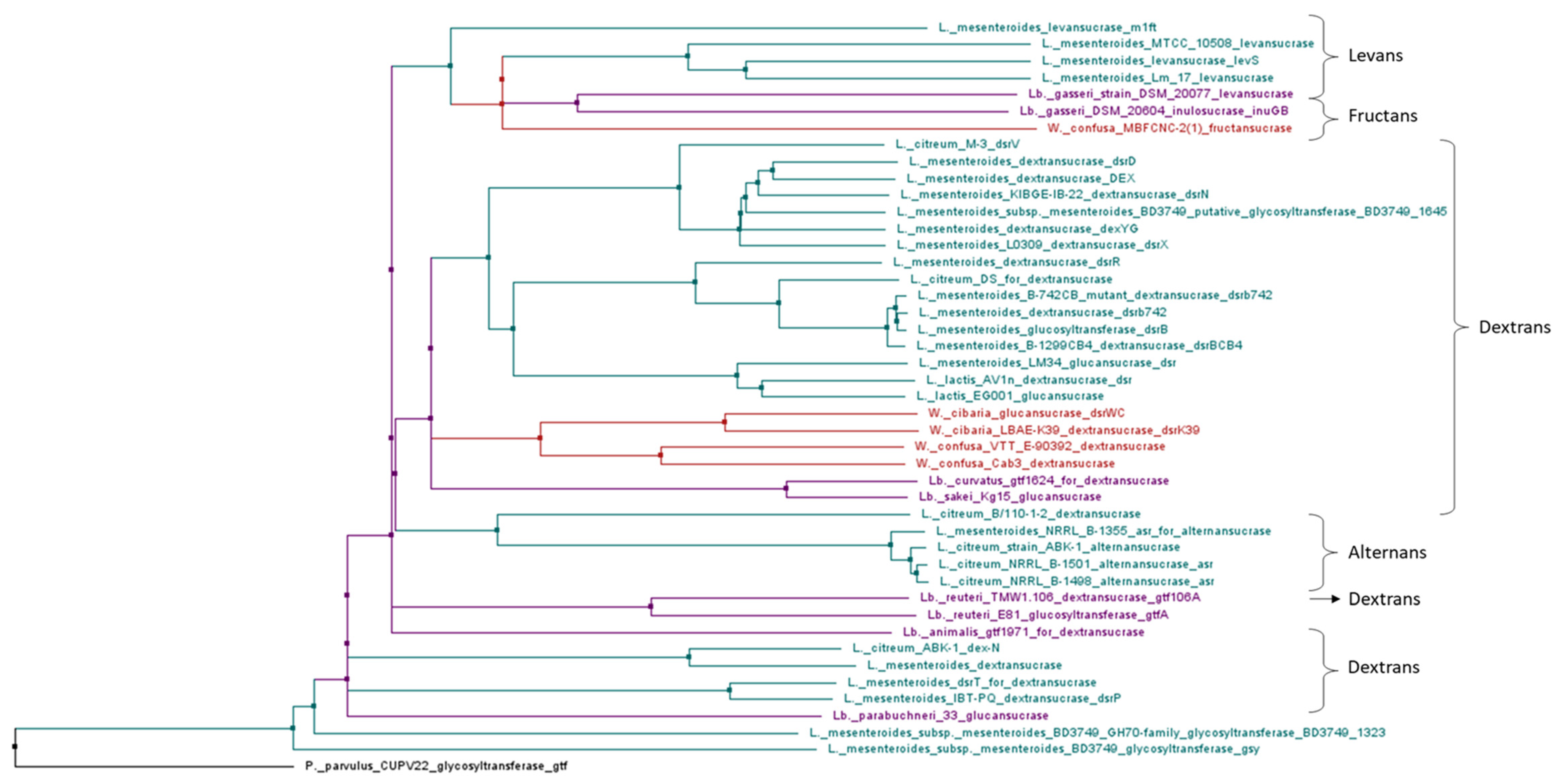
3.3. HoPS Production Pathway
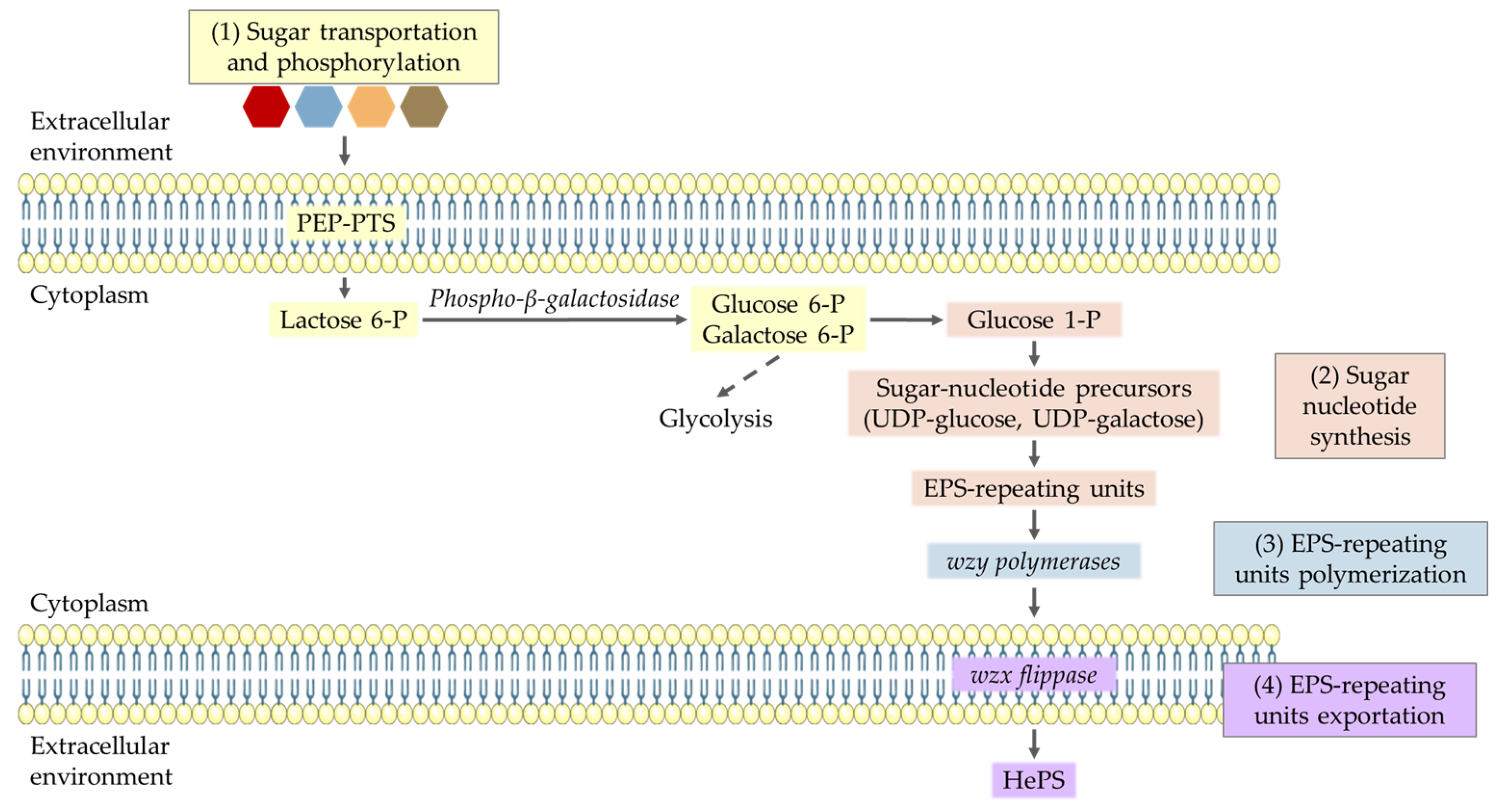
3.4. HePS Production Pathway
4. Enzyme Activities, Genes Involved and HoPS Synthesis Pathway Regulations
4.1. Dextransucrase Activity and In vitro Enzyme Properties
4.2. Genes Involved in Production of HoPS
5. LAB Producing EPS
5.1. L. mesenteroides and L. pseudomesenteroides
5.2. Leuconostoc lactis
5.3. Weissella cibaria
5.4. Lactobacillus Species
5.4.1. Lactobacillus plantarum
5.4.2. Lactobacillus fermentum
5.4.3. Lactobacillus rhamnosus
5.5. Other LAB Species
5.5.1. Pediococcus spp.
5.5.2. Bifidobacterium spp.
6. Production of EPS during Lactic Acid Fermentation of Fruit and Vegetables and Consequences on Food Quality
7. Health Benefits of EPS
7.1. Prebiotic Properties
7.2. Antioxidant Activity
7.3. Anti-Inflammatory Activity
7.4. Cholesterol-Lowering Activity
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic acid bacteria exopolysaccharides in foods and beverages: Isolation, properties, characterization, and health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Campbell-Sills, H.; El Khoury, M.; Favier, M.; Romano, A.; Biasioli, F.; Spano, G.; Sherman, D.J.; Bouchez, O.; Coton, E.; Coton, M.; et al. Phylogenomic analysis of Oenococcus oeni reveals specific domestication of strains to cider and wines. Genome Biol. Evol. 2015, 7, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Tang, D.; Zhang, Y. Comparing product stability of probiotic beverages using litchi juice treated by high hydrostatic pressure and heat as substrates. Innov. Food Sci. Emerg. Technol. 2014, 23, 61–67. [Google Scholar] [CrossRef]
- Juvonen, R.; Honkapää, K.; Maina, N.H.; Shi, Q.; Viljanen, K.; Maaheimo, H.; Virkki, L.; Tenkanen, M.; Lantto, R. The impact of fermentation with exopolysaccharide producing lactic acid bacteria on rheological, chemical and sensory properties of pureed carrots (Daucus carota L.). Int. J. Food Microbiol. 2015, 207, 109–118. [Google Scholar] [CrossRef]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by Archaea. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef]
- Ripari, V. Techno-functional role of exopolysaccharides in cereal-based, yogurt-like beverages. Beverages 2019, 5, 16. [Google Scholar] [CrossRef]
- Silva, L.A.; Lopes Neto, J.H.P.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]
- Singh, P.; Saini, P. Food and health potentials of exopolysaccharides derived from Lactobacilli. Microbiol. Res. J. Int. 2017, 22, 1–14. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria: Structures, physiochemical functions and applications in the food industry. Food Hydrocoll. 2019, 94, 475–499. [Google Scholar] [CrossRef]
- Oleksy, M.; Klewicka, E. Exopolysaccharides produced by Lactobacillus sp.: Biosynthesis and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 450–462. [Google Scholar] [CrossRef]
- Farinazzo, F.S.; Valente, L.J.; Almeida, M.B.; Simionato, A.S.; Thereza, M.; Fernandes, C.; Saori, C.; Mauro, I.; Aparecida, A.; Tomal, B.; et al. Characterization and antioxidant activity of an exopolysaccharide produced by Leuconostoc pseudomesenteroides JF17 from juçara fruits (Euterpe edulis Martius). Process Biochem. 2020, 91, 141–148. [Google Scholar] [CrossRef]
- Wade, M.E.; Strickland, M.T.; Osborne, J.P.; Edwards, C.G. Role of Pediococcus in winemaking. Aust. J. Grape Wine Res. 2018, 25, 7–24. [Google Scholar] [CrossRef]
- Cerning, J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol. Lett. 1990, 87, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.Y.M.; Reehana, N.; Jayaraj, K.A.; Ahamed, A.A.P.; Dhanasekaran, D.; Thajuddin, N.; Alharbi, N.S.; Muralitharan, G. Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int. J. Biol. Macromol. 2016, 93, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Ciqual: Table de Composition Nutritionnelle des Aliments. Available online: https://ciqual.anses.fr/ (accessed on 23 October 2020).
- Guerrero, S.N.; Alzamora, S.M. Effect of pH, Temperature and Glucose Addition on Flow Behaviour of Fruit Purees: II. Peach, Papaya and Mango Purées. J. Food Eng. 1998, 37, 77–101. [Google Scholar] [CrossRef]
- Laloknam, S.; Sirisopana, S.; Phornphisutthimas, S. Learning retention in undergraduate biology using a hands-on practical “enzyme detection from vegetables and fruits”. J. Chem. 2010, 4, 29–35. [Google Scholar]
- Yu, Y.J.; Chen, Z.; Chen, P.T.; Ng, I.S. Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Mauch, A.; Galle, S.; Gänzle, M.; Coffey, A.; Arendt, E.K.; Taylor, J.P.; Waters, D.M. Barley malt wort fermentation by exopolysaccharide-forming Weissella cibaria MG1 for the production of a novel beverage. J. Appl. Microbiol. 2013, 115, 1379–1387. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Besrour-Aouam, N.; Mohedano, M.L.; Fhoula, I.; Zarour, K.; Najjari, A.; Aznar, R.; Prieto, A.; Ouzari, H.I.; López, P. Different modes of regulation of the expression of dextransucrase in Leuconostoc lactis AV1n and Lactobacillus sakei MN. Front. Microbiol. 2019, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Dertli, E.; Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Cankurt, H.; Sagdic, O. Effects of in situ exopolysaccharide production and fermentation conditions on physicochemical, microbiological, textural and microstructural properties of Turkish-type fermented sausage (sucuk). Meat Sci. 2016, 121, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.S.; Xue, C. Enhanced exopolysaccharide production and biological activity of Lactobacillus rhamnosus ZY with calcium and hydrogen peroxide. Process Biochem. 2017, 52, 295–304. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; van Leeuwen, S.S.; Kralj, S.; Dijkstra, B.W.; Dijkhuizen, L. Glucansucrases: Three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J. Biotechnol. 2013, 163, 250–272. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Zhu, P.; Liu, Z.; Guo, B.; Ren, J. Improvement of exopolysaccharide production in Lactobacillus casei LC2W by overexpression of NADH oxidase gene. Microbiol. Res. 2015, 171, 73–77. [Google Scholar] [CrossRef]
- Zafar, S.B.; Siddiqui, N.N.; Shahid, F.; Qader, S.A.U.; Aman, A. Bioprospecting of indigenous resources for the exploration of exopolysaccharide producing lactic acid bacteria. J. Genet. Eng. Biotechnol. 2018, 16, 17–22. [Google Scholar] [CrossRef]
- Bounaix, M.S.; Robert, H.; Gabriel, V.; Morel, S.; Remaud-Siméon, M.; Gabriel, B.; Fontagné-Faucher, C. Characterization of dextran-producing Weissella strains isolated from sourdoughs and evidence of constitutive dextransucrase expression. FEMS Microbiol. Lett. 2010, 311, 18–26. [Google Scholar] [CrossRef]
- Kang, H.K.; Oh, J.S.; Kim, D. Molecular characterization and expression analysis of the glucansucrase DSRWC from Weissella cibaria synthesizing a α(1→6) glucan. FEMS Microbiol. Lett. 2009, 292, 33–41. [Google Scholar] [CrossRef]
- Amari, M.; Arango, L.F.G.; Gabriel, V.; Robert, H.; Morel, S.; Moulis, C.; Gabriel, B.; Remaud-Siméon, M.; Fontagné-Faucher, C. Characterization of a novel dextransucrase from Weissella confusa isolated from sourdough. Appl. Microbiol. Biotechnol. 2013, 97, 5413–5422. [Google Scholar] [CrossRef]
- Schmid, J.; Bechtner, J.; Vogel, R.F.; Jakob, F. A systematic approach to study the pH-dependent release, productivity and product specificity of dextransucrases. Microb. Cell Fact. 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Baruah, R.; Deka, B.; Goyal, A. Purification and characterization of dextransucrase from Weissella cibaria RBA12 and its application in in vitro synthesis of prebiotic oligosaccharides in mango and pineapple juices. LWT-Food Sci. Technol. 2017, 84, 449–456. [Google Scholar] [CrossRef]
- Dertli, E.; Mercan, E.; Arici, M.; Yilmaz, M.T.; Sağdiç, O. Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT 2016, 71, 116–124. [Google Scholar] [CrossRef]
- Heng, N.C.K.; Yeh, C.W.; Malik, A. Draft genome sequence of Weissella confusa MBF8-1, a glucansucrase- and bacteriocinproducing strain isolated from a homemade soy product. Genome Announc. 2017, 5, e01497-16. [Google Scholar] [CrossRef]
- Malik, A.; Sheilla, S.; Firdausi, W.; Handayani, T.; Saepudin, E. Sucrase activity and exopolysaccharide partial characterization from three Weissella confusa strains. HAYATI J. Biosci. 2015, 22, 130–135. [Google Scholar] [CrossRef][Green Version]
- Côté, G.L.; Skory, C.D. Cloning, expression, and characterization of an insoluble glucan-producing glucansucrase from Leuconostoc mesenteroides NRRL B-1118. Appl. Microbiol. Biotechnol. 2012, 93, 2387–2394. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wu, H.C.; Pan, S.F.; Lin, B.G.; Lin, Y.H.; Tung, W.C.; Li, Y.L.; Chiang, C.M.; Yanagida, F. Isolation and characterization of lactic acid bacteria from yan-taozih (pickled peaches) in Taiwan. Ann. Microbiol. 2013, 63, 607–614. [Google Scholar] [CrossRef]
- Montet, D.; Ray, R.C.; Zakhia-Rozis, N. Lactic acid fermentation of vegetables and fruits. In Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2014; pp. 108–140. ISBN 9781482223095. [Google Scholar]
- Sánchez, I.; Palop, L.; Ballesteros, C. Biochemical characterization of lactic acid bacteria isolated from spontaneous fermentation of “Almagro” eggplants. Int. J. Food Microbiol. 2000, 59, 9–17. [Google Scholar] [CrossRef]
- Abdalrahim, S.; Zohri, A.N.A.; Khider, M.; Kamal El-Dean, A.M.; Abulreesh, H.H.; Ahmad, I.; Elbanna, K. Phenotypic and genotypic characterization of exopolysaccharide producing bacteria isolated from fermented fruits, vegetables and dairy products. J. Pure Appl. Microbiol. 2019, 13, 1349–1362. [Google Scholar] [CrossRef]
- Ahmed, R.Z.; Siddiqui, K.; Arman, M.; Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr. Polym. 2012, 90, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Malang, S.K.; Maina, N.H.; Schwab, C.; Tenkanen, M.; Lacroix, C. Characterization of exopolysaccharide and ropy capsular polysaccharide formation by Weissella. Food Microbiol. 2015, 46, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Adesulu-Dahunsi, A.T.; Jeyaram, K.; Isiaka Sanni, A.; Banwo, K. Production of exopolysaccharide by strains of Lactobacillus plantarum YO175 and OF101 isolated from traditional fermented cereal beverage. PeerJ 2018, 2018, e5326. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Baruah, R.; Goyal, A. A food additive with prebiotic properties of an α-d-glucan from Lactobacillus plantarum DM5. Int. J. Biol. Macromol. 2014, 69, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Rojas, M.F.; Reinheimer, J.A.; Binetti, A.G. Lactobacillus fermentum: Could EPS production ability be responsible for functional properties? Food Microbiol. 2020, 90, 103465. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef]
- Salazar, N.; Prieto, A.; Leal, J.A.; Mayo, B.; Bada-Gancedo, J.C.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Production of exopolysaccharides by Lactobacillus and Bifidobacterium strains of human origin, and metabolic activity of the producing bacteria in milk. J. Dairy Sci. 2009, 92, 4158–4168. [Google Scholar] [CrossRef]
- Grosu-Tudor, S.-S.; Zamfir, M. Exopolysaccharide production by selected lactic acid bacteria isolated from fermented vegetables. Sci. Bull. Ser. Biotechnol. 2014, 18, 107–114. [Google Scholar]
- Lee, S.; Park, Y.S. Draft genome sequence of oligosaccharide producing Leuconostoc lactis CCK940 isolated from kimchi in Korea. Korean J. Microbiol. 2018, 54, 445–447. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT-Food Sci. Technol. 2018, 87, 432–442. [Google Scholar] [CrossRef]
- Sánchez, I.; Seseña, S.; Palop, L. Identification of lactic acid bacteria from spontaneous fermentation of “Almagro” eggplants by SDS-PAGE whole cell protein fingerprinting. Int. J. Food Microbiol. 2003, 82, 181–189. [Google Scholar] [CrossRef]
- Farias, N.; Soares, M.; Gouveia, E. Enhancement of the viability of Lactobacillus rhamnosus ATCC 7469 in passion fruit juice: Application of a central composite rotatable design. LWT-Food Sci. Technol. 2016, 71, 149–154. [Google Scholar] [CrossRef]
- Andrade, R.; Santos, E.; Azoubel, P.; Ribeiro, E. Increased survival of Lactobacillus rhamnosus ATCC 7469 in guava juices with simulated gastrointestinal conditions during refrigerated storage. Food Biosci. 2019, 32, 100470. [Google Scholar] [CrossRef]
- de Andrade Pires, B.; de Almeida Bianchini Campos, R.C.; Canuto, J.W.; de Melo Carlos Dias, T.; Martins, E.M.F.; Licursi, L.; de Castro Leite Júnior, B.R.; Martins, M.L. Lactobacillus rhamnosus GG in a mixed pineapple (Ananas comosus L. Merril) and jussara (Euterpe edulis Martius) beverage and its survival in the human gastrointestinal tract. LWT 2020, 134, 110028. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Wu, H.-C.; Yu, C.-R.; Chen, Z.-Y.; Lu, Y.-C.; Yanagida, F. Isolation and characterization of lactic acid bacteria from xi-gua-mian (fermented watermelon), a traditional fermented food in Taiwan | Italian Journal of Food Science. Ital. J. Food Sci. 2016, 28, 9–14. [Google Scholar]
- Xu, X.; Luo, D.; Bao, Y.; Liao, X.; Wu, J. Characterization of diversity and probiotic efficiency of the autochthonous lactic acid bacteria in the fermentation of selected raw fruit and vegetable juices. Front. Microbiol. 2018, 9, 2539. [Google Scholar] [CrossRef]
- Saarela, M.; Virkajärvi, I.; Alakomi, H.L.; Sigvart-Mattila, P.; Mättö, J. Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. Int. Dairy J. 2006, 16, 1477–1482. [Google Scholar] [CrossRef]
- Lu, Y.; Putra, S.D.; Liu, S.Q. A novel non-dairy beverage from durian pulp fermented with selected probiotics and yeast. Int. J. Food Microbiol. 2018, 265, 1–8. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Han, J.; Hang, F.; Guo, B.; Liu, Z.; You, C.; Wu, Z. Dextran synthesized by Leuconostoc mesenteroides BD1710 in tomato juice supplemented with sucrose. Carbohydr. Polym. 2014, 112, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 1–17. [Google Scholar] [CrossRef]
- Pan, L.; Han, Y.; Zhou, Z. In vitro prebiotic activities of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its effect on the gut microbiota of mice. J. Funct. Foods 2020, 67, 103853. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Pan, D.; Mei, X. Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Dinic, M.; Pecikoza, U.; Djokic, J.; Stepanovic-Petrovic, R.; Milenkovic, M.; Stevanovic, M.; Filipovic, N.; Begovic, J.; Golic, N.; Lukic, J. Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats. Front. Pharmacol. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wang, L.; Li, J.; Fu, R.; Wang, S.; Zhang, J. In vitro and in vivo anti-inflammatory activity of a succinoglycan Riclin from Agrobacterium sp. ZCC3656. J. Appl. Microbiol. 2019, 127, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, K.; Kozhummal Vaikkath, D.; Devendra, L.; Nampoothiri, K.M. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresour. Technol. 2018, 254, 264–267. [Google Scholar] [CrossRef]
- Lim, J.; Kale, M.; Kim, D.H.; Kim, H.S.; Chon, J.W.; Seo, K.H.; Lee, H.G.; Yokoyama, W.; Kim, H. Antiobesity effect of exopolysaccharides isolated from kefir grains. J. Agric. Food Chem. 2017, 65, 10011–10019. [Google Scholar] [CrossRef]



| Gene | Function | EPS | LAB Species | Ref. |
|---|---|---|---|---|
| gtf | Glucansucrase gene | Dextran | Lb. plantarum, Lb. curvatus, Lb. rossiae, Lb. sanfranciscensis, Lb. brevis, Lb. paralimentarius, W. paramesenteroides, L. mesenteroides, L. pseudomesenteroides, W. cibaria, W. confusa | [35,36] |
| ftf | Fructansucrase | Levan | W. confusa | [37] |
| lev | Levansucrase gene | Levan | Lb. paraplantarum, Lb. sanfranciscensis, Lb. paralimentarius, W. paramesenteroides, L. mesenteroides, L. pseudomesenteroides | [35] |
| dsr | Gene of extracellular Dsr enzyme | Dextran | Streptococcus, Leuconostoc and Lactobacillus strains | [23] |
| dps | Transmembrane glucosyltransferase (Gtf) gene | β-glucans | Pediococcus spp. | [14] |
| LAB Species | EPS Structure | Production Improvement Factor | Observed Effect on the Product | Ref. |
|---|---|---|---|---|
| L. mesenteroides | Levan-type and fructans | Sucrose | Strong acidity of smell and taste, poor sweetness, enhanced thickness | [6,42] |
| L. pseudomesenteroides | Dextrans with α-(1 → 4) linkages | Sucrose | [6,42] | |
| L. lactis | Dextrans, mainly glucopyranose units with 𝛼-(1 → 6) linkages and side chains made of a 𝛼-glucopyranose unit | [23] | ||
| W. cibaria | Dextrans with ⍺-(1 → 6) linkages and ⍺-(1 → 3) linked branches, or Fructans | [43,44] | ||
| Lb. plantarum | HePS containing fructose, arabinose, galactose, glucose, and mannose⍺-D-glucan with ⍺-(1 → 6) linkage and 𝛼-(1 → 3) branching | Glucose | [45,46,47] | |
| Lb. fermentum | β-glucan and two HePS composed of glucose and galactose | Glucose | [48] | |
| Lb. rhamnosus | EPS containing rhamnose, glucose and galactose | [49] | ||
| Pediococcus spp. | β-glucans composed of D-glucose with β-(1 → 3) linkages and β-(1 → 2) branches | Enhance viscosity, modulated mouthfeel | [14] | |
| Bifidobacterium spp. | HePS containing galactose, glucose and rhamnose | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guérin, M.; Silva, C.R.-D.; Garcia, C.; Remize, F. Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits. Fermentation 2020, 6, 115. https://doi.org/10.3390/fermentation6040115
Guérin M, Silva CR-D, Garcia C, Remize F. Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits. Fermentation. 2020; 6(4):115. https://doi.org/10.3390/fermentation6040115
Chicago/Turabian StyleGuérin, Marie, Christine Robert-Da Silva, Cyrielle Garcia, and Fabienne Remize. 2020. "Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits" Fermentation 6, no. 4: 115. https://doi.org/10.3390/fermentation6040115
APA StyleGuérin, M., Silva, C. R.-D., Garcia, C., & Remize, F. (2020). Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits. Fermentation, 6(4), 115. https://doi.org/10.3390/fermentation6040115







