Antioxidant Content of Aronia Infused Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
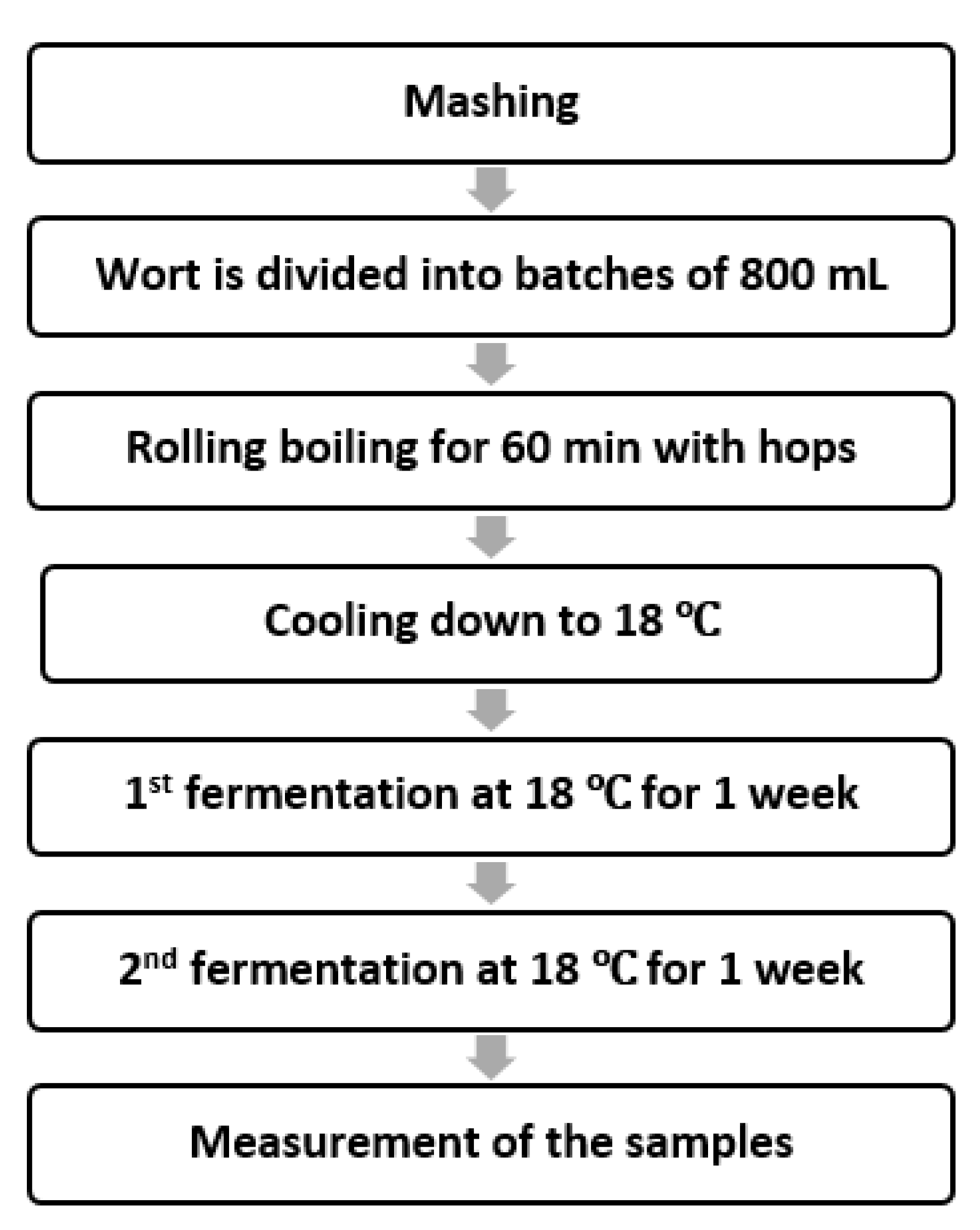
2.2. Brewing and Aronia Berry Infusion
2.3. Glucose Equivalent, pH and Color Analysis
2.4. Total Polyphenol Content (TPC) Assay
2.5. Ascorbic Acid Equivalent Antioxidant Capacity (AEAC) According to DPPH
2.6. Statistical Analysis
3. Results and Discussion
3.1. Carbohydrate Content
3.2. Color
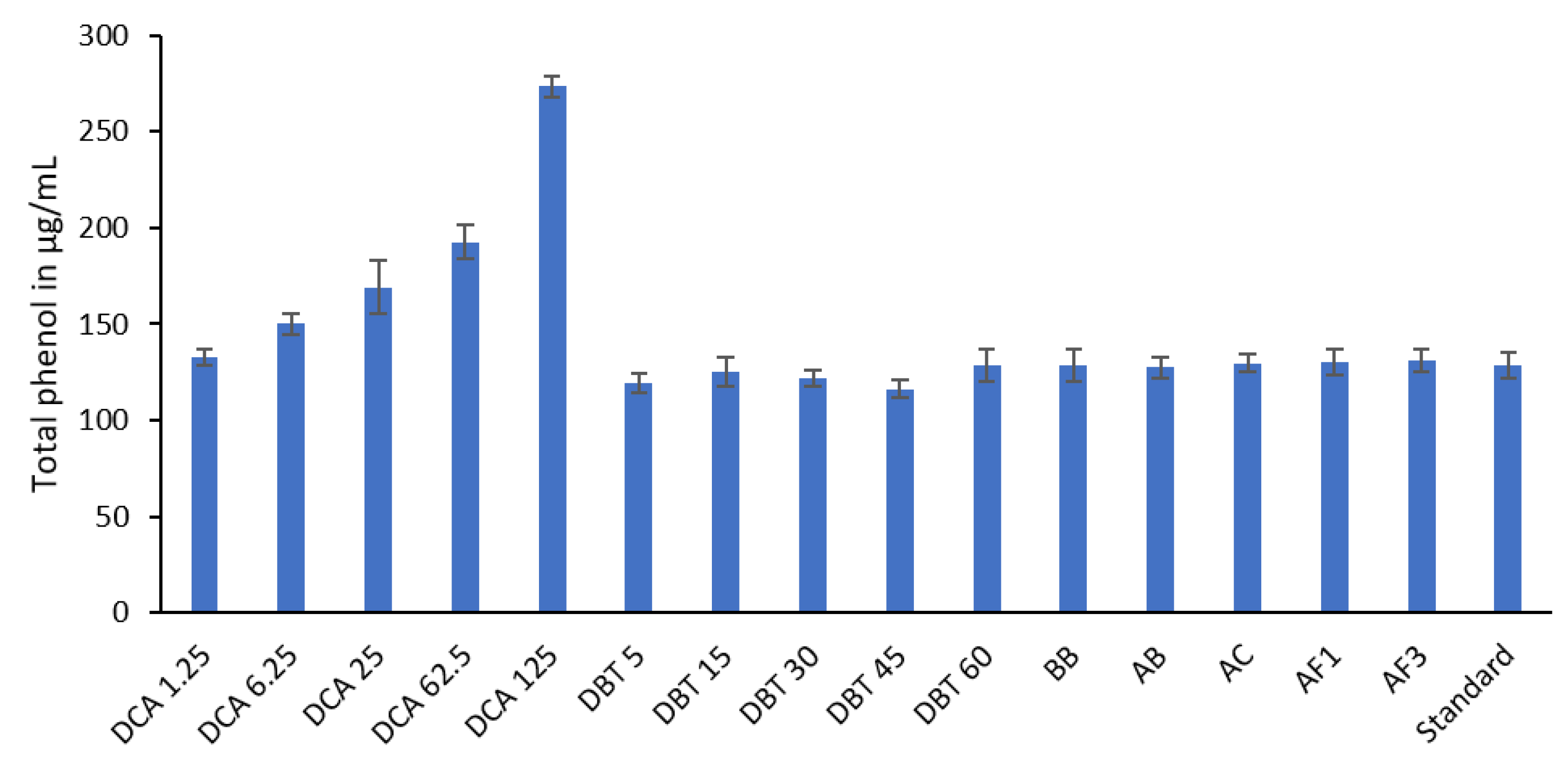
3.3. Total Phenol
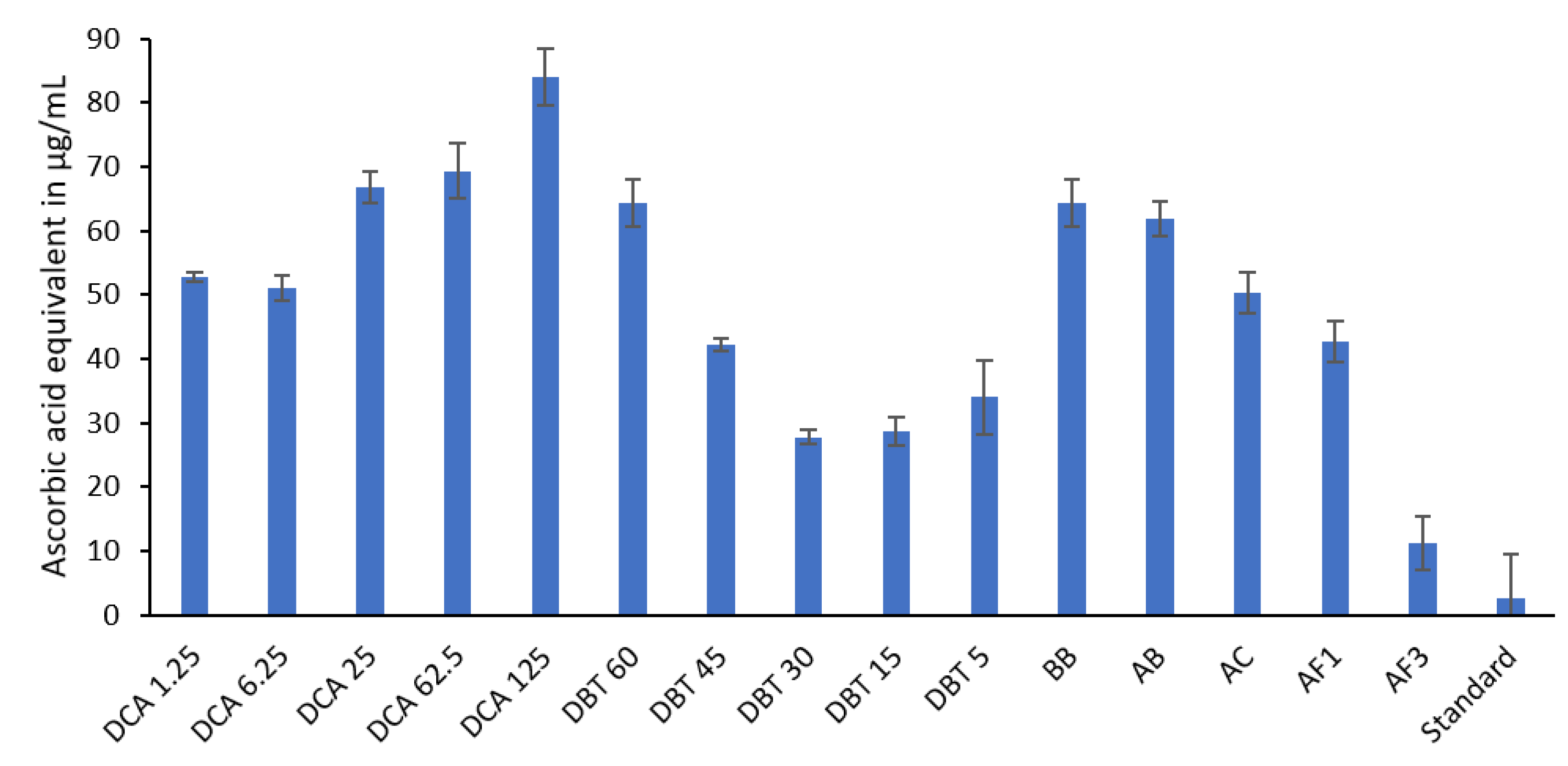
3.4. Ascorbic Acid Equivalent Antioxidant Capacity (AEAC) According to DPPH
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Pavsler, A.; Buiatti, S. Lager Beer. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 31–43. [Google Scholar] [CrossRef]
- Pavsler, A.; Buiatti, S. Non-lager Beer. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 17–30. [Google Scholar] [CrossRef]
- Cabras, I.; Bamforth, C. From reviving tradition to fostering innovation and changing marketing: The evolution of micro-brewing in the UK and US, 1980–2012. Bus. Hist. 2016, 58, 625–646. [Google Scholar] [CrossRef]
- Kellershohn, J.; Russell, I. Innovations in alcoholic beverage production. In Advances in Bioprocess Technology; Springer International Publishing: Cham, Switzerland, 2015; pp. 423–433. ISBN 9783319179155. [Google Scholar] [CrossRef]
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Prasad, M.P. In-Vitro Evaluation of Antioxidant Properties of Fermented Fruit Beer Samples. Int. J. Sci. Res. 2014, 3, 1545–1550. [Google Scholar]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Hall, M.B. Efficacy of reducing sugar and phenol-sulfuric acid assays for analysis of soluble carbohydrates in feedstuffs. Anim. Feed Sci. Technol. 2013, 185, 94–100. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and pH degradation kinetics of anthocyanins in natural food colorant prepared from black rice bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Tiitinen, K.; Vahvaselkä, M.; Laakso, S.; Kallio, H. Malolactic fermentation in four varieties of sea buckthorn (Hippophaë rhamnoides L.). Eur. Food Res. Technol. 2007, 224, 725–732. [Google Scholar] [CrossRef]
- Leiper, K.A.; Stewart, G.G.; McKeown, I.P.; Nock, T.; Thompson, M.J. Optimising Beer Stabilisation by the Selective Removal of Tannoids and Sensitive Proteins. J. Inst. Brew. 2005, 111, 118–127. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation | Description | Aronia Concentration | Point of Addition |
|---|---|---|---|
| DCA 1.25 | Different Aronia Concentration | 1.25 g/L | Before the boil |
| DCA 6.25 | Different Aronia Concentration | 6.25 g/L | Before the boil |
| DCA 25 | Different Aronia Concentration | 25 g/L | Before the boil |
| DCA 62.5 | Different Aronia Concentration | 62.5 g/L | Before the boil |
| DCA 125 | Different Aronia Concentration | 125 g/L | Before the boil |
| DBT 60 | Different Boiling Time | 12.5 g/L | 60 min before end of the boil |
| DBT 45 | Different Boiling Time | 12.5 g/L | 45 min before end of the boil |
| DBT 30 | Different Boiling Time | 12.5 g/L | 30 min before end of the boil |
| DBT 15 | Different Boiling Time | 12.5 g/L | 15 min before end of the boil |
| DBT 5 | Different Boiling Time | 12.5 g/L | 5 min before end of the boil |
| BB | Before Boil | 12.5 g/L | Before the boil |
| AB | After Boil | 12.5 g/L | At the end of the boil |
| AC | After Cooling | 12.5 g/L | After wort was cooled to 18 °C |
| AF 1 | After Fermentation start | 12.5 g/L | 1 day after start of fermentation |
| AF 3 | After Fermentation start | 12.5 g/L | 3 days after start of fermentation |
| Standard | Standard | - | - |
| Beer | pH | SRM | EBC |
|---|---|---|---|
| Standard | 4.38 | 6.77 ± 0.01 | 13.32 ± 0.03 |
| DCA 1.25 | 4.4 | 7.90 ± 0.12 | 15.54 ± 0.07 |
| DCA 6.25 | 4.81 | 8.31 ± 0.04 | 16.35 ± 0.24 |
| DCA 25 | 4.42 | 9.01 ± 0.03 | 17.73 ± 0.08 |
| DCA 62.5 | 4.42 | 9.89 ± 0.08 | 19.48 ± 0.07 |
| DCA 125 | 4.34 | 13.36 ± 0.08 | 26.31 ± 0.16 |
| DBT 5 | 4.38 | 8.02 ± 0.09 | 15.79 ± 0.17 |
| DBT 15 | 4.46 | 8.12 ± 0.03 | 15.98 ± 0.06 |
| DBT 30 | 4.35 | 9.69 ± 0.23 | 19.08 ± 0.45 |
| DBT 45 | 4.36 | 11.39 ± 0.16 | 22.43 ± 0.32 |
| DBT 60 | 4.48 | 8.6 ± 0.08 | 16.93 ± 0.15 |
| BB | 4.48 | 8.60 ± 0.08 | 16.93 ± 0.15 |
| AB | 4.25 | 12.03 ± 0.21 | 23.69 ± 0.41 |
| AC | 4.40 | 8.46 ± 0.02 | 16.65 ± 0.04 |
| AF 1 | 4.43 | 7.45 ± 0.11 | 14.67 ± 0.21 |
| AF 3 | 4.39 | 7.03 ± 0.01 | 13.84 ± 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahn, A.; Kim, J.; Bashir, K.M.I.; Cho, M.g. Antioxidant Content of Aronia Infused Beer. Fermentation 2020, 6, 71. https://doi.org/10.3390/fermentation6030071
Jahn A, Kim J, Bashir KMI, Cho Mg. Antioxidant Content of Aronia Infused Beer. Fermentation. 2020; 6(3):71. https://doi.org/10.3390/fermentation6030071
Chicago/Turabian StyleJahn, Alexander, Juyeong Kim, Khawaja Muhammad Imran Bashir, and Man gi Cho. 2020. "Antioxidant Content of Aronia Infused Beer" Fermentation 6, no. 3: 71. https://doi.org/10.3390/fermentation6030071
APA StyleJahn, A., Kim, J., Bashir, K. M. I., & Cho, M. g. (2020). Antioxidant Content of Aronia Infused Beer. Fermentation, 6(3), 71. https://doi.org/10.3390/fermentation6030071





