Suitability of the Lebanese “Ace Spur” Apple Variety for Cider Production Using Hanseniaspora sp. Yeast
Abstract
1. Introduction
2. Materials and Methods
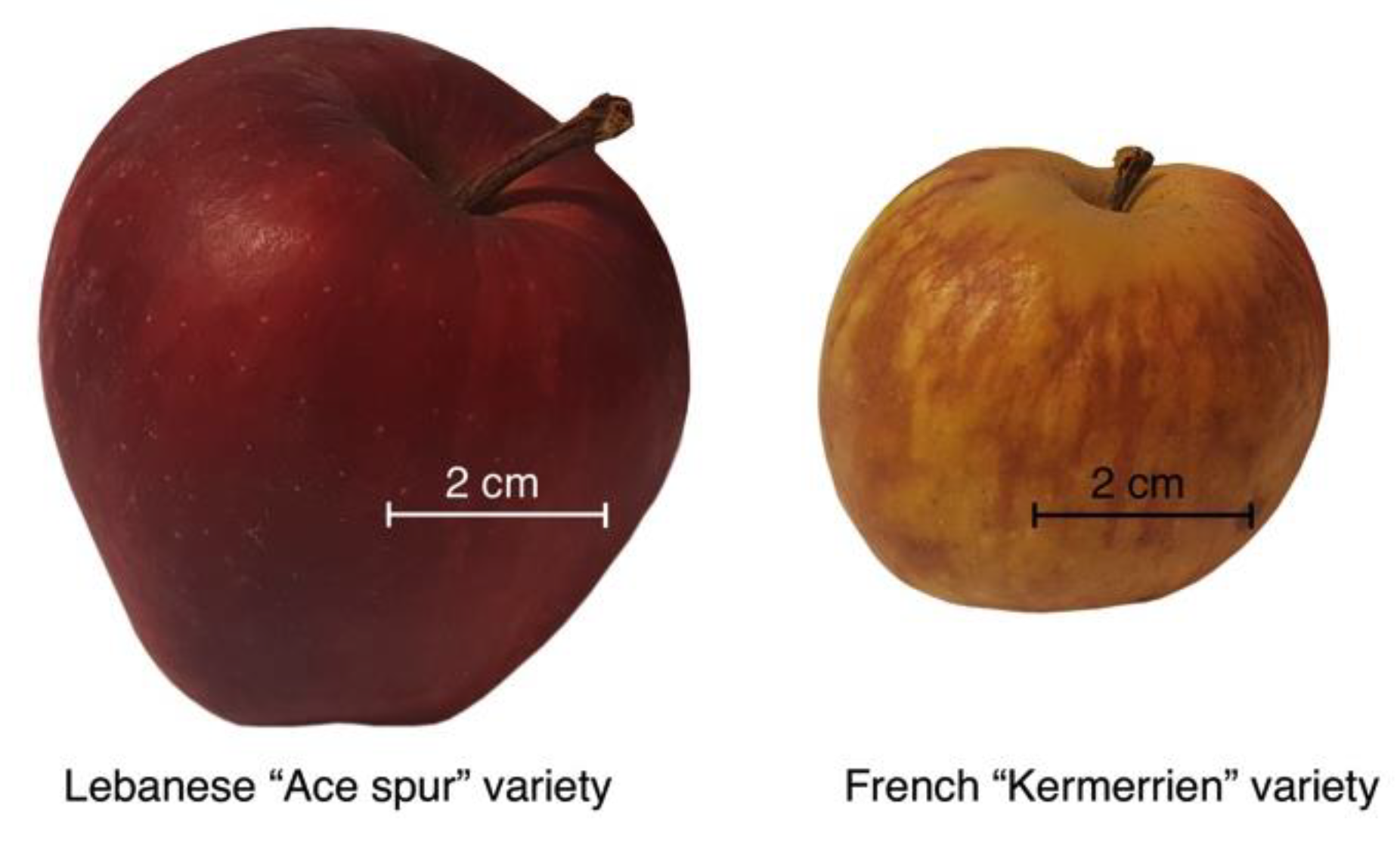
2.1. Apples and Chemicals
2.2. Determination of the Apple Properties
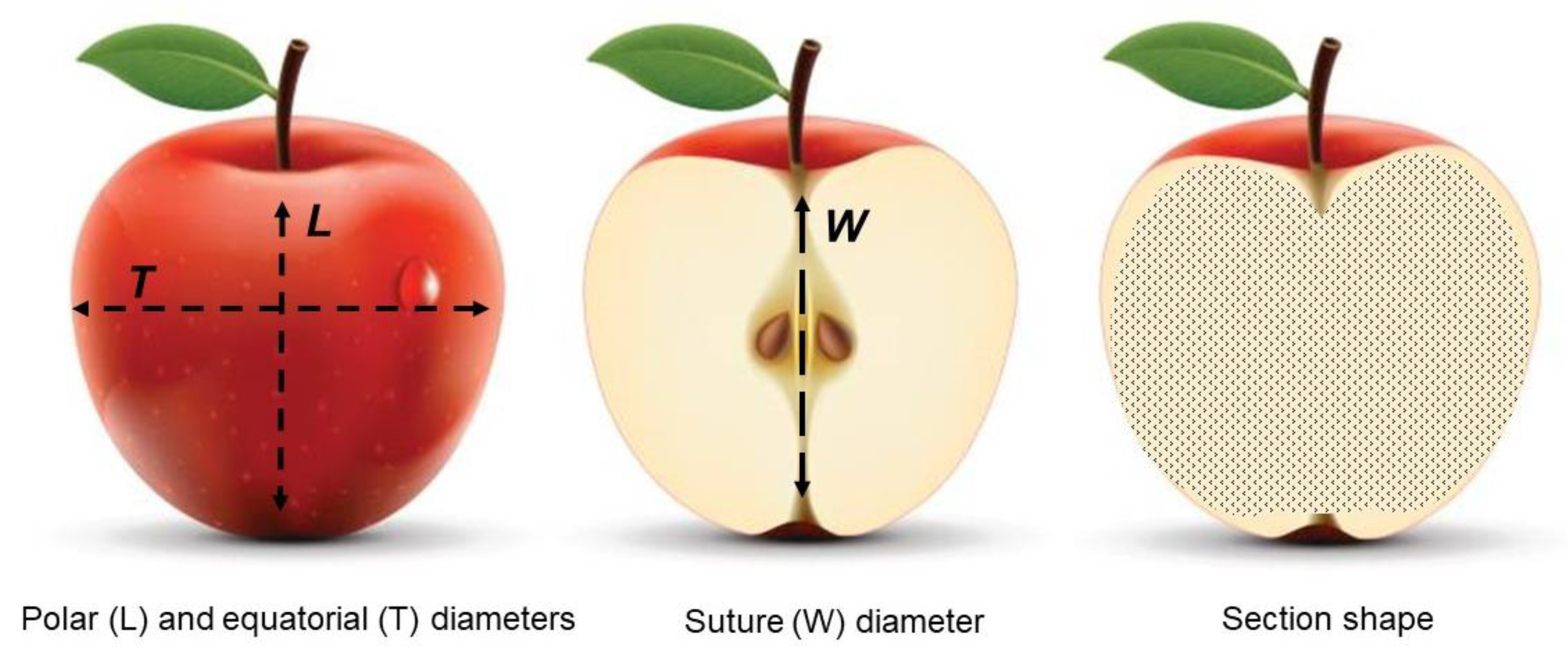
2.2.1. Determination of the Size and Shape Properties
2.2.2. Mechanical Analyses
Relaxation Test
Cutting/Slicing Test
2.2.3. Determination of the Water Content in Apples
2.3. Determination of the Biochemical Properties
2.3.1. Titratable Acidity
2.3.2. pH Measurement
2.3.3. Total Soluble Solids (Brix % (s, w))
2.3.4. Analysis of the Total Polyphenol Content
2.3.5. Determination of the Free Radical Scavenging Activities
2.4. Determination of the Fermentative Capacity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Apple Pomological Properties
3.2. Apples Composition
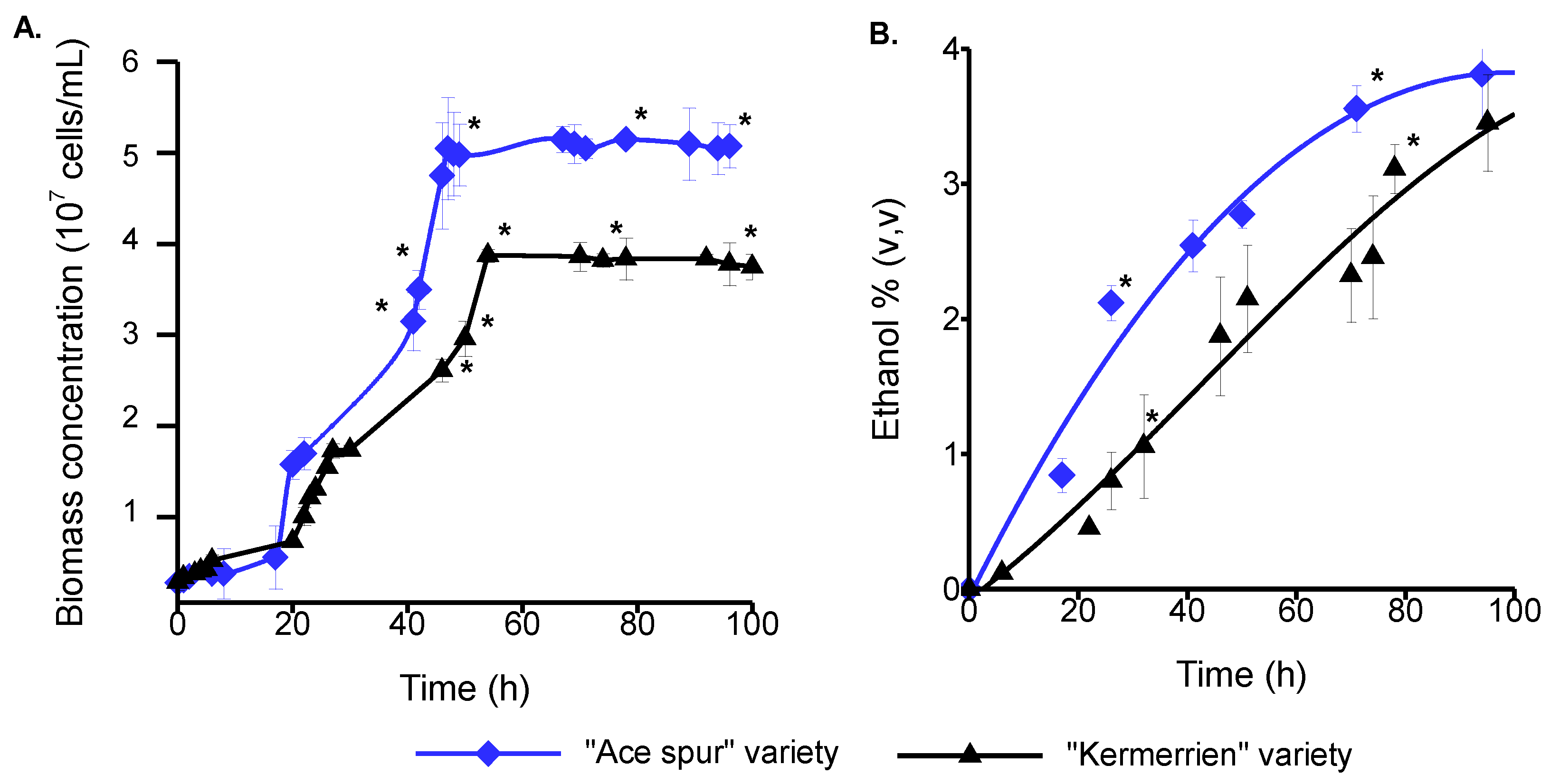
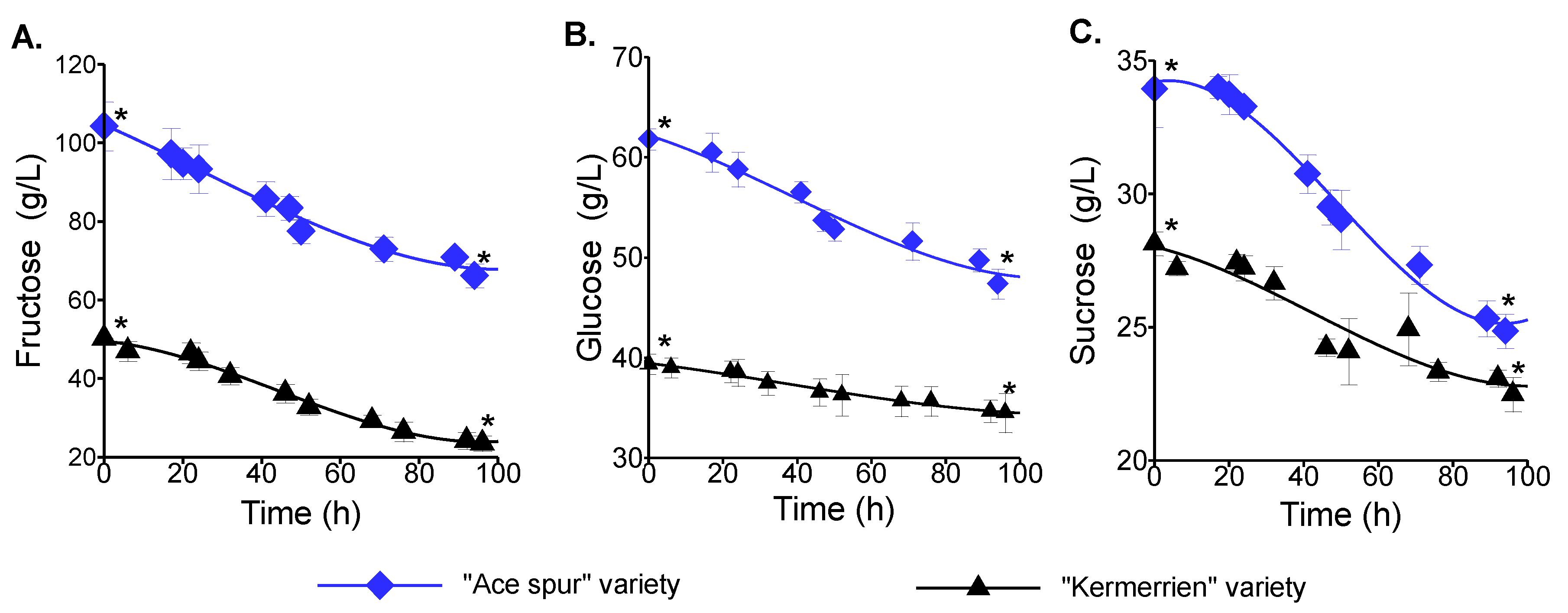
3.3. Fermentative Potential of the Lebanese Apple Juice
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture. Fresh Deciduous Fruit: World Markets and Trade (Apples, Grapes & Pears); Foreign Agricultural Service: Washington, DC, USA, 2018.
- Wilkie, J.D.; Sedgley, M.; Olesen, T. Regulation of floral initiation in horticultural trees. J. Exp. Bot. 2008, 59, 3215–3228. [Google Scholar] [CrossRef]
- Al-Absi, K. Thinning intensity of ‘Ace Spur Delicious’ and “Idared” apples with ethephon, benzyladenine and their combination. Jordan J. Agric. Sci. 2009, 5, 237–250. [Google Scholar]
- Beech, F.W. Cider making and cider research: A review. J. Inst. Brew. 1972, 78, 477–491. [Google Scholar] [CrossRef]
- Hidalgo, P.; Pueyo, E.; Pozo-Bayón, M.A.; Martínez-Rodríguez, A.J.; Martín-Alvarez, P.; Polo, M.C. Sensory and analytical study of rose sparkling wines manufactured by second fermentation in the bottle. J. Agric. Food Chem. 2004, 52, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodriguez, A.J.; Polo, M.C. Effect of the addition of bentonite to the tirage solution on the nitrogen composition and sensory quality of sparkling wines. Food Chem. 2003, 81, 383–388. [Google Scholar] [CrossRef]
- Pomme à Cidre: Les Variétés, Institut Français des Productions Cidricoles; Sées, France, 2009.
- Lea, A.G.H.; Drilleau, J.-F. Cidermaking. In Fermented Beverage Production; Lea, A.G.H., Piggott, J.R., Eds.; Springer US: Boston, MA, USA, 2003; pp. 59–87. [Google Scholar]
- Kvåle, A. Composition and quality of gravenstein apples as related to some environmental factors. Acta Agric. Scand. 1969, 19, 229–239. [Google Scholar] [CrossRef]
- Tablas Peruanas de Composición de Alimentos; Ministerio de Salud del Perú, Centro Nacional de Alimentación y Nutrición: Jesus María, Lima 11, Perú, 2009.
- Knee, M. Pome fruits. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Chapman & Hall: London, UK, 1993; pp. 325–346. [Google Scholar]
- Baldwin, E.A. Fruit flavor, volatile metabolism and consumer perceptions. In Fruit Quality and its Biological Basis; Knee, M., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2002; pp. 89–106. [Google Scholar]
- Sánchez-Moreno, C.; Pascual-Teresa, S.D.; Ancos, B.D.; Cano, M.P. Nutritional values of fruits. In Handbook of Fruits and Fruit Processing; Hui, Y.H., Ed.; John Wiley & Sons, Ltd.: Chichester, NH, USA, 2007; pp. 29–43. ISBN 978-0-470-27773-7. [Google Scholar]
- Sanz, M.L.; Villamiel, M.; Martínez-Castro, I. Inositols and carbohydrates in different fresh fruit juices. Food Chem. 2004, 87, 325–328. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Berthels, N.J.; Cordero Otero, R.R.; Bauer, F.F.; Thevelein, J.M.; Pretorius, I.S. Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 2004, 4, 683–689. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1995; ISBN 978-1-4419-5190-8. [Google Scholar]
- Fleet, G.H. Growth of yeasts during wine fermentations. J. Wine Res. 1990, 1, 211–223. [Google Scholar] [CrossRef]
- Riekstina-Dolge, R.; Kruma, Z.; Karklina, D.; Dimiņš, F. Physical-chemical parameters of Latvian apple juices and their suitability for cider production. Int. J. Nutr. Food Eng. 2014, 8, 263–267. [Google Scholar]
- Nour, V.; Trandafir, I.; Ionica, M.E. Compositional Characteristics of Fruits of several Apple (Malus domestica Borkh) Cultivars. Not. Bot. Hort. Agrobot. Cluj 2010, 38, 228–233. [Google Scholar]
- Campeanu, G.; Neata, G.; Darjanschi, G. Chemical composition of the fruits of several apple cultivars growth as biological crop. Not. Bot. Hort. Agrobot. Cluj 2009, 37, 161–164. [Google Scholar]
- Valois, S.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization of fermented cider apple cultivars grown in upstate New York. J. Am. Pomol. Soc. 2006, 60, 113–128. [Google Scholar]
- Worobo, R.W.; Splittoesser, D.F. Microbiology of fruit products. In Processing Fruits; Barrett, D.M., Somogyi, L., Ramaswamy, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Nogueira, A.; Guyot, S.; Marnet, N.; Lequéré, J.M.; Drilleau, J.-F.; Wosiacki, G. Effect of alcoholic fermentation in the content of phenolic compounds in cider processing. Braz. Arch. Biol. Technol. 2008, 51, 1025–1032. [Google Scholar] [CrossRef]
- Park, J. Characterizing and Improving the Oral Sensations and Preference of Polyphenol-Rich Aronia Berry Juice. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2014. [Google Scholar]
- Symoneaux, R.; Baron, A.; Marnet, N.; Bauduin, R.; Chollet, S. Impact of apple procyanidins on sensory perception in model cider (part 1): Polymerisation degree and concentration. LWT Food Sci. Technol. 2014, 57, 22–27. [Google Scholar] [CrossRef]
- Mangas, J.J.; Rodríguez, R.; Suárez, B.; Picinelli, A.; Dapena, E. Study of the phenolic profile of cider apple cultivars at maturity by multivariate techniques. J. Agric. Food Chem. 1999, 47, 4046–4052. [Google Scholar] [CrossRef]
- Arshad, M.; Shahnawaz, M.; Shahkeela, S.; Hussain, M.; Ahmad, M.; Khan, S.S. Significance of physical properties of apple fruit influenced by preharvest orchard management factors. Eur. J. Exp. Biol. 2014, 4, 82–89. [Google Scholar]
- Barnett, J.A. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850–1880. Yeast 2000, 16, 755–771. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R.; Maifreni, M. Glycerol and other fermentation products of apiculate wine yeasts. J. Appl. Microbiol. 1997, 82, 615–618. [Google Scholar] [CrossRef]
- de Arruda Moura Pietrowski, G.; dos Santos, C.M.E.; Sauer, E.; Wosiacki, G.; Nogueira, A. Influence of fermentation with Hanseniaspora sp. yeast on the volatile profile of fermented apple. J. Agric. Food Chem. 2012, 60, 9815–9821. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Berbegal, C.; Tufariello, M.; Grieco, F.; Spano, G.; Grieco, F. Impact of co-inoculation of Saccharomyces cerevisiae, Hanseniaspora uvarum and Oenococcus oeni autochthonous strains in controlled multi starter grape must fermentations. LWT 2019, 109, 241–249. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Portillo, M.D.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Mateo, J.J.; Maicas, S. Screening of Hanseniaspora strains for the production of enzymes with potential interest for winemaking. Fermentation 2016, 2, 1. [Google Scholar]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—A review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Maroun, R.G.; Louka, N.; Vorobiev, E. Ultrasound-assisted fermentation for cider production from Lebanese apples. Ultrason. Sonochem. 2020, 63, 104952. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Vorobiev, E.; Maroun, R.G.; Louka, N. Control of the sugar/ethanol conversion rate during moderate pulsed electric field-assisted fermentation of a Hanseniaspora sp. strain to produce low-alcohol cider. Innov. Food Sci. Emerg. Technol. 2020, 59, 102258. [Google Scholar] [CrossRef]
- Fıratlıgil-Durmuş, E.; Šárka, E.; Bubník, Z. Image vision technology for the characterisation of shape and geometrical properties of two varieties of lentil grown in turkey. Czech J. Food Sci. 2007, 26, 109–116. [Google Scholar] [CrossRef]
- Miloševi, T.; Miloševi, N.; Gliši, I.; Gliši, I.S. Determination of size and shape properties of apricots using multivariate analysis. Acta Sci. Pol. 2014, 13, 77–90. [Google Scholar]
- Mohsenin, N.N. Physical Properties of Plant and Animal Materials: Structure, Physical Characteristics, and Mechanical Properties; Gordon and Breach: New York, NY, USA, 1986. [Google Scholar]
- Jain, R.K.; Bal, S. Properties of pearl millet. J. Agric. Eng. Res. 1997, 66, 85–91. [Google Scholar] [CrossRef]
- Marquina, P.L.; Burgos, J.; Oria, R. Application of a compression-relaxation test for the characterization of burlat sweet cherry. J. Texture Stud. 2001, 32, 15–30. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Koubaa, M.; Mhemdi, H.; Vorobiev, E. Seed oil polyphenols: Rapid and sensitive extraction method and high resolution-mass spectrometry identification. Anal. Biochem. 2015, 476, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Al Daccache, M.; Salameh, D.; Maroun, R.; Louka, N. New Indigenous Yeast Strains “Hanseniaspora Meyeri-Libani” for the Elaboration of Cider. 12017/10-11265L, 4 October 2017. [Google Scholar]
- Pando Bedriñana, R.; Querol Simón, A.; Suárez Valles, B. Genetic and phenotypic diversity of autochthonous cider yeasts in a cellar from Asturias. Food Microbiol. 2010, 27, 503–508. [Google Scholar] [CrossRef]
- Valles, B.S.; Bedriñana, R.P.; Tascón, N.F.; Simón, A.Q.; Madrera, R.R. Yeast species associated with the spontaneous fermentation of cider. Food Microbiol. 2007, 24, 25–31. [Google Scholar] [CrossRef]
- Ercisli, S.; Sayinci, B.; Kara, M.; Yildiz, C.; Ozturk, I. Determination of size and shape features of walnut (Juglans regia L.) cultivars using image processing. Sci. Hortic. 2012, 133, 47–55. [Google Scholar] [CrossRef]
- Downes, J.W. Equipment for extraction and processing of soft and pome fruit juices. In Production and Packaging of Non-Carbonated Fruit Juices and Fruit Beverages; Ashurst, P.R., Ed.; Springer US: Boston, MA, USA, 1995; pp. 197–220. [Google Scholar]
- Wlodarska, K.; Pawlak-Lemanska, K.; Gorecki, T.; Sikorska, E. Perception of apple juice: A comparison of physicochemical measurements, descriptive analysis and consumer responses. J. Food Qual. 2016, 39, 351–361. [Google Scholar] [CrossRef]
- Riekstina-Dolge, R.; Kruma, Z.; Dimins, F.; Straumite, E.; Karklina, D. Phenolic composition and sensory properties of ciders produced from Latvian apples. Rural Sustain. Res. 2014, 31, 39–45. [Google Scholar] [CrossRef]
- Harker, F.R.; Amos, R.L.; Echeverríaa, G.; Gunson, F.A. Influence of texture on taste: Insights gained during studies of hardness, juiciness, and sweetness of apple fruit. J. Food Sci. 2006, 71, S77–S82. [Google Scholar] [CrossRef]
- Bayarri, S.; Calvo, C.; Costell, E.; Durán, L. Influence of color on perception of sweetness and fruit flavor of fruit drinks. Food Sci. Technol. Int. 2001, 7, 399–404. [Google Scholar] [CrossRef]
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.P. Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853. [Google Scholar] [CrossRef] [PubMed]
- Will, F.; Schulz, K.; Ludwig, M.; Otto, K.; Dietrich, H. The influence of enzymatic treatment of mash on the analytical composition of apple juice. Int. J. Food Sci. Technol. 2002, 37, 653–660. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A.; Tyrakowska, B. Quality of commercial apple juices evaluated on the basis of the polyphenol content and the teac antioxidant activity. J. Food Sci. 2003, 68, 1844–1849. [Google Scholar] [CrossRef]
- Bradshaw, T.L.; Kingsley-Richards, S.L.; Foster, J. Apple cultivar evaluations for cider making in Vermont, USA. Acta Hortic. 2018, 453–460. [Google Scholar] [CrossRef]
- Jarvis, B. The product and its manufacture—Chemistry and microbiology of cidermaking. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, MI, USA, 2003; pp. 1312–1318. ISBN 978-0-12-227055-0. [Google Scholar]
- Merwin, I.A.; Valois, S.; Padilla-Zakour, O.I. Cider apples and cider-making techniques in Europe and North America. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Ltd.: Chichester, NH, USA, 2008; pp. 365–415. ISBN 978-0-470-38014-7. [Google Scholar]
- Lachenmeier, D.W.; Gill, J.S.; Chick, J.; Rehm, J. The total margin of exposure of ethanol and acetaldehyde for heavy drinkers consuming cider or vodka. Food Chem. Toxicol. 2015, 83, 210–214. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Thierie, J.; Penninckx, M. Crabtree effect. In Encyclopedia of Industrial Biotechnology; Flickinger, M.C., Ed.; Wiley & Sons, Ltd.: Chichester, NH, USA, 2010; pp. 1–18. ISBN 978-0-470-05458-1. [Google Scholar]

| Apple Variety | “Ace Spur” | “Kermerrien” |
|---|---|---|
| Average weight (g) * | 117.13 ± 20.01 | 60.57 ± 8.34 |
| Equivalent mean diameter De (mm) * | 56.72 ± 3.44 | 44.10 ± 1.83 |
| Mean size Da (mm) * | 56.76 ± 3.34 | 44.12 ± 1.62 |
| Mean elongation E | 1.05 ± 0.07 | 1.14 ± 0.2 |
| Mean Surface area S (cm2) | 180.14 ± 5.4 | 119.17 ± 3.7 |
| Mean fruit volume V (cm3) * | 174.1 ± 4.8 | 49.22 ± 8.2 |
| Water content (%) | 82.34 ± 2.1 | 84.15 ± 1.5 |
| Compression force (N) * | 64.88 ± 15.7 | 118.37 ± 20.5 |
| Rupture force (N) | 14.85 ± 1.87 | 14.57 ± 2.5 |
| Apple Variety | “Ace Spur” | “Kermerrien” |
|---|---|---|
| Glucose (g/L) * | 55.69 ± 1.54 | 39.80 ± 1.60 |
| Fructose (g/L) * | 101.41 ± 4.08 | 57.84 ± 1.98 |
| Sucrose (g/L) | 35.98 ± 1.89 | 34.59 ± 1.23 |
| Total sugar content (g/L) * | 193.08 ± 7.51 | 132.32 ± 4.81 |
| Fructose—Glucose ratio * | 1.83 ± 0.08 | 1.45 ± 0.05 |
| °Brix (g/100 mL) * | 20.57 ± 1.80 | 13.57 ± 0.80 |
| Titratable acidity (g malic acid/L) * | 2.93 ± 0.66 | 15.28 ± 1.90 |
| Ph * | 4.90 ± 0.08 | 3.59 ± 0.09 |
| Total phenolic content (mg GAE/100 g dry matter) * | 419.83 ± 8.32 | 514.13 ± 13.75 |
| Total antioxidant activity (%) * | 93.15 ± 0.87 | 95.97 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Suitability of the Lebanese “Ace Spur” Apple Variety for Cider Production Using Hanseniaspora sp. Yeast. Fermentation 2020, 6, 32. https://doi.org/10.3390/fermentation6010032
Al Daccache M, Koubaa M, Maroun RG, Salameh D, Louka N, Vorobiev E. Suitability of the Lebanese “Ace Spur” Apple Variety for Cider Production Using Hanseniaspora sp. Yeast. Fermentation. 2020; 6(1):32. https://doi.org/10.3390/fermentation6010032
Chicago/Turabian StyleAl Daccache, Marina, Mohamed Koubaa, Richard G. Maroun, Dominique Salameh, Nicolas Louka, and Eugène Vorobiev. 2020. "Suitability of the Lebanese “Ace Spur” Apple Variety for Cider Production Using Hanseniaspora sp. Yeast" Fermentation 6, no. 1: 32. https://doi.org/10.3390/fermentation6010032
APA StyleAl Daccache, M., Koubaa, M., Maroun, R. G., Salameh, D., Louka, N., & Vorobiev, E. (2020). Suitability of the Lebanese “Ace Spur” Apple Variety for Cider Production Using Hanseniaspora sp. Yeast. Fermentation, 6(1), 32. https://doi.org/10.3390/fermentation6010032