Abstract
Metschnikowia pulcherrima (Mp) is a ubiquitous yeast that frequently appears in spontaneous fermentations. The current interest in Mp is supported by the expression of many extracellular activities, some of which enhance the release of varietal aromatic compounds. The low fermentative power of Mp makes necessary the sequential or mixed use with Saccharomyces cerevisiae (Sc) to completely ferment grape musts. Mp has a respiratory metabolism that can help to lower ethanol content when used under aerobic conditions. Also, Mp shows good compatibility with Sc in producing a low-to-moderate global volatile acidity and, with suitable strains, a reduced level of H2S. The excretion of pulcherrimin gives Mp some competitive advantages over other non-Saccharomyces yeasts as well as providing some antifungal properties.
1. Ecology and Physiology
Metschnikowia pulcherrima (Mp) is a globous/elliptical yeast that cannot be distinguished from Saccharomyces cerevisiae (Sc) by microscopy (Figure 1). Sometimes, it can be observed a single large, highly refractive oil droplet inside the cell. Mp is a teleomorph yeast belonging to an ascomycetous genus [1]. Its anamorph form is called Candida pulcherrima. Mp is a ubiquitous yeast that has been found in grapes, fruits (fresh and spoiled), flowers, nectars and tree sap fluxes. Several insects can work as vectors for this yeast. Mp strains can be identified through the use of selective and differential substrates; Mp strains showed both positive β-glucosidase enzyme activity and proteolytic activity [2]. Mp grows properly in either YPD or L-lysine media, and it can also can use arbutin as a carbon source in agar plates, indicating the expression of β-glucosidase activity (Figure 2) [3]. Recently, its nitrogen requirement was evaluated and slower consumption rates of ammonium were observed in Mp in comparison to other yeast genera [4]. This slow nitrogen uptake is indicative of its low fermentative ability [5].
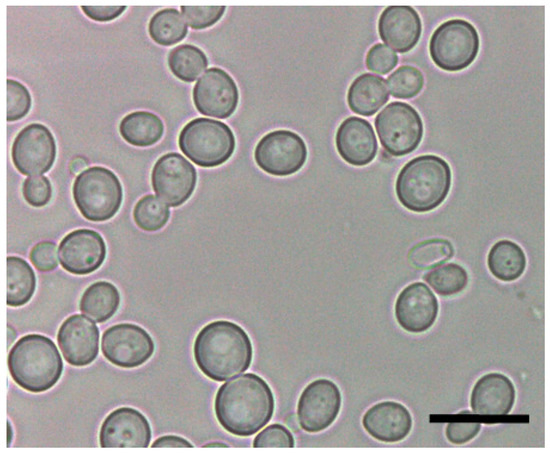
Figure 1.
Cell morphology and shape of Metschnikowia pulcherrima. Graphical scale 10 μm.
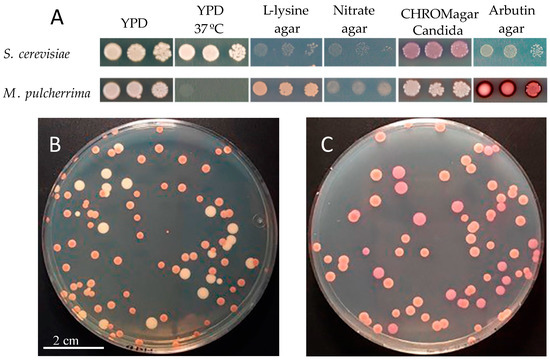
Figure 2.
(A) Development and colony appearance in several growth media and different culture conditions (temperature). (B) Metschnikowia pulcherrima (Mp) orange colonies, some of them surrounded with white halos and Saccharomyces cerevisiae (Sc) white/creamy colonies in YPD media. (C) Mp and Sc in CHROMagar® media. Sc: bigger colonies with light pink color, Mp: smaller orange colonies, some of them with white halos.
The β-glucosidase activity related to Mp has been associated with different intracellular β-glucosidases, with the identification of three different bands observed when using fluorogenic substrates via an electrophoretic technique [6]. Of these three bands, the major band has similar physicochemical properties to those found in other studied yeasts, with high activity in ethanol and glucose concentrations often found in wines but low stability below pH 4. Mp is unable to develop in YPD at 37 °C and shows very weak or no growth in nitrate agar (Figure 2). It is able to use glucose, sucrose, fructose, galactose and maltose as carbon sources but shows weak or inexistent development in lactose [7]. It can grow properly under low temperature (15–20 °C) and pH conditions (3–6) [8]. Under environmental stress conditions such as a shortage of nitrogen, its recognition in optical microscopy is easy thanks to the appearance of a fat globule inside the cell at the beginning of the sporulation process [8]. In its sporulated form, the asci of Metschnikowia are long and clavate, containing one to two acicular to filiform spores [1].
The fermentative power of Mp is low, with many strains easily reaching 4% v/v in ethanol [3], although previous studies have observed the production of ethanol up to 6–7% v/v [9]. This feature, together with the fact that the presence of Mp in freshly pressed must is about 19–39% of the yeast ecology [9], makes it necessary to use Mp together with other yeast with a high fermentative power such as Sc or Schizosaccharomyces pombe to fully ferment grape sugars [10]. Its volatile acidity is also quite moderate, ranging from 0.3 to 0.4 g/L expressed as acetic acid [3]. Moreover, some strains are able to decrease the formation of H2S during fermentation [11].
The fermentative performance of Mp is lower than that observed for other non-Saccharomyces species. The CO2 production during fermentation yielded lower amounts for Mp than for Sc with 4.5 g per 100 mL vs. 12.9 g per 100 mL, respectively [12]. Mp has an intermediate acetoin production during alcoholic fermentation with respect to other species, such as S. cerevisiae and B. bruxellensis with low acetoin production and C. stellata and K. apiculata with the highest production of acetoin. The metabolic pathway for the production of this secondary metabolite from fermentation is shown in Figure 3. In addition, the amount of 2,3-butanediol produced by Mp is usually lower than that produced by Sc.
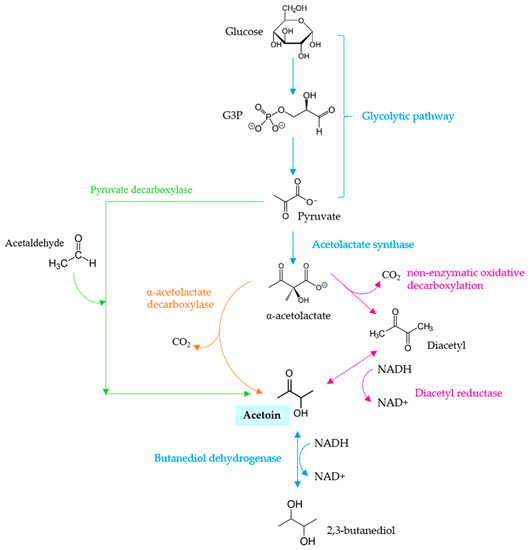
Figure 3.
Metabolic route for the biosynthesis of acetoin by yeasts (adapted from Romano and Suzzi, [13]).
In mixed cultures with S. cerevisiae, viability was found to decrease rapidly after a few days of fermentation because of the low resistance to the ethanol produced by S. cerevisiae [14,15]. The use of emerging physical technologies that are able to strongly reduce the wild yeast content in grapes [16] can facilitate the prevalence of Mp during a longer period until the sequential inoculation of Sc, thus also increasing its effect on the sensory profile of the wines.
The sensibility of Mp to SO2 is lower than that observed in Sc, Saccharomycodes ludwigii or S. pombe, but Mp shows a medium resistance compared with other non-Saccharomyces species [7]. A certain sensibility to some antimicrobials such as carvacrol and thymol has also been observed [17]. Regarding the use of dimethyl dicarbonate (DMDC), the growth of Mp strains during the fermentation of grape must is delayed, but not inhibited, after the addition of 400 mg/L DMDC [18]. The total inhibition of the microbial population can be achieved with 500 mg/L of DMDC. Sc can survive the addition of 200 mg/L DMDC, whereas the growth of other species of the genus Saccharomyces is inhibited with 150 mg/L DMDC.
2. Antimicrobial Bio-Tool
Mp can be used as a biological control agent thanks to its ability to produce natural antimicrobial compounds, namely pulcherrimin, an insoluble red pigment with antifungal activity. This peculiar antimicrobial activity is produced by the depletion of iron in the medium through the precipitation of iron(III) ions caused by the interaction with pulcherriminic acid, a precursor of pulcherrimin secreted by Mp. In this way, the environment becomes inhospitable to other microorganisms that require iron for their development. Pulcherrimin has shown effective inhibitory activity against several yeasts: Candida tropicalis and Candida albicans, as well as the Brettanomyces/Dekkera, Hanseniaspora and Pichia genera; and fungi: Botrytis cinerea, as well as Penicillium, Alternaria and Monilia spp. [19,20,21,22,23,24]. However, S. cerevisiae seems not to be affected by this antimicrobial activity [21,22]. Therefore, the use of Mp as a selected starter in sequential or mixed biotechnologies with Sc could be of great interest in modern enology.
Mp, as well as other yeast species such as Wickerhamomyces anomala (formerly Pichia anomala) and Torulaspora delbrueckii (Td), has a broad killer spectrum against some spoilage yeasts [25,26], of which C. glabrata had the highest sensitivity against the toxins from this species [27]. Mp has also been described as biofungicide capable of effectively reducing the incidence of Botrytis development in postharvest fruits [28]. Its antagonistic mechanism is mainly based on its competition for nutrients [29].
3. Aroma Compounds
The single use of Mp has led to excessive production of ethyl acetate with negative sensory repercussions [30]. However, the mixed use of Mp with Saccharomyces uvarum reduces the production of ethyl acetate, simultaneously favoring the formation of 2-phenyl ethanol and 2-phenylethyl acetate [30]. The use of co-inoculations of this type (mixed fermentations with Mp/Sc) has produced high contents of acetate esters and β-damascenone with lower levels of C6 alcohols in ice wines made from the Vidal blanc grape variety [31]. An improvement in the aromatic complexity of the wines can be obtained by the use of Mp as a co-starter with Sc [3,32], mainly due to its high production of esters derived from its intense extracellular enzymatic activity [10,33]. Similarly, sequential fermentations with Mp showed a higher production of higher alcohols, with particularly high concentrations of isobutanol and phenylethanol [4].
4. Enzymatic Activities
Activities of the following enzymes have been described in Mp: pectinase, protease, glucanase, lichenase, β-glucosidase, cellulase, xylanase, amylase, sulphite reductase, lipase and β-lyase [11,33,34,35]. This is because Mp one of the non-Saccharomyces yeast species able to express more extracellular hydrolytic enzymes. Its high proteolytic activity makes it a very interesting fermentation partner for Sc, since the amino acids released (including those from autolysis) can serve as a source of nutrients for Sc [36]. In addition, its intense glucosidase activity [2], higher under aerobic conditions [37], promotes the release of varietal aromas from the grape by hydrolyzing bound monoterpenes. However, it is important to always remember that the intensity of the enzymatic activity depends not only on the species, but also on the strain [32].
Concerning aroma enhancement, the expression of β-D-glucosidase favors the release of free terpenes and this activity has been evaluated using the substrates 4-methylumbelliferyl-β-D-glucoside (MUG) and p-nitrophenyl-β-D-glucoside (pNPG), showing a good intensity with medium-to-low degradation of color by the effect on anthocyanin glucosides [38]. The commercial Mp L1781 (Flavia™ MP346, Lallemand) expresses α-arabinofuranosidase; this activity helps to release precursors of volatile terpenes [39,40] (Figure 4) and thiols [32,41], which help to enhance fruity smells in some varieties. This strain has shown an enzymatic specific activity of 0.22 U/mg when used as a dry yeast or fresh culture [41]. This has been measured by the hydrolysis of 11 μmol de p-nitrophenyl-α-L-arabinofuranosidase (pNPA) per minute [42].
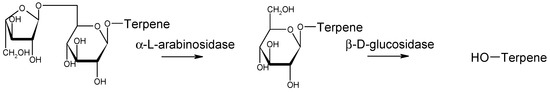
Figure 4.
Effect of sequential α-arabinofuranosidase and β-D-glucosidase activities on the transformation of bonded terpenes into free forms, enhancing the aromatic profile.
Intracellular β-glucosidase of Mp has been purified by ion-exchange chromatography on amino agarose gel [6] and subsequently characterized. The optimum catalytic activity was observed at 50 °C and pH 4.5. The enzyme shows hydrolytic activity on β-(1→4) and β-(1→2) glycosidic bonds. The stability in alcoholic media (12% v/v) is good but it is affected by low pH.
5. Aerobic Metabolism/Alcohol Degree Reduction
The sequential use of Mp and Sc has proved to be somewhat effective in lowering the ethanol content of wine [11,43,44,45,46]. This is connected with the aerobic respiratory metabolisms of Mp that, in suitable aeration conditions, can aerobically metabolize more than 40% of sugars, thus significantly reducing the ethanol yield. An example of this application can be seen in the study developed by Contreras et al. (2014), where an average reduction in the alcoholic strength of 1.6% v/v was achieved when Mp was used in sequential fermentation with Sc (inoculated on the fourth day) in the production of red wine of the Syrah variety from a must with 240 g/L of sugars (potential alcoholic strength of 14% v/v). Therefore, the use of certain non-Saccharomyces yeast species, such as Mp, has been suggested as a biotechnological strategy aimed at producing wines with lower levels of ethanol [47]. In this last study, a kind of “collaboration” was seen between populations of Mp and S. uvarum, that is, a synergistic effect, achieving a lower ethanol production than in pure fermentations with each yeast. Recently, Mestre Furlani et al. (2017) evaluated the metabolic behavior of different non-Saccharomyces native yeasts to reduce the ethanol content during winemaking. They report that two out of the three strains of Mp isolated from grapes have a sugar to ethanol conversion ratio greater than >19 g/L/% v/v [48]. This confirms the usefulness of Mp to obtain wines with lower ethanol content.
6. Improvement of Wine Color Stability
Some non-Saccharomyces adsorb lower contents of anthocyanins during fermentation than Sc [49]. In Sc, the adsorption can range between 1 and 6% in total content of anthocyanins [50], but can reach up to 30% for some specific anthocyanins [51]. Adsorption is influenced by the composition and structure of the yeast cell wall. Mp shows a low adsorption of anthocyanins in cell walls when compared with other yeasts such as Sc, Td or Lachancea thermotolerans (Lt) in grape skin agar (Figure 5), according to the methodology described by Caridi et al. [52].
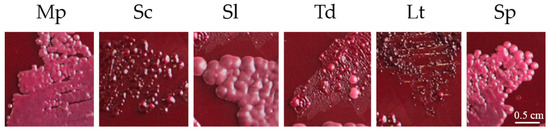
Figure 5.
Adsorption of grape anthocyanins in yeast cell walls (Saccharomyces and non-Saccharomyces) during growth in a specific plating medium containing pigments. Metschnikowia pulcherrima (Mp), Saccharomyces cerevisiae (Sc), Saccharomycodes ludwigii (Sl), Torulaspora delbrueckii (Td), Lachancea thermotolerans (Lt), Schizosaccharomyces pombe (Sp).
The effect of Mp in the formation of stable pigments (pyranoanthocyanins and polymers) during fermentation has been studied in sequential fermentations with Sc and S. pombe [10].
7. Conclusions
The versatility of Metschnikowia pulcherrima lies in its ability to ferment in combination with other yeast species as well as modulate the synthesis of secondary metabolites of fermentation to improve the sensory profile of the wine. It is characterized by a medium fermentation power and a high enzymatic capacity to release aromatic precursors from the grape. In addition, this yeast has potential as a biocontrol agent in order to limit competition with other yeasts in the fermentation medium.
The abovementioned applications and features of Metschnikowia pulcherrima may be of great interest in order to address one of the major concerns in today’s winemaking industry, such as excessive alcoholic strengths and the increasing prevalence in the market of flat wines from a sensory point of view. Mp could help solve these issues. The only important thing is to select the proper combination, as well as the right time and ratio of inoculation, between Mp and another yeast species capable of completing the alcoholic fermentation.
Author Contributions
A.M., C.E. and I.L.: literature review, writing, and editing; A.M.: images design; J.M.d.F.: literature review and critical reading; M.A.B.: critical reading; J.A.S.-L.: critical reading.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
References
- Kurtzman, C.P.; Fell, J.W. The Yeasts: A Taxonomic Study, 4th ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1998; ISBN 9780444813121. [Google Scholar]
- Fernández, M.; Ubeda, J.F.; Briones, A.I. Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. Int. J. Food Microbiol. 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilisation of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1, ISBN 9780470010341. [Google Scholar]
- González-Pombo, P.; Pérez, G.; Carrau, F.; Guisán, J.M.; Batista-Viera, F.; Brena, B.M. One-step purification and characterization of an intracellular β-glucosidase from Metschnikowia pulcherrima. Biotechnol. Lett. 2008, 30, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Isolation, selection and identification techniques for non-Saccharomyces yeasts of oenological interest. In Biotechnological Progress and Beverage Consumption; Volume 19: The Science of Beverages; Grumezescu, A., Holban, A.-M., Eds.; Elsevier Academic Press: Cambridge, MA, USA, Chapter 15, in press.
- Santamauro, F.; Whiffin, F.M.; Scott, R.J.; Chuck, C.J. Low-cost lipid production by an oleaginous yeast cultured in non-sterile conditions using model waste resources. Biotechnol. Biofuels 2014, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Del Fresno, J.M.; Loira, I.; Morata, A.; Tesfaye, W.; del Carmen González, M.; Suárez-Lepe, J.A. Formation of polymeric pigments in red wines through sequential fermentation of flavanol-enriched musts with non-Saccharomyces yeasts. Food Chem. 2018, 239, 975–983. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A.; Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; et al. Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from Douro wine region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Romano, P.; Granchi, L.; Caruso, M.; Borra, G.; Palla, G.; Fiore, C.; Ganucci, D.; Caligiani, A.; Brandolini, V. The species-specific ratios of 2,3-butanediol and acetoin isomers as a tool to evaluate wine yeast performance. Int. J. Food Microbiol. 2003, 86, 163–168. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Origin and production of acetoin during wine yeast fermentation. Appl. Environ. Microbiol. 1996, 62, 309. [Google Scholar]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Escott, C.; Loira, I.; Morata, A.; Bañuelos, M.A.; Antonio Suárez-Lepe, J. Wine Spoilage Yeasts: Control Strategy. In Yeast—Industrial Applications; Morata, A., Loira, I., Eds.; InTech: London, UK, 2017; p. 98. [Google Scholar]
- Delfini, C.; Gaia, P.; Schellino, R.; Strano, M.; Pagliara, A.; Ambrò, S. Fermentability of grape must after inhibition with Dimethyl Dicarbonate (DMDC). J. Agric. Food Chem. 2002, 50, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Csutak, O.; Vassu, T.; Sarbu, I.; Stoica, I.; Cornea, P. Antagonistic activity of three newly isolated yeast strains from the surface of fruits. Food Technol. Biotechnol. 2013, 51, 70–77. [Google Scholar]
- Janisiewicz, W.J.; Tworkoski, T.J.; Kurtzman, C.P. Biocontrol potential of Metchnikowia pulcherrima strains against blue mold of apple. Phytopathology 2001, 91, 1098–1108. [Google Scholar] [CrossRef]
- Kántor, A.; Hutková, J.; Petrová, J.; Hleba, L.; Kačániová, M. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 282–285. [Google Scholar] [CrossRef]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, D.; Ciavorella, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol. Technol. 2008, 49, 121–128. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia strains isolated from botrytized grapes antagonize fungal and bacterial growth by iron depletion. Appl. Environ. Microbiol. 2006, 72, 6716–6724. [Google Scholar] [CrossRef]
- Lopes, C.A.; Sáez, J.S.; Sangorrín, M.P. Differential response of Pichia guilliermondii spoilage isolates to biological and physico-chemical factors prevailing in Patagonian wine fermentations. Can. J. Microbiol. 2009, 55, 801–809. [Google Scholar] [CrossRef]
- Sangorrín, M.P.; Lopes, C.A.; Jofré, V.; Querol, A.; Caballero, A.C. Spoilage yeasts from Patagonian cellars: Characterization and potential biocontrol based on killer interactions. World J. Microbiol. Biotechnol. 2008, 24, 945–953. [Google Scholar] [CrossRef]
- Lopes, C.A.; Sangorrín, M.P. Optimization of killer assays for yeast selection protocols. Rev. Argent. Microbiol. 2010, 42, 298–306. [Google Scholar]
- Spadaro, D.; Ciavorella, A.; Dianpeng, Z.; Garibaldi, A.; Gullino, M.L. Effect of culture media and pH on the biomass production and biocontrol efficacy of a Metschnikowia pulcherrima strain to be used as a biofungicide for postharvest disease control. Can. J. Microbiol. 2010, 56, 128–137. [Google Scholar] [CrossRef]
- Piano, S.; Neyrotti, V.; Migheli, Q.; Gullino, M.L. Biocontrol capability of Metschnikowia pulcherrima against Botrytis postharvest rot of apple. Postharvest Biol. Technol. 1997, 11, 131–140. [Google Scholar] [CrossRef]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef]
- Zhang, B.-Q.; Shen, J.-Y.; Duan, C.-Q.; Yan, G.-L. Use of indigenous Hanseniaspora vineae and Metschnikowia pulcherrima co-fermentation with Saccharomyces cerevisiae to improve the aroma diversity of Vidal Blanc icewine. Front. Microbiol. 2018, 9, 2303. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The Role and Use of Non-Saccharomyces Yeasts in Wine Production. Available online: https://pdfs.semanticscholar.org/2c05/c11843cafd3d1ed97dcbefce97964537386a.pdf (accessed on 7 June 2019).
- Ganga, M.A.; Martínez, C. Effect of wine yeast monoculture practice on the biodiversity of non-Saccharomyces yeasts. J. Appl. Microbiol. 2004, 96, 76–83. [Google Scholar] [CrossRef]
- Reid, V.J.; Theron, L.W.; Du Toit, M.; Divol, B. Identification and partial characterization of extracellular aspartic protease genes from Metschnikowia pulcherrima IWBT Y1123 and Candida apicola IWBT Y1384. Appl. Environ. Microbiol. 2012, 78, 6838–6849. [Google Scholar] [CrossRef]
- Romano, P.; Capece, A.; Jespersen, L. Taxonomic and ecological diversity of food and beverage yeasts. In Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006; pp. 13–53. [Google Scholar]
- Mendes Ferreira, A.; Clímaco, M.C.; Mendes Faia, A. The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components-a preliminary study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef]
- Manzanares, P.; Rojas, V.; Genovés, S.; Vallés, S. A preliminary search for anthocyanin-β-D-glucosidase activity in non-Saccharomyces wine yeasts. Int. J. Food Sci. Technol. 2000, 35, 95–103. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. A 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Gunata, Z.; Bitteur, S.; Brillouet, J.-M.; Bayonove, C.; Cordonnier, R. Sequential enzymic hydrolysis of potentially aromatic glycosides from grape. Carbohydr. Res. 1988, 184, 139–149. [Google Scholar] [CrossRef]
- Ganga, M.A.; Carriles, P.; Raynal, C.; Heras, J.M.; Ortiz-Julien, A.; Dumont, A. Vincular la Metschnikowia pulcherrima y la Saccharomyces cerevisiae Para una Máxima Revelación del Aroma en Vinos Blancos. Available online: https://www.lallemandwine.com/wp-content/uploads/2014/10/Flavia-Lee-el-documento.pdf (accessed on 7 June 2019).
- Gunata, Z.; Brillouet, J.M.; Voirin, S.; Baumes, R.; Cordonnier, R. Purification and some properties of an alpha-L-arabinofuranosidase from Aspergillus niger. Action on grape monoterpenyl arabinofuranosylglucosides. J. Agric. Food Chem. 1990, 38, 772–776. [Google Scholar] [CrossRef]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef]
- Varela, J.; Varela, C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019, 56, 88–96. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Suárez Lepe, J.A. Influence of yeasts in wine colour. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; InTech: London, UK, 2016; pp. 298–300. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Caridi, A.; Sidari, R.; Kraková, L.; Kuchta, T.; Pangallo, D. Assessment of color adsorption by yeast using Grape Skin Agar and impact on red wine color. OENO One 2015, 49, 195–203. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).