Effects of Ultradisperse Humic Sapropel Suspension on Microbial Growth and Fermentation Parameters of Barley Distillate
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
3. Results and Discussion
3.1. Microbial Assessment
3.2. Moisture and Starch Content of Barley Grains
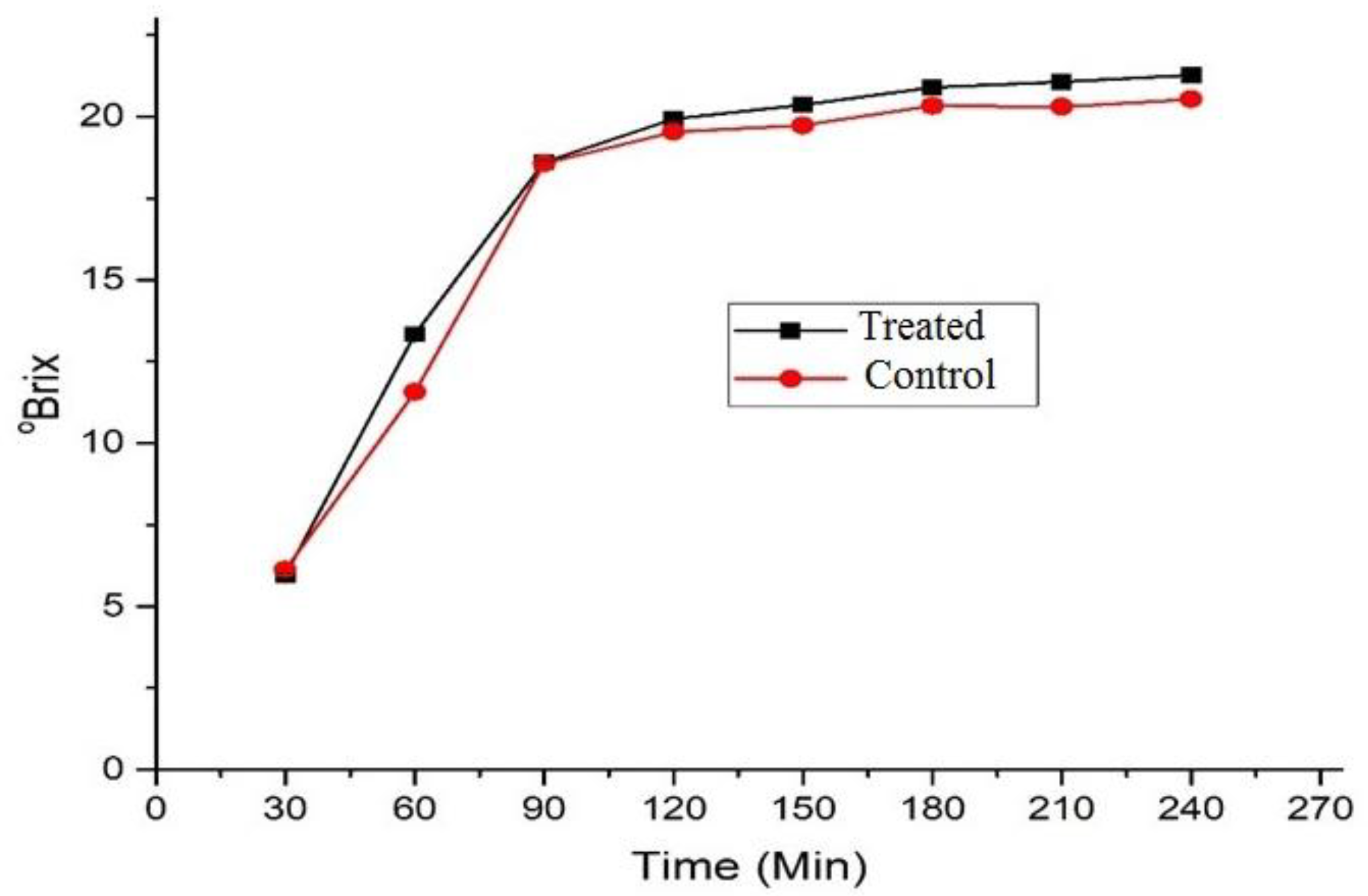
3.3. °Brix
3.4. Free Alpha Amino Nitrogen (FAN) of Mash
3.5. Variation of Carbon Dioxide during Fermentation
3.6. Titratable Acidity
3.7. Ethyl Alcohol Analysis: Volatile Compounds Determination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raneses, A.; Hanson, K.; Shapouri, H. Economic impacts from shifting cropland use from food to fuel biomass bioenergy. Biomass Bioenergy 1998, 15, 417–422. [Google Scholar] [CrossRef]
- Sheetal, B.G.; Patil, I.D. Utilization of cereal grains for bioethanol production. IJSSBT 2015, 3, 5–9. [Google Scholar]
- Murghagh, J.E. Production of neutral spirits and preparation of gin and vodka. In The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries, 3rd ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University Press: Nottingham, UK, 1999; pp. 195–210. [Google Scholar]
- Bischoff, K.M.; Liu, S.; Leathers, T.D.; Worthington, R.E.; Rich, J.O. Modelling bacterial contamination of fuel ethanol fermentation. Biotechnol. Bioeng. 2009, 103, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Hynes, S.H.; Thomas, K.C.; Ingledew, M.W. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. J. Appl. Environ. Microbiol. 1997, 63, 4158–4163. [Google Scholar]
- Lushia, W.; Heist, P. Antibiotic-resistant bacteria in fuel ethanol fermentations. Ethanol. Prod. Mag. 2005, 80–82. [Google Scholar]
- Kireicheva, L.V.; Khokhlova, O.B. Sapropels: Composition, Properties, Applications; Roma: Amsterdam, The Netherlands, 1998; p. 120. Available online: http://elib.ieek.timacad.ru/opac/index.php?url=/notices/index/IdNotice:5485/Source:default (accessed on 26 February 2019). (In Russian)
- Barakova, N.V.; Sharova, N.Y.; Juškauskaite, A.R.; Mityukov, A.S.; Romanov, V.A.; Nsengumuremyi, D. Fungicidal activity of ultradisperse humic sapropel suspensions. Agron. Res. 2017, 15, 639–648. [Google Scholar]
- Nsengumuremyi, D.; Barakova, N.V.; Romanov, V.A.; Mityukov, A.S.; Guzeva, A.V. The effect of sapropel extracts on microflora and physicochemical parameters of dried distillers grain. Agron. Res. 2018, 16, 1457–1465. [Google Scholar]
- Adadi, P.; Obeng, A.K. Assessment of bacterial quality of honey produced in Tamale metropolis (Ghana). J. Food Drug Anal. 2017, 25, 369–373. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization Technical Committee 34 (ISO/TC 34). ISO 712: 2009 Cereals and Cereal Products Determination of Moisture Content. 2009. Available online: https://www.iso.org/obp/ui/#!iso:std:44807:en (accessed on 8 October 2018).
- International Organization for Standardization Technical Committee 93 (ISO/TC 93). ISO10520:1997 Native Starch Determination of Starch Content, Ewers Polarimetric. 1997. Available online: https://www.iso.org/standard/18589.html (accessed on 26 February 2019).
- Lie, S. The EBC-Ninhydrin method for determination of free alpha amino nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- GOST 12788-87. Methods of Determining Acidity in Beer. Determination of Acidity by Direct Titration of the Sample with Phenolphthalein. 1987. Available online: https://standartgost.ru/g/ГОСТ_12788-87 (accessed on 8 October 2018). (In Russian).
- GOST 30536-2013. Vodka and Ethyl Alcohol: Vodka and Ethanol from Food Raw Material. Gas-Chromatographic Express-Method for Determination of Toxic Microadmixtures Content. 2013. Available online: http://docs.cntd.ru/document/1200104836 (accessed on 8 October 2018). (In Russian).
- GOST 32039-2013. Vodka and Ethanol from Food Raw Material. Gas-Chromatographic Method for Determination of Authenticity. 2013. Available online: http://docs.cntd.ru/document/1200107259 (accessed on 8 October 2018). (In Russian).
- Wu, M.; Song, M.; Liu, M.; Jiang, C.; Li, Z. Fungicidal activities of soil humic/fulvic acids as related to their chemical structures in greenhouse vegetable fields with cultivation chronosequence. Sci. Rep. 2016, 6, 32858. [Google Scholar] [CrossRef] [PubMed]
- Shtin, S.M. Lake Sapropels Complex Utilization; MSU: Moscow, Russia, 2005; p. 213. (In Russian) [Google Scholar]
- Kosov, V.I. Sapropel. Resources, Engineering, Geoecology, Saint Petersburg, Nauka, Russia. 2007, p. 244. Available online: https://xn--90ax2c.xn--p1ai/catalog/000200_000018_RU_NLR_bibl_1156546/ (accessed on 26 February 2019). (In Russian).
- Schepetkin, I.; Khlebnikov, A.; Kwon, B.S. Medical drugs from humus matter: Focus on mumie. Drug Dev. Res. 2002, 57, 140–159. [Google Scholar] [CrossRef]
- Buzlama, A.V.; Chernov, Y.N. Pharmacological properties, action pathways and prospectives of humic substances in medicine: An analysis. Eksperimental’naia i Klinicheskaia Farmakologiia 2010, 73, 43–48. (In Russian) [Google Scholar] [PubMed]
- Cheng, M.L.; Ho, H.Y.; Huang, Y.W.; Lu, F.J.; Chiu, D.T.Y. Humic acid induces oxidative DNA damage, growth retardation, and apoptosis in human primary fibroblasts. Exp. Biol. Med. 2003, 228, 413–423. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Managing microbial spoilage in cereals and baking products. In Food Spoilage Microorganisms; de Blackburn, C., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2006; pp. 194–212. [Google Scholar]
- Hill, A.E. Microbiological stability of beer. In Beer a Quality Perspective; Bamforth, C., Ed.; Academic Press: Cambridge, UK, 2009; pp. 163–184. [Google Scholar]
- Suzuki, K. 125th Anniversary review: Microbiological instability of beer caused by spoilage bacteria. J. Inst. Brew. 2011, 117, 131–155. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, C.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, W.; Zhang, E.; Sheng, J.; Yu, X.; Xiong, F. Morphological and physicochemical properties of starches isolated from three taro bulbs. Starch/Stärke 2018, 70, 1700168. [Google Scholar] [CrossRef]
- Patron, N.J.; Greber, B.; Fahy, B.; Laurie, D.A.; Parker, M.L.; Denyer, K. The lys5 mutations of barley reveal the nature and importance of plastidial ADP-Glc transporters for starch synthesis in cereal endosperm. Plant Physiol. 2004, 135, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, M. Enzymes in Brewing. MBAA TQ 2015, 52, 156–164. [Google Scholar]
- Nelson, O.A.; Hulett, G.A. The moisture content of cereals. J. Ind. Eng. Chem. 1920, 12, 40–45. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Cereals and cereal products. In Food Chemistry, 4th ed.; Belitz, H.-D., Grosch, W., Schieberle, P., Eds.; Springer: Berlin, Germany, 2009; pp. 670–675. [Google Scholar]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing: Science and Practice; Woodhead: Cambridge, UK, 2004; Volume 1, p. 881. [Google Scholar]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation, 1st ed.; Blackwell Science: Oxford, UK, 2001; p. 659. [Google Scholar]
- Muller, R.E. The effects of mashing temperature and mash thickness on wort carbohydrate composition. J. Inst. Brew. 1991, 97, 85–92. [Google Scholar] [CrossRef]
- Heggart, H.M.; Margaritis, A.; Pilkington, H.; Stewart, R.J.; Dowhanick, T.M.; Russell, I. Factors affecting yeast viability and vitality characteristics: A review. Master Brew. Assoc. Am. Tech. Q. 1999, 36, 383–406. [Google Scholar]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Inhibition by soil humic acids of native and acetylated proteolytic enzymes. Soil Biol. Biochem. 1971, 3, 157–160. [Google Scholar] [CrossRef]
- Bobrov, V.A.; Fedorin, M.A.; Leonova, G.A.; Markova, Y.N.; Orlova, L.A.; Krivonogov, S.K. Investigation into the elemental composition of sapropel from Lake Kirek (West Siberia) by SR XFA technique. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2012, 6, 458–463. [Google Scholar] [CrossRef]
- Platonov, V.V.; Chunosov, S.N.; Fridzon, K.Y. Biological action of sapropel. Fund Res. 2014, 9–11, 2474–2480. (In Russian) [Google Scholar]
- Tyl, C.; Sadler, G.D. pH and Titratable Acidity. In Food Analysis; Nielsen, S., Ed.; Springer: Basel, Switzerland, 2017; pp. 389–405. [Google Scholar]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Shatunova, S.A.; Petrov, A.S. Production and analysis of non-traditional beer supplemented with sea buckthorn. Agron. Res. 2017, 15, 1831–1845. [Google Scholar]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Shatunova, S.A. Biotechnological production of non-traditional beer. AIP Conf. Proc. 2017, 1886, 020098. [Google Scholar] [CrossRef]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Barakova, N.V. Production and investigations of antioxidant rich beverage: Utilizing Monascus purpureus IHEM LY2014-0696 and various malts. Agron. Res 2018, 16, 1312–1321. [Google Scholar]
- Gray, W.D. Studies on the alcohol tolerance of yeasts. J. Bacteriol. 1941, 42, 561–574. [Google Scholar] [PubMed]
- O’Rourke, T. The role of pH in brewing. Brewer Int. 2002, 2, 21–23. [Google Scholar]
- Lewis, M.J.; Young, T.W. Brewing, 2nd ed.; Springer: Berlin, Germany, 2002; p. 398. [Google Scholar]
- André, M.O.M. Continuous Fermentation of Alcohol-Free Beer—Bioreactor Hydrodynamics and Yeast Physiology. Ph.D. Thesis, University of Minho, Braga, Portugal, 2012. [Google Scholar]
- Charapitsa, S.V.; Kavalenka, A.N.; Kulevich, N.V.; Makoed, N.M.; Mazanik, A.L.; Sytova, S.N.; Zayats, N.I.; Kotov, Y.N. Direct determination of volatile compounds in spirit drinks by gas chromatography. J. Agric. Food Chem. 2013, 61, 2950–2956. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.G.; Koolwal, S.M.; Butala, H.D. Fusel oil: Composition, removal and potential utilization. Int. Sugar J. 2002, 104, 51–58. [Google Scholar]
- Stanisz, M.; Sapińska, E.; Pielech-Przybylska, K. Characteristics of contaminants present in raw spirits. Zeszyty Naukowe Chemia Spożywczai Biotechnologia 2009, 73, 105–121. [Google Scholar]
- Tarko, T. Components of the aroma of alcoholic beverages. Laboratorium 2006, 11, 39–42. (In Polish) [Google Scholar]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Metabolism of Wort by Yeast: Brewing Science and Practice; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Vallejo-Cordoba, B.; González-Córdova, A.F.; del Carmen Estrada-Montoya, M. Tequila volatile characterization and ethyl ester determination by solid phase microextraction gas chromatography/mass spectrometry analysis. J. Agric. Food Chem. 2004, 52, 5567–5571. [Google Scholar] [CrossRef] [PubMed]
- Branyik, T.; Vicente, A.A.; Dostalek, P.; Teixeira, J.A. A review of flavour formation in continuous beer fermentations. J. Inst. Brew. 2008, 114, 3–13. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Luís, F.; Guido, L.F. Impact of wort amino acids on beer flavour: A review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Berry, D.R.; Slaughter, J.C. Alcoholic beverage fermentations. In Fermented Beverage Production, 2nd ed.; Lea, A.G.H., Piggott, J.R., Eds.; Springer Science & Business Media: New York, NY, USA, 2003; pp. 25–39. [Google Scholar]
- Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of aldehydes in beer using solid-phase microextraction with on-fiber derivatization and gas chromatography/mass spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The analysis of raw spirits—A review of methodology. J. Inst. Brew. 2016, 122, 5–10. [Google Scholar] [CrossRef]


| Sampling Points | Treated | Control |
|---|---|---|
| Grains | 1.145 ± 0.120 × 104 cfu/g | 3.425 ± 0.33 × 105 cfu/g |
| Mashed wort | 1.55 ± 0.212 × 103 cfu/mL | 2.904 ± 0.141 × 104 cfu/mL |
| Wash | 2.07 ± 0.127 × 102 cfu/mL | 3.335 ± 0.205 × 103 cfu/mL |
| Sample | Starch Content (%) | Moisture Content (%) |
|---|---|---|
| Treated | 58.8 ± 0.2 | 10.57 ± 0.03 |
| Control | 58.3 ± 0.3 | 11. 62 ± 0.02 |
| Parameter | Control, mg/L | Treated, mg/L |
|---|---|---|
| Free Amino Nitrogen (FAN) | 41.42 ± 0.01 | 35.14 ± 0.02 |
| Time (hours) | Control (g) | Treated (g) |
|---|---|---|
| 24 | 6.950 ± 0.031 | 6.870 ± 0.020 |
| 48 | 8.474 ± 0.012 | 8.508 ± 0.022 |
| 72 | 8.846 ± 0.04 | 8.824 ± 0.013 |
| Day | Control (°) | Treated (°) |
|---|---|---|
| 1 | 0.396 ± 0.015 | 0.443 ± 0.040 |
| 2 | 0.443 ± 0.040 | 0.443 ± 0.040 |
| 3 | 0.406 ± 0.015 | 0.510 ± 0.010 |
| Number | Compounds | Control Sample, mg/dm3 | Treated Sample, mg/dm3 |
|---|---|---|---|
| 1 | Acetaldehyde | 0.2581 | 0.4766 |
| 2 | Ethyl acetate | 0.2784 | 0.9619 |
| 3 | Methanol * | 0.0002 | 0.0004 |
| 4 | 2-propanol | 0.6711 | 1.1315 |
| 5 | Ethanol * | 5.0884 | 7.6540 |
| 6 | 1-propanol | 16.1658 | 30.0025 |
| 7 | Isobutanol | 14.8179 | 31.0407 |
| 8 | 1-butanol | 0.2595 | × |
| 9 | Isoamylol | 38.495 | 83.834 |
| 10 | 1-pentanol | × | 0.1992 |
| 11 | Hexanol | × | 0.4580 |
| 12 | Benzaldehyde | 14.3795 | 17.5549 |
| 13 | 2-phenylethanol | 34.4960 | 33.9260 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nsengumuremyi, D.; Adadi, P.; Ukolova, M.V.; Barakova, N.V. Effects of Ultradisperse Humic Sapropel Suspension on Microbial Growth and Fermentation Parameters of Barley Distillate. Fermentation 2019, 5, 24. https://doi.org/10.3390/fermentation5010024
Nsengumuremyi D, Adadi P, Ukolova MV, Barakova NV. Effects of Ultradisperse Humic Sapropel Suspension on Microbial Growth and Fermentation Parameters of Barley Distillate. Fermentation. 2019; 5(1):24. https://doi.org/10.3390/fermentation5010024
Chicago/Turabian StyleNsengumuremyi, Daniel, Parise Adadi, Maria V. Ukolova, and Nadezhda V. Barakova. 2019. "Effects of Ultradisperse Humic Sapropel Suspension on Microbial Growth and Fermentation Parameters of Barley Distillate" Fermentation 5, no. 1: 24. https://doi.org/10.3390/fermentation5010024
APA StyleNsengumuremyi, D., Adadi, P., Ukolova, M. V., & Barakova, N. V. (2019). Effects of Ultradisperse Humic Sapropel Suspension on Microbial Growth and Fermentation Parameters of Barley Distillate. Fermentation, 5(1), 24. https://doi.org/10.3390/fermentation5010024





