Abstract
The genus Zygosaccharomyces is generally associated to wine spoilage in the winemaking industry, since a contamination with strains of this species may produce re-fermentation and CO2 production in sweet wines. At the same time, this capacity might be useful for sparkling wines production, since this species may grow under restrictive conditions, such as high ethanol, low oxygen, and harsh osmotic conditions. The spoilage activity of this genus is also found in fruit juices, soft drinks, salad dressings, and other food products, producing besides package expansion due to gas production, non-desired compounds such as ethanol and esters. Despite these drawbacks, Zygosaccharomyces spp. produces high ethanol and acetoin content in wines and may play an important role as non-Saccharomyces yeasts in differentiated wine products. Control strategies, such as the use of antimicrobial peptides like Lactoferricin B (Lfcin B), the use of dimethyl dicarbonate (DMDC) or non-thermal sterilization techniques may control this spoilage genus in the food industry.
1. The Genus Zygosaccharomyces
There were nine species accepted in the genus Zygosaccharomyces at the beginning of the century [1], and by then, the genus also included Zygosaccharomyces rouxii (Z. rouxii). Nonetheless, the number of species of the genus Zygosaccharomyces has increased rapidly over the past years and the classification of the genus by 2014 included the following species: Z. bailii, Z. bisporus, Z. gambellarensis, Z. kombuchaensis, Z. lentus, Z. machadoi, Z. mellis, Z. parabaillii, Z. pseudobailii, Z. pseudorouxii, Z. rouxii, Z. sapae, and Z. siamensi [2]. Out of the recently isolated species, Z. pseudorouxii is closely related to the species Z. sapae. Some of these species affect the food and beverage industries as spoiling microorganisms, and others are associated with fermentations and sweet foodstuff like honey. From the aforementioned species, only Z. rouxii and Z. bailii have their genome sequenced [2]. In this way, from the osmophilic yeasts, being the first cause of fruit juice spoilage, the genus Zygosaccharomyces is the most frequently described [3].
The genus Zygosaccharomyces is related to an important genus in winemaking, Saccharomyces, and at the same time, is a genus involved in food and beverage spoilage [4]. This genus is considered a spoiling microorganism since it shows high tolerance to osmotic stress, and therefore, the Zygosaccharomyces species can grow in harsh environments with high sugars concentration. In this regard, contamination by these microorganisms is often seen in fruit juices, sauces, carbonated soft drinks, salad dressings, ketchup [1], sugar syrups, candied fruit, jams and preserves, tomato sauce, and wines [5]. Wine is subjected to spoilage by this genus, since it is also capable of growing at very low pH values.
This genus can also resist extreme conditions in the presence of organic acids, low oxygen levels, and high concentration of permitted preservatives [6]. These preservatives, commonly used in the food production industry, comprise the use of sorbic acid, benzoic acid, acetic acid, and ethanol [5] and up to 200 mg/L SO2 in winemaking.
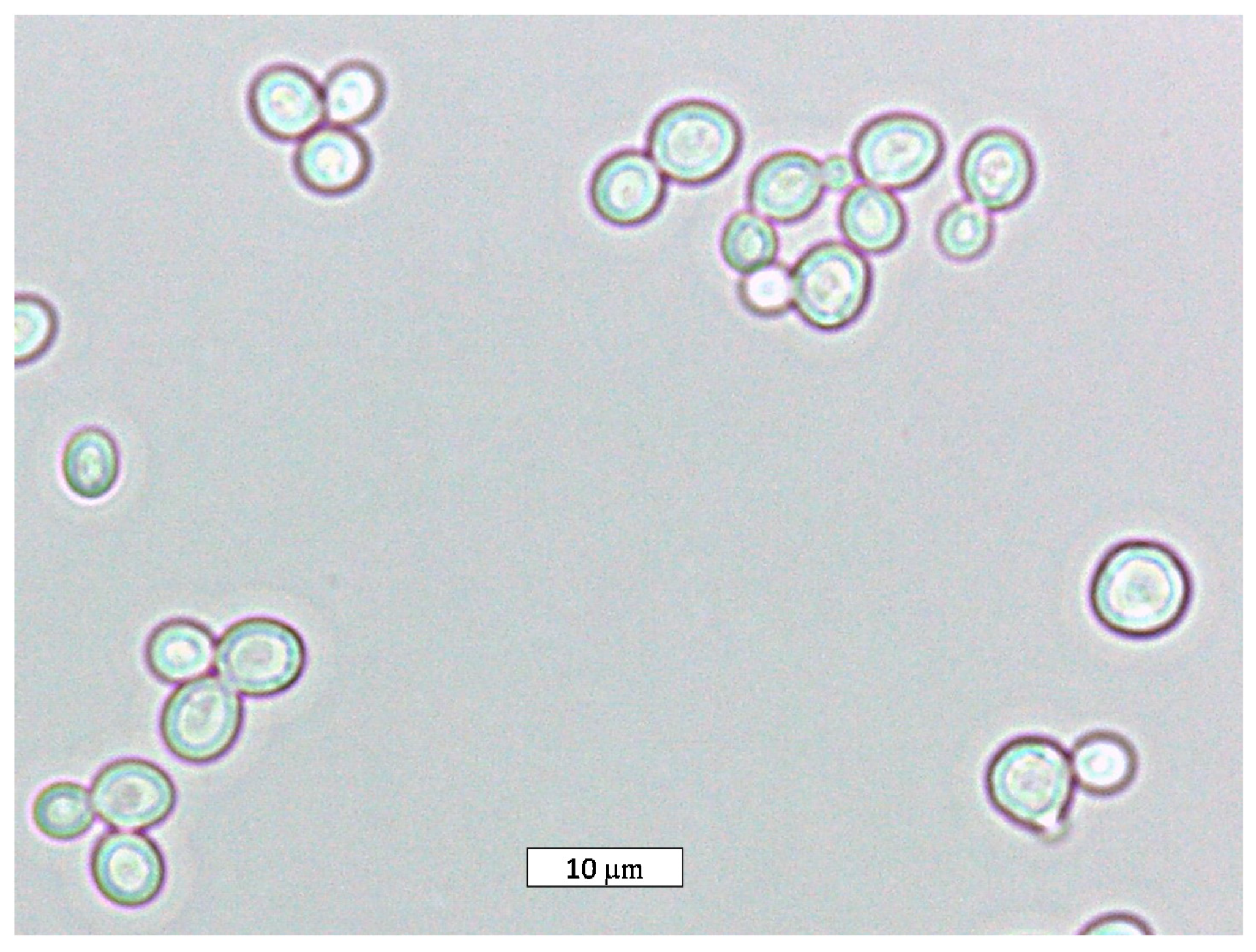
The physiology, the metabolism, and the spoilage/industrial activity of the species Z. rouxii, where the morphology is shown in Figure 1, will be described in the following sections.
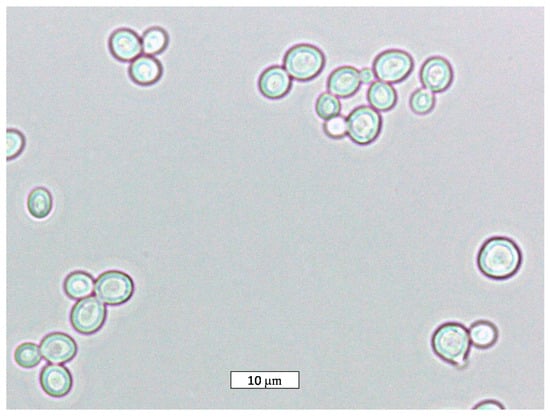
Figure 1.
Optical microscopic picture of the species Z. rouxii (100× magnification).
2. Physiology and Metabolism of Z. rouxii
The species Z. rouxii and Z. bailii are associated with food spoilage, especially affecting those products with a high concentration of sugar and/or salt, low pH, or week-organic acids content.
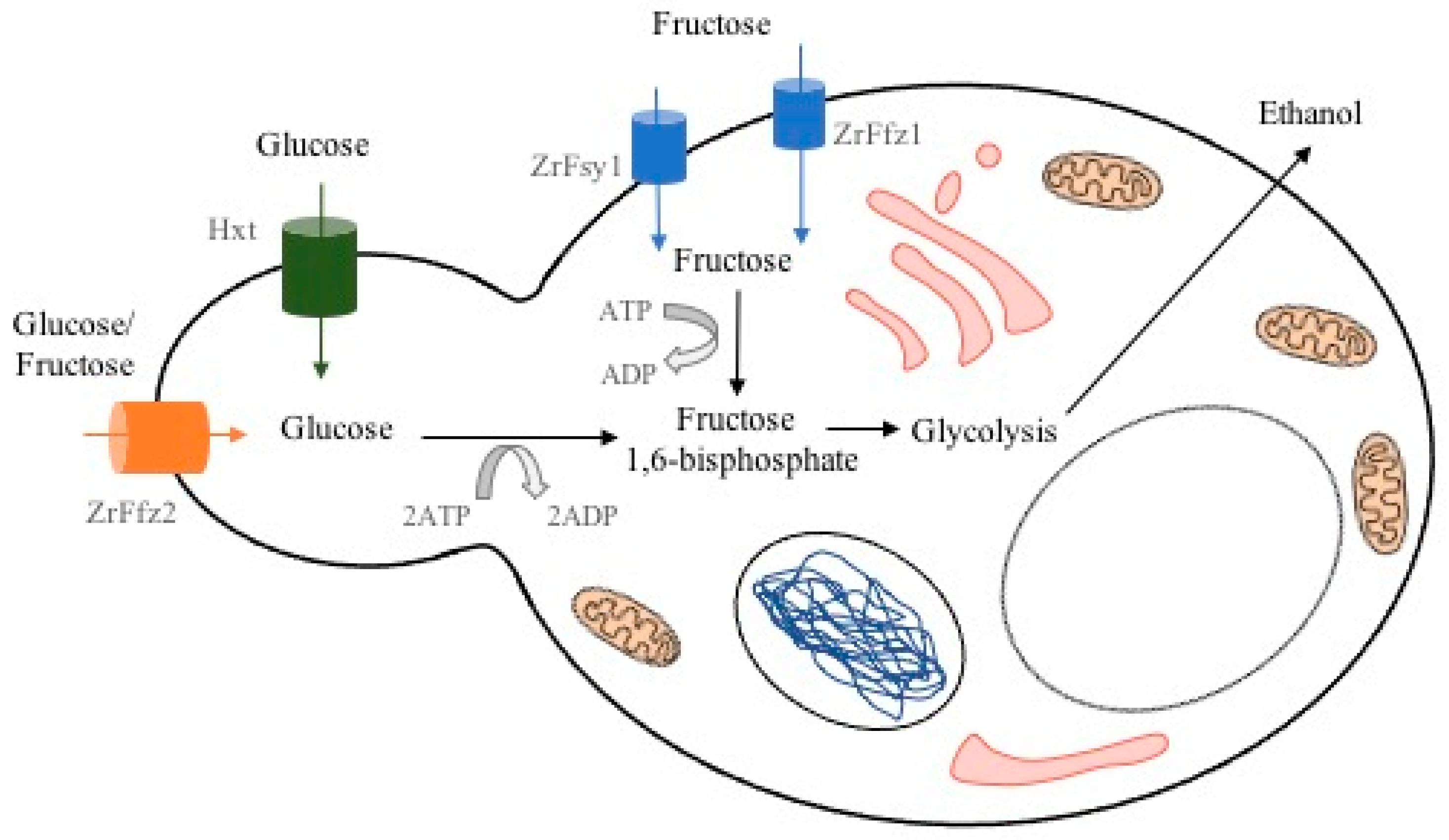
From these species, Z. rouxii is able to endure very low water activity (aw) environments, and due to this, it is one of the most xerophilic organisms known. It can grow in food with up to 70% glucose in their composition, and some strains survive to even higher concentrations, 5 M (>90% w/v) and 5.5 M glucose (saturated glucose solution) [7,8]. Moreover, it opts to consume fructose over glucose, being the reason why it is considered a fructophilic yeast. As a fructophilic yeast, Z. rouxii possesses genes FFZ that encode specific fructose facilitators and proteins, which have been characterized for both species, Z. rouxii [9] and Z. bailii [10]. The fructose transporter systems mediate the uptake of hexoses via a facilitated diffusion mechanism. Figure 2 is a schematic representation of such transporters during ethanol fermentation. ZrFfz1 and ZrFsy1 are fructose transporters, Hxt is a hexose transporter, and ZrFfz2 is a fructose/glucose transporter. The intermediate reactions in the production of fructose 1,6-bisphosphate from glucose and fructose, double phosphorylation in glucose, and phosphorylation in fructose, are not shown.
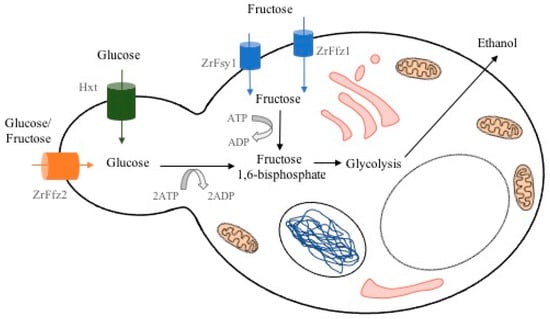
Figure 2.
Fructose and glucose transporters during Z. rouxii ethanol fermentation.
The yeast species Zygosaccharomyces rouxii is usually haploid and heterothallic [11]. Besides being osmophilic and xerophilic, it is also considered acid-tolerant. The species can adapt and grow in acidic media at pH values of 2.2 or even as low as 1.8 [7,11], being the reason why this species could either spoil products, such as grape juice concentrates [12] or be used industrially in the production of soy sauce and miso paste [11]. Total inhibition is achieved at pH values below 1.7, although this might be difficult to achieve at an industrial scale [13]. Assuring pH 2.2, food products such as concentrated grape juice, could extend their shelf-life significantly for storage or shipping overseas.
In terms of inhibition temperature, 47 °C is needed to reduce log2 CFU/mL in inoculums of spoilage yeast species Z. rouxii, P. guillermondii, and Z. lentus [7]; nonetheless, some strains require higher temperatures, between 55 °C and 60 °C, to inhibit their growth.
Water activity tolerance is one physiological difference between the species Zygosaccharomyces rouxii and Zygosaccharomyces bailii. The species Z. rouxii can tolerate low water activity (aw) environments, whilst Z. bailii requires environments with (aw) of at least 0.85 [7]. This characteristic makes it difficult for Z. bailii to survive in high sugar foods, such as syrups and candied fruits.
The yeast species Zygosaccharomyces rouxii, as well as other osmotolerant microorganisms, adjusts its internal osmotic pressure to tolerate high concentrations of salt (NaCl) of about 3–4 M (ca. 20% w/v) [14]. The mechanism is the efflux of sodium cations (Na+) from cells under high concentration of salt [11]. A change in the fluidity of the lipidic cell membranes has been observed when exposing yeast cells to 15% NaCl [14]. The lipid composition of the cell membrane and the plasma membranes changed by means of a decrease in the degree of saturation, an increase in ergosterol concentration, and a decrease in the phospholipid to protein ratio. The accumulation of glycerol as a compatible solute is a mechanism that Z. rouxii follows to survive to high osmolarity. This protects the cell against lysis [11].
Another metabolic feature of this yeast is the production of volatiles in high-sugar food matrices at an early stage; this would indicate the presence of spoilage yeast Z. rouxii. Ethanol, acetone, ethyl acetate, acetaldehyde, or 3-methyl-1-butanol could be detected by analytical techniques and even before the human nose is able to [15].
3. Food Spoilage Activity
As already described, the physiologies of Zygosaccharomyces rouxii, comprising the osmotolerance, the xerophilic ability, the fructophilic capacity, and the weak-acid tolerance, are responsible for causing food spoilage. The food products prone to growing spoilage microorganisms include juice concentrates, sugar syrups, honey, jams, confectionary products, and dried fruits [16]. Among several fruit concentrate juices, those with a higher incidence of Z. rouxii, even after 16 months stored at −18 °C, are cherry and orange juices; grape concentrated juice grew populations of C. stellate, K, thermotolerans, P. anomala, S. cerevisiae, and Z. rouxii [17]. Despite the presence of other yeast species in non-spoiled juices, 100% of the yeasts isolated in spoiled concentrated grape juice, belonged to the species Zygosaccharomyces rouxii [18].
One of the most obvious effects observed in food products after the contamination of spoilage yeasts, is the production of excess gas. This gas compromises the integrity of the food package as it can swell containers, and it could also be responsible for “blown” cans or exploding glass bottles. This excess gas is the result of the fermentation of sugars by yeasts, during the product’s shelf-life. The volume of gas produced is variable and so is the pressure inside the food package; this effect depends on the fermentative yeast species and their fermentation power. In this matter, three Zygosaccharomyces species (Z. lentus, Z. bailii, and Z. rouxii) produced larger amounts of gas in comparison with other spoilage yeast genera (P. guillermondii, C. halophila and C. magnolia), as evidence of the high fermenting capacity of Zygosaccharomyces genus in food with high sugar content [7].
Taking all this into consideration, the efforts in controlling the spoilage yeasts should not just focus on the conservation alternatives using preservatives, but also in the production facilities in terms of hygienic practices to avoid the contamination of piping, containers, and any other equipment and machinery. The quick detection of spoilage microorganisms, would solve contamination in the initial stage and save related costs.
4. Detection
Food spoilage caused by Zygosaccharomyces rouxii strains, is perceived by consumers due to the formation of non-desired odors affecting the products. In products where the aroma profile is synonymous of quality, like apple juice, the presence of such aromas may contribute to increased product waste. Electronic noses (e-nose) may detect the contamination by Zygosaccharomyces rouxii strains, even at populations as low as log2 CFU/mL, during the production stage or in the product’s shelf life [19]. The electronic noses work with gas sensory array technology and are able to detect changes in the volatile pattern associated with microorganism spoilage [20]. Organic acids and esters, are compounds detectable by e-noses which indicate microorganism activity. The production of such volatiles is then related to a certain microorganism; this characteristic makes this technique even able to distinguish contamination coming from different species [21]. No sample preparation is needed when using the e-nose technique, but a series of sensors able to distinguish among aromatic compounds, nitrogen oxide, ammonia, alkanes, methane, Sulphur compounds, alcohol, etc. are required [15]. These electronic noses could be coupled with chemometric analysis, for the early diagnosis of the contamination by Z. rouxii strains in apple juice [19]. Other analytical techniques, more expensive than e-noses, but quite extended on the identification and quantification of spoilage yeast metabolites among other compounds, are gas chromatography and liquid chromatography. Gas chromatography coupled with mass-spectrometry, detects concentrations of guaiacol, 2,6-dibromophenol, and 2,6-dichlorophenol in kiwi juices [22]; ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS), is suitable to identify molecules from different mycotoxins present in cereal syrups [23]. The selection of the appropriate analytical technique is also related to the stability and solubility of the target molecules, and no less important is the sample preparation, which may include solid phase extraction, partitioning via salting-out interfaces between aqueous and organic solvent layers, etc.
Although several analytical techniques might help in identifying the presence of Zygosaccharomyces genus in food products and in winemaking by the detection of molecules associated to its metabolic activity, the use of primers with plasmid DNA in multiplex polymerase chain reaction (PCR) would also allow the identification between Z. rouxii and Z. bailii, as well as to differentiate this genus from the species S. cerevisiae [24]. This method was used in the late twentieth century and it later allowed the identification of the genes TPS1 and TPS2, encoding trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase, respectively [25], for the synthesis of trehalose; or the identification of the nucleotide sequence of the genes Nq+/H+-antiporter (ZSOD2 and ZSOD22) related to the salt tolerance of this yeast species [26]. Lately, the use of pre-treatment methodologies in PCR analysis, has allowed the identification of the food-spoilage yeast Z. rouxii in real apple juice samples. Double washing dielectrophoretic (DEP) manipulation of yeast cells, is one of such pre-treatments [27]. The DEP device washes out PCR inhibitors and improves the analysis.
The use of selective high-sugar medium, such as PYGF broths with 300 g/L glucose and 300 g/L fructose, may also help in isolating osmotolerant and fructophilic yeast strains present in food matrices, for further identification during microbiological controls and detection [15].
5. Control Strategies
Zygosaccharomyces bailii species has shown high resistance to food preservatives, such as sorbic acid, benzoic acid, acetic acid, cinnamic acid, ethanol, and to heat. On the other hand, they lacked resistance to peracetic acid or hypochlorite, suggesting the possibility of using biocidal cleaning agents to control their population in production facilities [7]. Regarding sanitization practices, it is interesting to pay attention to the materials used in the food industry. Frisón, Chiericatti, Aríngoli, Basílico, and Basílico [28] have compared the effect of different sanitizing materials, such as peracetic acid, monochloramine, iodophor, and quaternary ammonium compounds, in a variety of surfaces, including wood, glass, PVC plastic, and stainless steel, against the yeast species Zygosaccharomyces rouxii. The results obtained revealed that peracetic acid was effective to avoid contamination by Z. rouxii, and it was preferred over the rest of the products tested due to its higher safety. Stainless steel was completely sanitized with all the compounds tested, being the reason why it shall be more appropriate to use this material in the diverse food industry sectors. Regarding limiting growing conditions for Z. rouxii, these have been determined as glucose concentration above 5M, temperature above 46.5 °C, and pH lower than 2.2 [7]. This yeast could also produce 31.5 mL of gas in substrates with 2% glucose, and up to 102 mL of gas in substrates with 18% glucose. Table 1 summarizes the comparison of different preservatives and disinfectants, and the minimum concentration for their inhibitory effect.

Table 1.
Minimum inhibitory concentration of preservatives and disinfectants against Zygosaccharomyces rouxii strains.
Zygosaccharomyces rouxii, similar to Z. bailii, has a high resistance to different chemical compounds used as food preservatives. Hydroxycinnamic acids, such as caffeic acid and p-coumaric acid, have a rather low inhibitory effect of around 15%, whilst preservatives like potassium sorbate, sodium benzoate, dimethyldicarbonate, and vanillin can inhibit the growth of this yeast species up to 40% [29]. The acetic acid has an impact in the respiratory activity of the halo-tolerant yeast Zygosaccharomyces rouxii R-1, and it also inhibits the formation of cytochromes. Z. rouxii was significantly inhibited, and its growth was considerably reduced, in the presence of 0.5% acetic acid and also in media containing NaCl above 18% [30].
The use of thermo-sonication, ultrasounds in combination with heat, to inactivate Zygosaccharomyces rouxii strains at different pH and water activity conditions [31], results in a reduction of >log5 CFU/mL of yeast population. The higher the temperature of sonication, the greater the effect of the temperature in the inactivation. The use of ultrasounds under these conditions produces irreversible cell damage contributing to yeast inactivation; the synergetic contribution of lower water activity (aw) and pH decreases with lower temperatures of sonication. Therefore, this approach might be useful as an alternative to traditional pasteurization of fruit juices.
Dielectric barrier discharge (DBD) plasma at 90W for 140 s, have shown to reduce log5 viable Z. rouxii cells in apple juice [6]. The DBD plasma have produced alterations in the permeability of the Z. rouxii cell membranes, and as a result, the release of intracellular macromolecules, such as nucleic acids and proteins. The disruptions caused in the cell membrane are observable with scanning electron microscopy (SEM) imaging. During DBD plasma processing, reactive species like H2O2 and NO2 were produced, and these reactive compounds would contribute to the inactivation of Z. rouxii together with the alterations produced in the membrane permeability. A drawback on the use of DBD plasma, is the negative effect on color parameters of apple juices treated. The juices reported higher acidic values, whilst on the other hand, the content of reducing sugars, total soluble solids, and total phenolics remained practically without change. DBD plasma might then be used as effective control technique to inactivate Z. rouxii in apple juice.
Other control strategies may involve the use of antimicrobial peptides such as Lactoferricin B (Lfcin B) and the use of killer toxins.
Lfcin B is a peptide produced after the gastric digestion of protein lactoferrin from bovine origin, and according to Escott, Loira, Morata, Bañuelos, and Suárez-Lepe [32], it has antibacterial and antifungal properties, besides being considered a peptide with antiviral, antitumor, anti-inflammatory, and immunoregulatory properties. These properties have shown to have an effective antimicrobial effect against spoilage yeasts, such as Dekkera bruxellensis and Zygosaccharomyces spp. in wine production.
Killer toxins, such as Pichia membranifaciens killer toxins (PMKT), may interact with other antimicrobial agents like metabisulphite, to avoid the spoilage by Zygosaccharomyces spp. in beverages with a high sugar concentration [33]. The interaction effect would reduce the amount of metabisulphite needed as antimicrobial, and therefore, reducing the potential negative effect on health and to the environment. Both yeasts, Saccharomyces spp. and non-Saccharomyces spp., have shown to possess the ability to produce killer toxins during spontaneous wine fermentation, as it is, in the case of the toxins K1, K2, K28, and Klus from S. cerevisiae. In some cases, the activity observed in killer toxins is due to β-glucanases. These β-glucanases are used to produced synthetic preparations as antimicrobial agents against spoilage yeasts Dekkera bruxellensis and Zygosaccharomyces bailii [34].
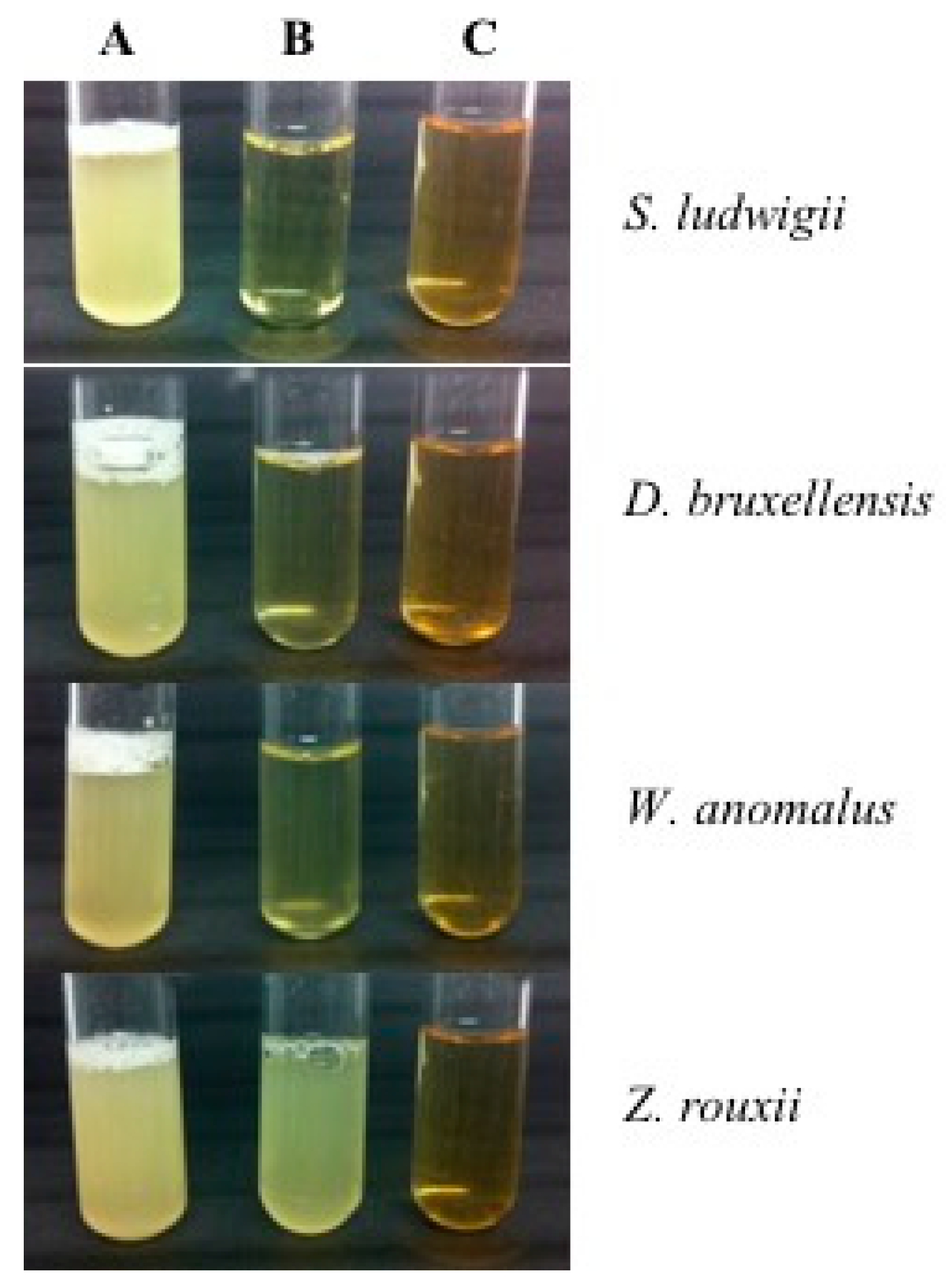
To assess the effect of β-glucanases on the inhibition of different spoilage yeasts species, including the species Zygosaccharomyces rouxii, an experiment was carried out by Escott et al. [32], and the results are shown in Figure 3. It could be observed that all control growing media have fermented as there was CO2 production in the tubes. After using β-glucanase 1, the optical density (OD) did not change for S. ludwigii, suggesting a complete inhibition effect, while D. bruxellensis and W. anomalus had slightly increased OD suggesting a high inhibition effect. Finally, lesser effects were observed in Z. rouxii as there was CO2 production and higher OD, suggesting partial inhibition effect. On the other hand, the use of β-glucanase 2 has successfully inhibited the growth of all spoilage yeast strains evaluated.
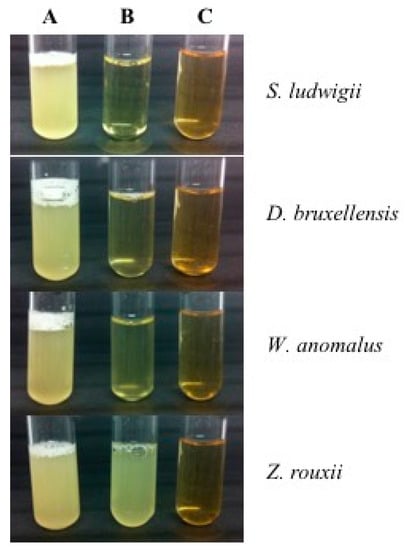
Figure 3.
Use of β-glucanases as yeasts inhibitors. The tubes contain the yeasts species S. ludwigii, D. bruxellensis, W. anomalus and Z. rouxii in yeast extract peptone dextrose (YEPD) liquid growing media with same optical density. (A) control, (B) β-glucanase 1 and (C) β-glucanase 2.
Sulphur dioxide (SO2) has two purposes in the winemaking industry, to avoid microbiological spoilage of musts and wines, and to act as an antioxidant of wines, especially to avoid browning of white wines [35]. The role of SO2 in red wines could be detrimental towards the anthocyanin content. Oenologists prefer the use of SO2 to preserve wines, rather than using it during winemaking. According to research, the minimal concentration of free SO2 needed to inhibit the growth of Z. rouxii populations is strain related and goes from 160–185 mg/L [36] to 217–262 mg/L (Table 1).
6. Food Applications
Soy sauce is probably the main product elaborated with the use of Zygosaccharomyces rouxii. The species Z. rouxii, as osmotolerant yeast, makes feasible the production of soy sauce and miso paste industrially [11]. This species contributes to enhancing the flavor of soy sauce during its production, since this yeast is able to increase the concentration of certain aromatic volatile compounds. These compounds comprise the formation of larger amounts of 3-methyl-1-butanol (isoamyl alcohol), 2-methil-1-butanol (amyl alcohol), and 2-methyl-1-propanol (isobutyl alcohol) [37]. Some strains are even prone to forming larger amounts of acetoin than others [4], contributing to the overall flavor formation.
Parallel to the production of soy sauce and miso paste, Z. rouxii is used industrially in the production of other salted condiments, such as balsamic vinegar [38,39].
In addition, Z. rouxii has also been used for the production of certain compounds of interest. Hecquet, Sancelme, Bolte and Demuynck [40] showed that Z. rouxii produced 4-hydroxy-2,5-dimethyl-3(2H)-furanone when this yeast grows aerobically with D-fructose 1,6-bisphosphate (10%) as precursor. 4-hydroxy-2,5-dimethyl-3(2H)-furanone is used in the food industry as an additive; it exhibits caramel-like odors and has a relatively low perception threshold. Similarly, Saha, Sakakibara and Cotta [41] isolated a strain of Z. rouxii, that produced d-arabitol as the main metabolic product from glucose. In this way, this yeast shows potential to be used for production of xylitol from glucose via the d-arabitol route. Xylitol is a five-carbon sugar alcohol, used as a natural food sweetener. In addition, Z. rouxii has been used in solid-state fermentation, to produce extra-cellular l-glutaminase [42].
Other uses of Zygosaccharomyces rouxii strains are given in a patent application in the United States of America, that proposes the commercial utilization of a novel yeast strain of the species Zygosaccharomyces rouxii and its fermented metabolites as probiotics, as well as antioxidant and antimicrobial agents in foods and cosmetics [43].
Finally, the use of genome shuffling technique was successfully used to improve the flavor formation. This improvement impacted the formation of flavor components and amino acid nitrogen, with the result of enhancing the quality of soy sauce [44].
7. Alcohol-Fermentation Applications
Latest research, has shown the potential of using strains from the species Z. rouxii in the production of low-alcohol beer [45]. The use of these nonconventional yeast strains, as well as yeasts from the species S. ludwigii, could become an alternative to current practices in the production of beer with ethanol content between 0.5 and 1.2% v/v. The use of Z. rouxii strains, compared to S. ludwigii strains, has produced ethanol and diacetyl in larger amounts, above the taste threshold in beers.
Regarding wine production, there is scarce research on the potential use of Zygosaccharomyces rouxii strains in winemaking. Although some metabolic features of strains from the genus Zygosaccharomyces, may be interesting in the production of wines.
Species from the yeast Zygosaccharomyces genus, found in grapes and musts are able to increase the production of higher alcohols, at the same time that acetoin is reduced [4]. Although the contribution of acetoin to wine aroma profile is difficult to assess and its threshold is rather high (150 mg/L) [46], concentration above 300 mg/L is expected to produce butter aromas not pleasant in wine [47]. Zygosaccharomyces spp. generally produce a lower amount of acetoin in comparison with high-acetoin producer yeasts, such as the genera Kloeckera and Hanseniaspora. Higher alcohols, or fusel alcohols, like isoamyl alcohol, amyl alcohol, and isobutyl alcohol, are also found during the production of soy sauce by Z. rouxii [37]. These volatile compounds may contribute to the aroma profile of wines.
Wines trials with lower ethanol concentration were produced at laboratory scale, with yeast strains of the species Torulaspora delbrueckii and Zygosaccharomyces bailii; and S. cerevisiae was used to ensure completion of fermentation after 50% of sugars were consumed by non-Saccharomyces yeasts. These strains allowed the production of wines with less ethanol concentration, under limited aerobic conditions (5 to 10 mL/min). The ethanol reduction depended on the aeration regime. Both strains, T. delbrueckii and Z. bailii, were able to reduce ethanol in 1.5% (v/v) and 2.0% (v/v), respectively, in comparison to anaerobic fermentation carried out with S. cerevisiae as control [48]. The media used in this analysis comprised the preparation of chemically defined grape juice having 100 g glucose, 100 g fructose, 0.2 g citric acid, 3 g malic acid, inorganic salts and nitrogen sources.
One drawback of having uncontrolled Zygosaccharomyces spp. yeasts in sweet wine production is the potential re-fermentation, producing turbidity and CO2 production [33], although this effect does not produce off-characters to wine. This, and the high production of acetic acid, has limited the possibilities of using this yeast species in winemaking production in the past years [49].
On the other hand, other Zygosaccharomyces species studied like Z. fermentati and Z. bailii, produce lower levels of H2S and malic acid degradation, respectively [50]. These characteristics might be useful for wine production, where mixed fermentations with these and other spoilage non-Saccharomyces yeasts, and S. cerevisiae could be performed. Laboratory testing has shown that most detrimental metabolites produced by spoilage yeasts in pure culture or spoiled juices, are reduced in mixed fermentations; the production of polysaccharides increased improving body of wines and this also had a positive effect on aroma and protein stability [51].
The main concern in the winemaking industry, comes from the fact that these strains have high stress tolerance and may produce off-metabolites. Nonetheless, there are commercial Zygosaccharomyces yeast products prepared for stuck fermentations and potentially suitable for musts from riper grapes where the concentration of sugars, particularly fructose, is higher, and this could limit the implantation of other fermentative strains, such as S. cerevisiae [52].
8. Conclusions
Although the presence of yeast strains of the genus Zygosaccharomyces in many food products may represent a quality control danger and negative economic impact, the controlled used of some strains may positively contribute to enhancing organoleptic parameters of a particular range of products in the food industry. The potential use of these strains in winemaking is still controversial for their high spoilage activity, but it might also be an alternative to current technologically challenging conditions, such as stuck fermentations or the use of high fructose riper grape musts. Further studies on the impact of using the species Zygosaccharomyces rouxii, a yeast species which might not always endanger wine production, at all levels in the winemaking industry are promising.
Author Contributions
C.E. and J.M.d.F. performed the bibliographic revision and drafted the manuscript, I.L. and A.M. coordinated the bibliographic work and revised the manuscript and J.A.S.-L. revised and corrected the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esteve-Zarzoso, B.; Zorman, T.; Belloch, C.; Quero, A. Molecular Characterisation of the Species of the Genus Zyosaccharomyces. Syst. Appl. Microbiol. 2003, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hulin, M.; Wheals, A. Rapid Identification of Zygosaccharomyces with Genus-Specific Primers. Int. J. Food Microbiol. 2014, 173, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Z.; Long, F.; Guo, C.; Niu, C.; Yuan, Y.; Yue, T. Combined Effect of Sugar Content and PH on the Growth of a Wild Strain of Zygosaccharomyces Rouxii and Time for Spoilage in Concentrated Apple Juice. Food Control. 2016, 59, 298–305. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Higher Alcohol and Acetoin Production by Zygosaccharomyces Wine Yeasts. J. Appl. Bacteriol. 1993, 75, 541–545. [Google Scholar] [CrossRef]
- Steels, H.; James, S.A.; Roberts, I.N.; Stratford, M. Zygosaccharomyces Lentus: A Significant New Osmophilic, Preservative-Resistant Spoilage Yeast, Capable of Growth at Low Temperature. J. Appl. Microbiol. 1999, 87, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of Dielectric Barrier Discharge Plasma on the Inactivation of Zygosaccharomyces Rouxii and Quality of Apple Juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Stratford, M.; Steels, H.; Fernández-Espinar, M.; Querol, A. Physiological Characterization of Spoilage Strains of Zygosaccharomyces Bailii and Zygosaccharomyces Rouxii Isolated from High Sugar Environments. Int. J. Food Microbiol. 2007, 114, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.; Solieri, L.; Giudici, P. Adaptive Response and Tolerance to Sugar and Salt Stress in the Food Yeast Zygosaccharomyces Rouxii. Int. J. Food Microbiol. 2014, 185, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.J.; Sychrová, H.; Prista, C.; Loureiro-Dias, M.C. The Osmotolerant Fructophilic Yeast Zygosaccharomyces Rouxii Employs Two Plasma-Membrane Fructose Uptake Systems Belonging to a New Family of Yeast Sugar Transporters. Microbiology 2011, 157, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Pina, C.; Gonçalves, P.; Prista, C.; Loureiro-Dias, M.C. Ffz1, a New Transporter Specific for Fructose from Zygosaccharomyces Bailii. Microbiology 2004, 150, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Wolfe, K.H. Recent Allopolyploid Origin of Zygosaccharomyces Rouxii Strain ATCC 42981. Yeast 2008, 25, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.C.; Torres Palazzolo, C.; Cuello, R.; González, M.; Guevara, F.; Ponsone, M.L.; Mercado, L.A.; Martínez, C.; Combina, M. Incidence of Osmophilic Yeasts and Zygosaccharomyces Rouxii during the Production of Concentrate Grape Juices. Food Microbiol. 2017, 64, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.C.; Arroyo López, F.N.; Lerena, M.C.; Mercado, L.; Torres, A.; Combina, M. Effects of pH and Sugar Concentration in Zygosaccharomyces Rouxii Growth and Time for Spoilage in Concentrated Grape Juice at Isothermal and Non-Isothermal Conditions. Food Microbiol. 2014, 38, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hosono, K. Effect of Salt Stress on Lipid Composition and Membrane Fluidity of the Salttolerant Yeast Zygosaccharomyces Rouxii. J. Gen. Microbiol. 1992, 138, 91–96. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Long, F.; Guo, C.; Yuan, Y.; Yue, T. Detection of Zygosaccharomyces rouxii and Candida tropicals in a High Sugar Medium by a Metal Oxide Sensor-Based Electronic Nose and Comparison with Test Panel Evaluation. J. Food Prot. 2015, 78, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G. Yeast Spoilage of Foods and Beverages. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2010; pp. 53–63. [Google Scholar]
- Deak, T.; Beuchat, L.R. Yeasts Associated with Fruit Juice Concentrates. J. Food Prot. 1993, 56, 777–782. [Google Scholar] [CrossRef]
- Combina, M.; Daguerre, C.; Massera, A.; Mercado, L.; Sturm, M.E.; Ganga, A.; Martinez, C. Yeast Identification in Grape Juice Concentrates from Argentina. Lett. Appl. Microbiol. 2007, 46, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Z.; Long, F.; Guo, C.; Yuan, Y.; Yue, T. Early Detection of Zygosaccharomyces Rouxii—Spawned Spoilage in Apple Juice by Electronic Nose Combined with Chemometrics. Int. J. Food Microbiol. 2016, 217, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Arakawa, E.; Kita, J.I.; Aoyama, Y.; Manome, Y.; Ikeda, K.; Yamamoto, K. Detection of Aeromonas Hydrophila in Liquid Media by Volatile Production Similarity Patterns, Using a FF-2A Electronic Nose. Sensors 2013, 13, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, E.; Falasconi, M.; Concina, I.; Mantero, G.; Bianchi, F.; Mattarozzi, M.; Musci, M.; Sberveglieri, G. Electronic Nose and Alicyclobacillus spp. Spoilage of Fruit Juices: An Emerging Diagnostic Tool. Food Control 2010, 21, 1374–1382. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, T.; Yuan, Y. Alicyclobacillus Contamination in the Production Line of Kiwi Products in China. PLoS ONE 2013, 8, e67704. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A.M. Simple and Efficient Methodology to Determine Mycotoxins in Cereal Syrups. Food Chem. 2015, 177, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.M.; McKee, R.A. Rapid Identification of Saccharomyces cerevisiae, Zygosaccharomyces bailii and Zygosaccharomyces rouxii. Int. J. Food Microbiol. 1992, 16, 63–67. [Google Scholar] [CrossRef]
- Kwon, H.; Yeo, E.; Hahn, S.; Bae, S.; Kim, D.; Byun, M. Cloning and Characterization of Genes Encoding Trehalose-6-Phosphate Synthase (TPS1) and Trehalose-6-Phosphate Phosphatase (TPS2) from Zygosaccharomyces rouxii. FEMS Yeast Res. 2003, 3, 433–440. [Google Scholar] [CrossRef]
- Iwaki, T.; Higashida, Y.; Tsuji, H.; Tamai, Y.; Watanabe, Y. Characterization of a Second Gene (ZSOD22) of Na+/H+ Antiporter from Salt-Tolerant Yeast Zygosaccharomyces rouxii and Functional Expression of ZSOD2 and ZSOD22 in Saccharomyces cerevisiae. Yeast 1998, 14, 1167–1174. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Huttener, M.; Alvarez, J.M.; Homs-Corbera, A.; Samitier, J.; Torrens, E.; Juárez, A. Dielectrophoresis Chips Improve PCR Detection of the Food-Spoiling Yeast Zygosaccharomyces rouxii in Apple Juice. Electrophoresis 2015, 36, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Frisón, L.N.; Chiericatti, C.A.; Aríngoli, E.E.; Basílico, J.C.; Basílico, M.Z. Effect of Different Sanitizers against Zygosaccharomyces Rouxii. J. Food Sci. Technol. 2014, 52, 4619–4624. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.C.; Arroyo López, F.N.; Lerena, M.C.; Mercado, L.; Torres, A.; Combina, M. Evaluation of Different Chemical Preservatives to Control Zygosaccharomyces Rouxii Growth in High Sugar Culture Media. Food Control 2014, 50, 349–355. [Google Scholar] [CrossRef]
- Kusumegi, K.; Yoshida, H.; Tomiyama, S. Inhibitory Effects of Acetic Acid on Respiration and Growth of Zygosaccharomyces Rouxii. J. Ferment. Bioeng. 1998, 85, 213–217. [Google Scholar] [CrossRef]
- Kirimli, S.; Kunduhoglu, B. Inactivation of Zygosaccharomyces Rouxii Using Ultrasound at Different Temperatures, PH and Water Activity Conditions. Ital. J. Food Sci. 2016, 28, 64–72. [Google Scholar]
- Escott, C.; Loira, I.; Morata, A.; Bañuelos, M.; Suárez-Lepe, J. Wine Spoilage Yeasts: Control Strategy. In Yeast-Industrial Applications; Morata, A., Loira, I., Eds.; InTech: Rijeka, Croatia, 2017; pp. 89–116. [Google Scholar]
- Alonso, A.; Belda, I.; Santos, A.; Navascués, E.; Marquina, D. Advances in the Control of the Spoilage Caused by Zygosaccharomyces Species on Sweet Wines and Concentrated Grape Musts. Food Control 2015, 51, 129–134. [Google Scholar] [CrossRef]
- Enrique, M.; Ibáñez, A.; Marcos, J.F.; Yuste, M.; Martínez, M.; Vallés, S.; Manzanares, P. β-Glucanases as a Tool for the Control of Wine Spoilage Yeasts. J. Food Sci. 2010, 75, M41–M45. [Google Scholar] [CrossRef] [PubMed]
- Usseglio-Tomasset, L. Properties and Use of Sulphur Dioxide. Food Addit. Contam. 1992, 9, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Warth, A.D. Resistance of Yeast Species to Benzoic and Sorbic Acids and to Sulfur Dioxide. J. Food Prot. 1985, 48, 564–569. [Google Scholar] [CrossRef]
- Jansen, M.; Veurink, J.; Euverink, G. Growth of the Salt-Tolerant Yeast Zygosaccharomyces Rouxii in Microtiter Plates: Effects of NaCl, PH and Temperature on Growth and Fusel Alcohol Production From. FEMS Yeast Res. 2003, 3, 313–318. [Google Scholar] [PubMed]
- Solieri, L.; Cassanelli, S.; Giudici, P. A New Putative Zygosaccharomyces Yeast Species Isolated from Traditional Balsamic Vinegar. Yeast 2007, 24, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Solieri, L.; Giudici, P. Yeasts Associated to Traditional Balsamic Vinegar: Ecological and Technological Features. Int. J. Food Microbiol. 2008, 125, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, L.; Sancelme, M.; Bolte, J.; Demuynck, C. Biosynthesis of 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone by Zygosaccharomyces Rouxii. J. Agric. Food Chem. 1996, 44, 1357–1360. [Google Scholar] [CrossRef]
- Saha, B.C.; Sakakibara, Y.; Cotta, M.A. Production of D-Arabitol by a Newly Isolated Zygosaccharomyces Rouxii. J. Ind. Microbiol. Biotechnol. 2007, 34, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Sabu, A.; Pandey, A.; Szakacs, G.; Soccol, C.R. Extra-Cellular l-Glutaminase Production by Zygosaccharomyces Rouxii under Solid-State Fermentation. Process Biochem. 2002, 38, 307–312. [Google Scholar] [CrossRef]
- Ok, T. Method of Utilization of Zygosaccharomyces Rouxii. U.S. Patent US20030219456A1, 21 May 2002. [Google Scholar]
- Cao, X.; Hou, L.; Lu, M.; Wang, C.; Zeng, B. Genome Shuffling of Zygosaccharomyces Rouxii to Accelerate and Enhance the Flavour Formation of Soy Sauce. J. Sci. Food Agric. 2010, 90, 281–285. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of New Strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to Produce Low-Alcohol Beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. MINIREVIEW Origin and Production of Acetoin during Wine Yeast Fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar] [PubMed]
- Romano, P.; Suzzi, G.; Zironi, R.; Comi, G. Biometric Study of Acetoin Production in Hanseniaspora guilliermondii and Kloeckera apiculata. Appl. Environ. Microbiol. 1993, 59, 1838–1841. [Google Scholar] [PubMed]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The Application of Non-Saccharomyces Yeast in Fermentations with Limited Aeration as a Strategy for the Production of Wine with Reduced Alcohol Content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage Yeasts in the Wine Industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Potential Use for Zygosaccharomyces Species in Winemaking. J. Wine Res. 1993, 4, 87–94. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a Future for Non-Saccharomyces Yeasts: Selection of Putative Spoilage Wine Strains to Be Used in Association with Saccharomyces cerevisiae for Grape Juice Fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Nor Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).