Recent Developments on the Origin and Nature of Reductive Sulfurous Off-Odours in Wine
Abstract
1. Introduction
2. Origin of Reductive Off-Odors
- (1)
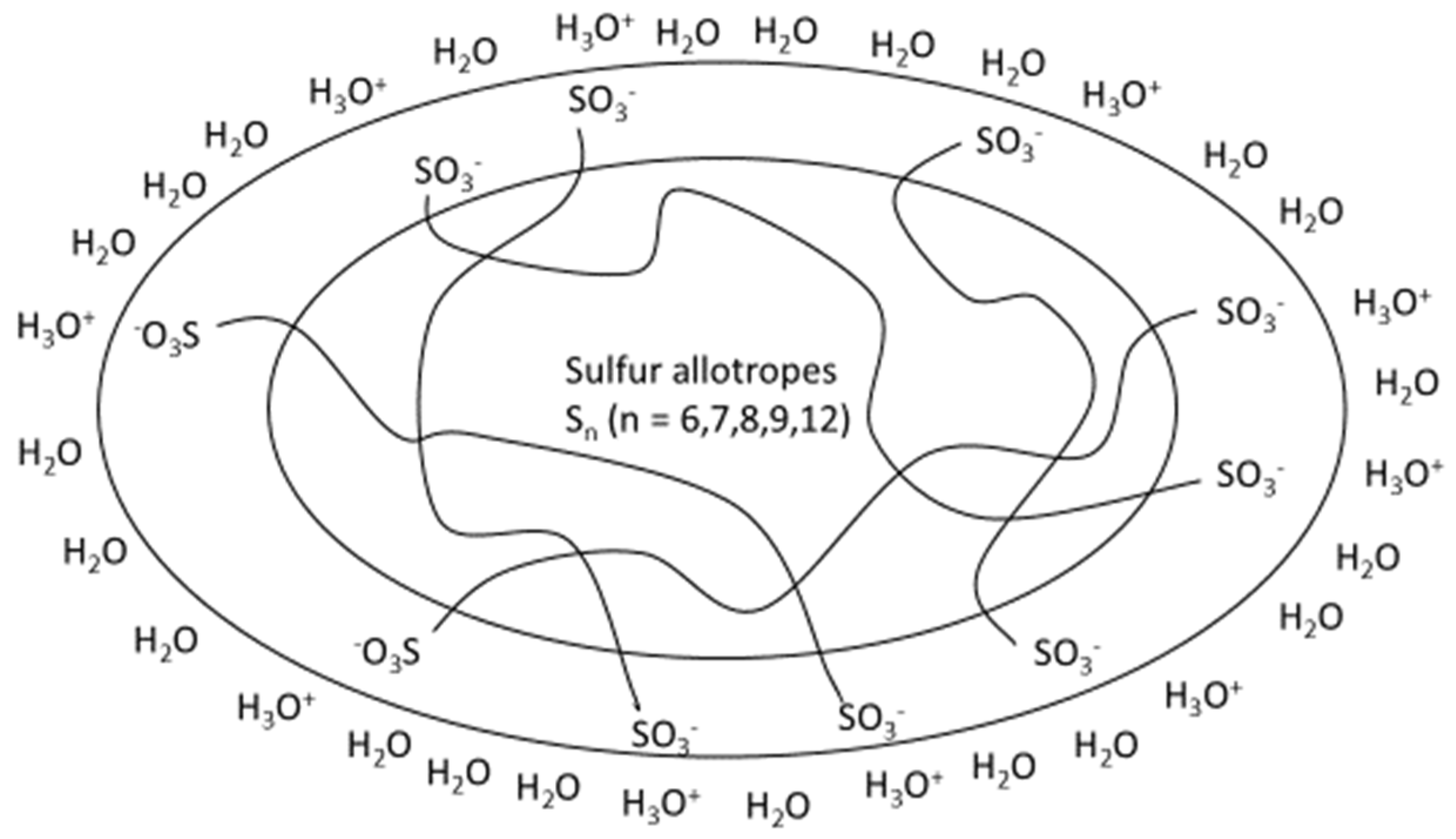
- Elemental sulfur [4,5] has been used as a pesticide since antiquity and is still used for the purpose of fighting fungal diseases in grapes. Elemental sulfur in all its allotropes and modifications is not by far inert and participates considerably in the formation of precursors like polysulfanes and polythionates.
- (2)
- (3)
- (4)
- From polythionates formed by the reaction of yeast-borne hydrogen sulfide with added sulfur dioxide (‘Wackenrodersche Flüssigkeit”, from here on called “Wackenroder solution” (WS)).
- (5)
- (6)
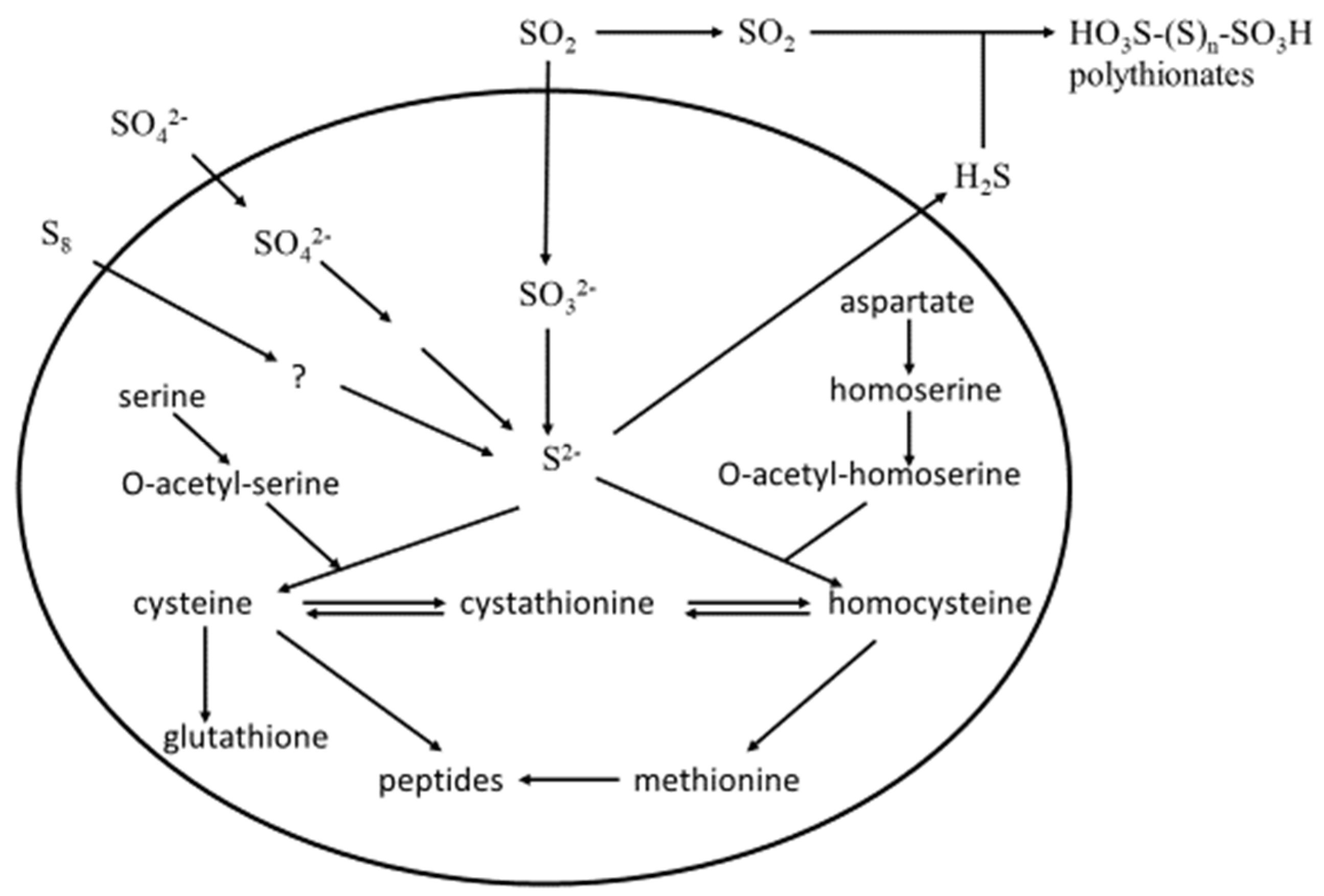
- Some evidence also exists for S-amino acids serving as precursors, as described below:
3. The Role of Elemental Sulfur
4. Polythionates and Polydithionates
5. History
5.1. Polythionates in Wine as Precursors
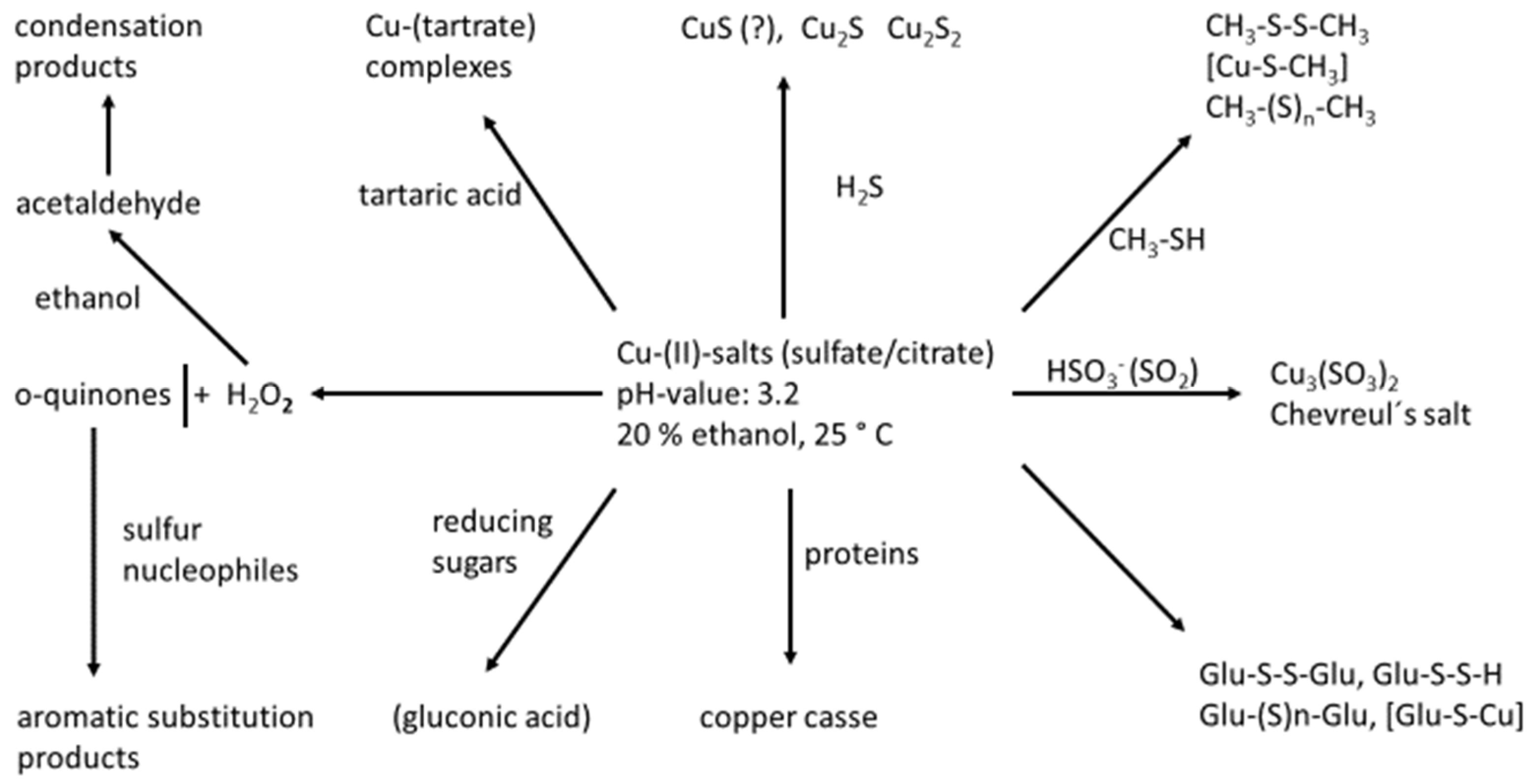
5.2. The Role of Copper
- (1)
- (2)
- Reaction with “off-odor” VSCs MeSH and EtSH leads to the formation of disulfides, mixed disulfides [51], including those with H2S, which leads to hydropersulfides (R-S-S-H), the formation of dialkylpolysulfanes and alkylpolysulfanes, as well as mixed forms with organyl residues (R1-(S) n-R2); the formation of copper-(I)-mercaptides and soluble complexed forms of these [53].
- (3)
- If wines are treated with copper (II) salts alone, almost the total disappearance of thiols like 3-MH and the concomitant formation of disulfides were observed [51].
- (4)
- (5)
- (6)
- Glucose (reducing aldoses): Copper (II) sulfate is used for the quantitative determination of reducible sugars in wine analytics according to Rebelein. Although this method works in strong alkaline solution (the aldehyde group is oxidized to a carboxyl group; glucose is converted to gluconic acid), there still might be some evidence that copper (II) is converted to Cu (I) at normal wine pH of around 3.0–3.5.
- (7)
- Copper addition also induces the formation of hazes in wine by reaction with proteins.
- (8)
- Copper, especially in combination with iron ions, catalyzes the oxidation of wine o-diphenols and other phenolics like caftaric acid, cyanidin, catechin, epicatechin, gallic acid and its derivatives, to form highly reactive o-quinones. The impact on sulfur-containing aroma active compounds lies in their high nucleophilicity, giving rise to irreversible 1,4-Michael additions [61].
- (9)
- o-Quinones are also made responsible for the development of volatile sulfur compounds by the Strecker reaction with cysteine and methionine [9].
- (10)
5.3. Some Remarks on the Oxidation States of Copper/Sulfur Compounds
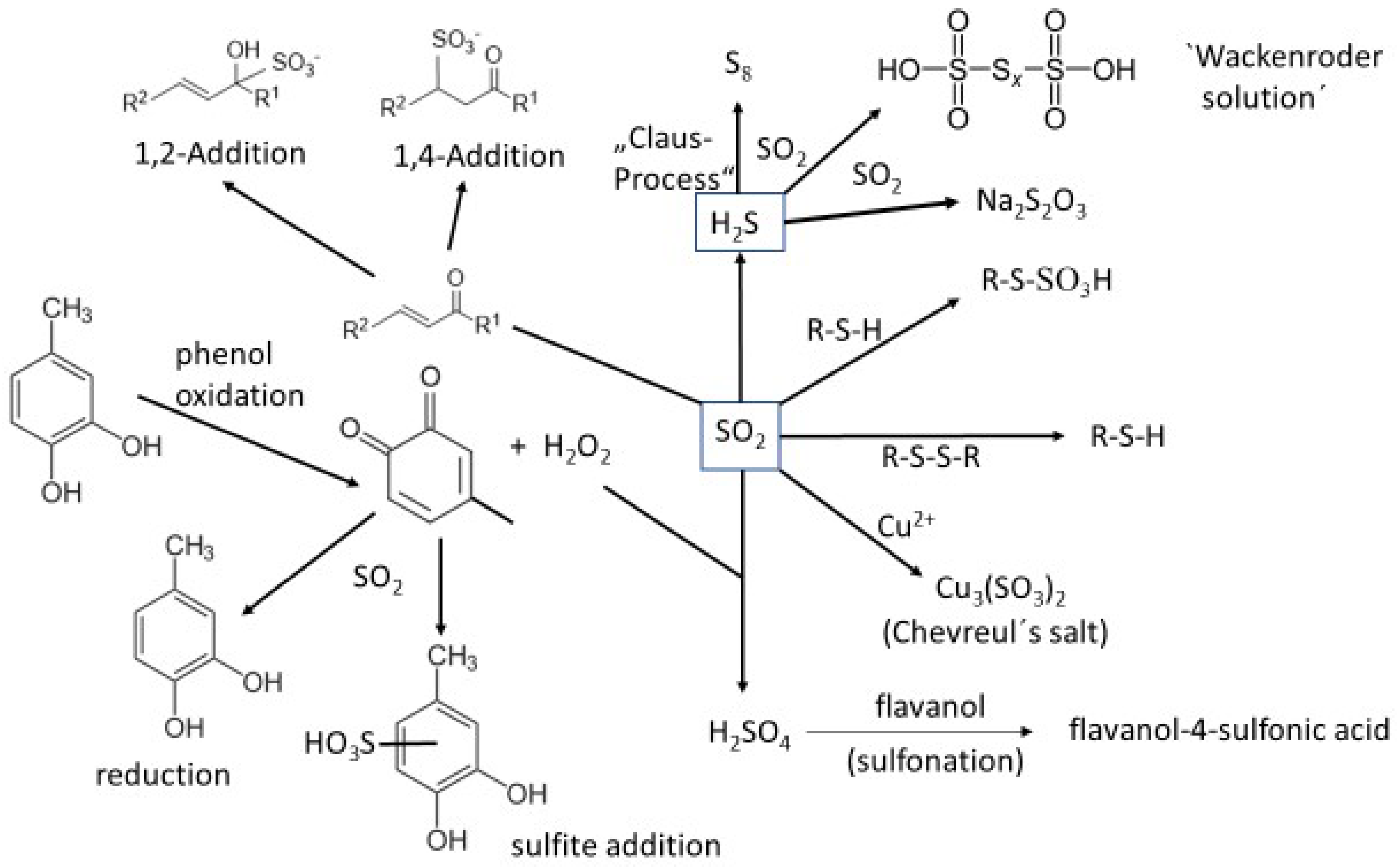
5.4. The Role of Sulfur Dioxide
- (1)
- The oxidation of wine o-diphenols leads to o-quinones and hydrogen peroxide, which oxidize sulfite to sulfuric acid. Highly reactive aromatic compounds like phenols, indoles, etc., may undergo electrophilic aromatic substitution (sulfonation) [66].
- (2)
- SO2 (as HSO3–) itself is a strong nucleophile, which reacts with o-quinones in a Michael addition-type reaction under the irreversible formation of aromatic sulfonic acids [61].
- (3)
- SO2 reacts with various carbonyl compounds forming bisulfite-addition products (“bound SO2”). α,β-unsaturated ketones (i.e., the wine-relevant aroma compound β-damascenone) form 1,4-addition products [67].
- (4)
- At elevated temperatures, H2S and SO2 comproportionate to elemental sulfur. This reaction is used in the technical “Claus-process” for desulfuration of crude gas.
- (5)
- At ambient temperature, H2S and SO2 react to the so-called “Wackenroder solution”, a mixture of polythionates, H2S, SO2 and elemental sulfur (see Section 5).
- (6)
- The formation of (at wine pH) instable thiosulfuric acid takes also part in the formation of “Wackenroder solution”.
- (7)
- Thiols are powerful reducing agents that may be oxidized by sulfur (IV) compounds to mixtures of polysulfanes. The first step of this reaction is the formation of S-sulfonated thiols.
- (8)
- Recently, various sulfonated compounds were observed in wine for the first time (e.g., S-sulfonated cysteine and glutathione) [66].
- (9)
- The addition of SO2 to anthocyanins results in colorless 4-sulfonic acids.
- (10)
- The addition of copper (II) to sulfite-containing solutions leads to the formation of a mixed valence copper sulfite (“Chevreul’s salt”) [68].
- (11)
- SO2 may cleave disulfide bonds under the formation of thiols (“sulfitolysis”) [69].
- (12)
- The so-called “sulfite degradation” of sulfur chains in elemental sulfur, polysulfanes and polythionates is part of the very complicated sulfur chemistry in aqueous systems (including wine).
6. Discussion of Polydithionates: The Answer to Some Unsolved Questions?
- (1)
- Wine contains H2S and MeSH in non-volatile and odorless forms. An analytical method has been developed to detect H2S and MeSH present in bonded forms [71]. We claim polythionates to be part of the non-volatile and odorless forms.
- (2)
- Ferreira and Franco-Luesma [70] pointed out that each wine has a specific ability to bind reversibly H2S and mercaptans. “If free H2S and MeSH are added to different wines, some of these molecules remain volatile, and hence can be easily smelled, while there are some others in which the same molecules are so strongly bonded that they cannot be measured (and hence smelled) as free forms” [70] (bonded forms include polythionates; → see Daltons’ experiment [38]). The reversible binding of H2S in polythionates lies in the labile character of these compounds. They easily decay into H2S, SO2 and elemental sulfur.
- (3)
- Oxygen only reacts in converting H2S and mercaptans into their free forms. Bound or complexed forms (including polythionates) require longer periods of treatment to release the free forms [37].
- (4)
- Even under strict oxygen-free conditions, H2S is able to slowly, but irreversibly (?) react to something (we claim polythionates) in some wine.
- (5)
- The increments of free H2S observed when wine is stored in anoxia are mostly the release of complexed forms: This is valid for polythionates and other components of the “Wackenroder solution”, if considered as part of the “complexed forms” [29].
- (6)
- Analytical methods to detect H2S, MeSH and other VSCs in the headspace do not work, if they suffer interactions in wines occasionally so strong that in some wines, they are not released to the wine headspace. In contrast, a previously-developed method in which the wine is strongly diluted with brine revealed that such interactions are reversible, demonstrating that wines contain a large pool of reversible complexed (as polythionates) non-volatile forms of H2S and mercaptans [72].
- (7)
- TCEP (Tris (2-carboxyethyl) phosphine) was evaluated for its ability to release complexed H2S from wine. Surprisingly, even though TCEP was much less effective than brine at releasing H2S from copper sulfide complexes in the recovery experiments, higher concentrations of H2S released by TCEP were observed in six of the seven wines (up to 25.3 µg/L). These results suggest the presence of additional TCEP-releasable sources (polythionates) of H2S (or thiol interferences) in wine [48]. Since TCEP acts specifically to break -S-S-bonds, polythionates will also be attacked with the release of H2S.
- (8)
- Elemental sulfur pesticide residues on grapes can not only produce H2S during fermentation, but are also able to form precursors capable of generating additional H2S after bottle storage. Among these precursors, glutathione tri- and polysulfanes (Glu-S-Sn-S-Glu) have been detected, but also for the first time, tetrathionate (S4O62−). These precursors are polar, wine-soluble intermediates that can be converted into H2S following TCEP (triscarboxyethyl phosphine) addition [29]. The identity of these intermediates was unclear, but could potentially involve polysulfide (polythionates) adducts. The authors claim sulfitolysis of polysulfanes as the reason; the classical formation by the “Wackenroder reaction” from the wine components H2S and SO2 is not mentioned. These findings come closest to our “polythionate theory” made in (6).
- (9)
- Wine producers know that if a wine has a tendency to develop or had reductive off-odors, it is possible that the off-flavor is reoccurring during the storage of the bottle, in particular if closures with low permeability to oxygen are used. This indicates that the oenological treatments affect the odor-active compounds, but not their diverse precursors [56,70]. Polythionates might belong to this type of precursor.
- (10)
- Up to this date, these precursors have not been identified. One reason why the progress in research has been so difficult lies in the complicated chemistry of sulfur. This element can be present in different redox states, has the ability to concatenate (polysulfanes and polythionic acids), making bonds with itself, and can form a large number of combinations [16]. These statements support our polythionates theory.
- (11)
- Copper, acting on unknown precursors, was associated with large increases of H2S in Shiraz wines [73]. The unknown precursors may be polythionates born in the fermentation step. There is no remarkable increase in model wine when treated with copper, due to the lack of these precursors.
- (12)
- This suggests that a pool of yet to be identified precursor compounds (polythionates) is also involved in modulating final concentrations in wine through copper-catalyzed reactions [74] (since copper salts are active in cleaving -S-S-bonds, these bonds will be cleaved in polythionates, as well).
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goode, J.; Harrop, S. Wine faults and their prevalence: Data from the world’s largest blind tasting. In Proceedings of the Les XXes Entretiens Scientifiques Lallemand, Horsens, Denmark, 15 May 2008; pp. 7–9. [Google Scholar]
- Kreitman, G.Y.; Elias, R.J.; Jeffery, D.W.; Sacks, G.L. Loss and formation of malodourous volatile sulfhydryl compounds during wine storage. Crit. Rev. Food Sci Nutr. 2018, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Regulation of hydrogen sulfide liberation in wine producing. Saccharomyces cerevisiae by assimilable nitrogen. Appl. Environ. Microbiol. 1995, 61, 461–467. [Google Scholar] [PubMed]
- Rauhut, D.; Kürbel, H. The production from H2S from elemental sulfur residues during fermentation and its influence on the formation of sulfur metabolites causing off-flavours in wine. Vitic. Enol. Sci. 1994, 49, 27–36. [Google Scholar]
- Rauhut, D. Usage and formation of sulfur compounds. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 255–291. [Google Scholar]
- Rauhut, D. Yeast-Production of sulfur compounds. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 183–223. [Google Scholar]
- Huang, C.-W.; Walker, M.E.; Fredrizzi, B.; Gardner, J.V. Hydrogen sulfide and its roles in Saccharomyces cerevisiae in wine making context. FEMS Yeast Res. 2017, 17, fox058. [Google Scholar] [CrossRef] [PubMed]
- Rauhut, D.; Kürbel, H.; Dittrich, H.H.; Grossmann, M. Properties and differences of commercial yeast strains with respect to their formation of sulfur compounds. Vitic. Enol. Sci. 1996, 51, 187–192. [Google Scholar]
- Pripis-Nicolau, L.; de Revel, G.; Bertrand, A.; Maujean, A. Formation of Flavor Components by the Reaction of Amino Acid and Carbonyl Compounds under Mild Conditions. J. Agric. Food Chem. 2000, 48, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. Thiaminpyrophosphat-ein natürlich vorkommendes Iminiumsalz. Z. Naturforsch. B 2014, 69, 489–500. [Google Scholar] [CrossRef]
- Lloyd, D. Hydrogen sulfide: Clandestine microbial messenger? TRENDS Microbiol. 2006, 14, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Murray, D.B. The temporal architecture of eukaryotic growth. FEBS Lett. 2006, 580, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Reschke, S.; Tran, T.; Bekker, M.; Wilkes, E.; Johnson, D. Using copper more effectively in winemaking. Wine Vitic. J. 2015, 9, 35–39. [Google Scholar]
- Görtges, S. Böckserbeseitigung mit Kupfercitrat. Der Deutsche Weinba 2009, 5, 24–25. [Google Scholar]
- Steidl, R. Der Einsatz von Silberchlorid zur Böckserbekämpfung. Mitt. Klosterneubg. 2010, 3, 92–95. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. (Eds.) Appearance of Reduced Aromas during Bottle Storage. In Understanding Wine Chemistry; John Wiley & Sons: Chichester, UK, 2016; pp. 397–399. [Google Scholar]
- Clark, A.C.; Wilkes, E.N.; Scollary, G.R. Chemistry of Copper in white wine: A. review. Aust. J. Grape Wine Res. 2015, 21, 339–350. [Google Scholar] [CrossRef]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Evolution of Volatile Sulfur Compounds during Wine Fermentation. J. Agric. Food Chem. 2015, 63, 8017–8024. [Google Scholar] [CrossRef] [PubMed]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Hydrogen sulfide production during yeast fermentation causes the accumulation of ethanethiol, S-ethyl thioacetate and diethyl disulfide. Food Chem. 2016, 209, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. Iminiumsalz-Strukturen bei der durch Pyridoxalphosphat (Vitamin B6) katalysierten Bildung von Aromastoffen und Fehlaromen im Wein. Z. Naturforsch. B 2018, 73, 521–533. [Google Scholar] [CrossRef]
- Clark, A.C.; Grant-Peerce, P.; Cleghorn, N.; Scollary, G.R. Copper (II) addition to white wines containing hydrogen sulfide: Residual copper concentration and activity. Aust. J. Grape Wine Res. 2015, 21, 30–39. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Day, M.P.; Holt, H.; Wilkes, E.; Smith, P.A. Effect of oxygen exposure during fermentation on volatile sulfur compounds in Shiraz wine and a comparison of strategies for remediation of reductive character. Aust. J. Grape Wine Res. 2016, 22, 24–35. [Google Scholar] [CrossRef]
- Starkenmann, C.; Chappuis, C.J.-F.; Niclass, Y.; Deneulin, P. Identification of Hydrogen Disulfanes and Hydrogen Trisulfanes in H2S Bottle, in Flint, and in Dry Mineral White Wine. J. Agric. Food Chem. 2016, 64, 9033–9044. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, N.; Kuroda, H.; Yamada, O.; Goto-Yamamoto, N. Factors Affecting Dimethyl Trisulfide Formation in Wine. Food Sci. Technol. Res. 2017, 23, 241–248. [Google Scholar] [CrossRef]
- Gijs, L.; Perpète, P.; Timmermans, A.; Collin, S. 3-Methylthiopropionaldehyde as Precursor of Dimethyl Trisulfide in Aged Beers. J. Agric. Food Chem. 2000, 48, 6196–6199. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y. Characteristic Aroma Compounds of Chinese Dry Rice Wine by Gas-Chromatography-Olfactometry and Gas-Chromatography-Mass Spectroscopy. In Flavor Chemistry of Wine and Other Alcoholic Beverages; ACS Symposium Series 2012; American Chemical Society: Washington, DC, USA, 2012; Chapter 16; pp. 277–301. [Google Scholar]
- Isogai, I.; Utsonomiya, H.; Kanda, R.; Iwata, H. Changes in Aroma Compounds of Sake during Aging. J. Agric. Food Chem. 2005, 53, 4118–4123. [Google Scholar] [CrossRef] [PubMed]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulphur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Jastrembski, J.A.; Allison, R.B.; Friedberg, E.; Sacks, G.L. Role of Elemental Sulfur in Forming Latent Precursors of H2S in Wine. J. Agric. Food Chem. 2017, 65, 10542–10549. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, F.C. On the Reactivity of Nanoparticulate Elemental Sulfur: Experimentation and Field Observations. Ph.D. Thesis, Indiana University, Bloomington, IN, USA, December 2017. [Google Scholar]
- Steudel, R.; Eckert, B. Solid sulfur allotropes. In Elemental Sulfur and Sulfur-Rich Compounds; Topics in Current Chemistry Book Series; Springer: Berlin/Heidelberg, Germany, 2003; Volume 230, 79p. [Google Scholar]
- Araujo, L.D.; Vannevel, S.; Buica, A.; Callerot, S.; Fredrizzi, B.; Kilmartin, P.A.; Du Toit, W.J. Indications of the prominent role of elemental sulfur in the formation of the varietal thiol 3-mercapto- hexanol in Sauvignon blanc. Food Res. Int. 2017, 98, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Krahn, R. Mechanismen der Schwefelwasserstofferzeugung durch Saccharomyces Cerevisiae für die Biotechnologische Immobilisierung von Schwermetallen; Inaugural-Dissertation: Düsseldorf, Germany, 2000. [Google Scholar]
- Kleinjan, W.E.; Keizer, A.; Janssen, A.J.H. Biologically Produced Sulfur. Top. Curr. Chem. 2003, 230, 167–188. [Google Scholar]
- Steudel, R. Aqueous Sulfur Sols. Top. Curr. Chem. 2003, 230, 153–166. [Google Scholar]
- Nedjma, M.; Hoffmann, N. Hydrogen sulfide reactivity with thiols in the presence of copper (II) in hydroalcoholic solutions. J. Agric. Food Chem. 1996, 44, 3935–3938. [Google Scholar] [CrossRef]
- Vela, E.; Purificíon, H.-O.; Franco-Luesma, E.; Ferreira, V. Micro-oxygenation does not eliminate hydrogen sulfide and mercaptans from wine; it simply shifts redox and complex-related equilibria to reversible oxidized species and complexed forms. Food Chem. 2018, 243, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.; Wolff, F. Ein neues System des Chemischen Theils der Naturwissenschaft; Verlag Eduard Hitzig: Berlin, Germany, 1813; Volume 2, pp. 190–196. [Google Scholar]
- Wackenroder, H. Über eine neue Säure des Schwefels. Arch. Pharm. 1846, 97, 272–288. [Google Scholar] [CrossRef]
- Tomozo, K. Analytical Chemistry of Polythionates and Thiosulfate. A. Review. Anal. Sci. 1990, 6, 3–14. [Google Scholar] [CrossRef]
- Blasius, E.; Burmeister, W. Untersuchung der Wackenroderschen Reaktion mit Hilfe der radiopapierchromatographischen Methode. Fresenius’ Z. Anal. Chem. 1959, 168, 1–15. [Google Scholar] [CrossRef]
- Stamm, H.; Seipold, O.; Goehring, M. Zur Kenntnis der Polythionsäuren und ihrer Bildung. 4. Mitteilung. Die Reaktionen zwischen Polythionsäuren und schwefliger Säure bzw. Thioschwefelsäure. Z. Allg. Anorg. Chem. 1941, 247, 93–98. [Google Scholar] [CrossRef]
- Drozdova, Y.; Steudel, R. The Reaction of H2S with SO2: Molecular Structures, Energies, and Vibrational Data of Seven Isomeric Forms of H2SO3. Chem. A Eur. J. 1995, 1, 193–198. [Google Scholar] [CrossRef]
- Schmidt, M.; Heinrich, H. Beitrag zur Lösung des Problems der Wackenroderschen Flüssigkeit. Über Säuren des Schwefels, XII. Angew. Chem. 1958, 70, 572–573. [Google Scholar]
- Thomas, D.; Surdin-Kerjan, Y. Metabolism of Sulfur Amino Acids in Saccharomyces cerevisiae. Mol. Biol. Rev. 1997, 61, 503–532. [Google Scholar]
- Ugliano, M.; Henschke, P.A. Yeasts and wine flavour. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 313–392. [Google Scholar]
- Swiegers, J.H.; Pretorius, I.S. Modulation of volatile sulfur compounds by wine yeast. Appl. Microbiol. Biol. 2007, 74, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jastrzembski, J.A.; Sacks, G.L. Copper-Complexed Hydrogen Sulfide in Wine. Measurement by Gas Detection Tubes and Comparison of Release Approaches. Am. J. Enol. Vitic. 2017, 68, 191–199. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.-B.; Aagard, O.; Waters, E.J. Evolution of 3-mercaptohexanol, hydrogen sulfide, and methyl mercaptan during bottle storage of Sauvignon blanc wines. Effect of glutathione, copper, oxygen exposure, and closure-derived oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Vela, E.; Hernández-Orte, P.; Franco-Luesma, E.; Ferreira, V. The effects of copper fining on wine content in sulfur off-odors and on their evolution during accelerated anoxic storage. Food Chem. 2017, 231, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Roland, A.; Delpech, S.; Dagan, L.; Ducasse, M.-A.; Cavelier, F.; Schneider, R. Innovative Analysis of 3-mercaptohexan-1-ol, 3-mercaptohexylacetate and their corresponding disulfides in wine by stable isotope dilution assay and nano-liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1468, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Reaction Mechanisms of Metals with Hydrogen Sulfide and Thiols in Model Wine. Part 1: Copper-Catalyzed Oxidation. J. Agric. Food Chem. 2016, 64, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Copper (II)-Mediated Hydrogen Sulfide and Thiol Oxidation to Disulfides and Organic Polysulfanes and Their Reductive Cleavage in in Wine. Mechanistic Elucidation and Potential Applications. J. Agric. Food Chem. 2017, 65, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R. Künftig ohne Kupfer; Heft 11/18; Der Deutsche Weinbau: Neustadt an der Weinstraße, Germany, 2018; pp. 16–18. [Google Scholar]
- Altmayer, B. Genehmigung auf der Kippe; Heft 5/18; Das Deutsche Weinmagazin: Neustadt an der Weinstraße, Germany, 2018; pp. 32–35. [Google Scholar]
- Müller, N.; Rauhut, D. Neuere Erkenntnisse zur Behandlung von reduktiven Noten und Böcksern im Wein. In Deutsches Weinbau Jahrbuch; Eugen Ulmer: Stuttgart, Germany, 2018; pp. 83–94. [Google Scholar]
- Calban, T.; Sevim, F.; Lacin, O. Investigation of Precipitation Conditions of Chevreul’s Salt. Int. J. Chem. Mol. Eng. 2016, 10, 1021–1024. [Google Scholar]
- Pietsch, E.H.E. Gmelin’s Handbook; Verlag Chemie: Weinheim, Germany, 1958; Volume 8, p. 484. [Google Scholar]
- OIV. International Code of Oenological Practices; International Organisation of Vine and Wine: Pairs, France, 2015. [Google Scholar]
- Ribéreau-Gayon, G.Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine Stabization and Treatment; John Wiley & Sons: Chichester, UK, 2006; Volume 2. [Google Scholar]
- Nikolantonaki, M.; Chichuc, J.; Teissedre, P.-L.; Darriet, P. Reactivity of volatile thiols with polyphenols in a wine-model medium: Impact of oxygen, iron, and sulfur dioxide. Anal. Chim. Acta 2010, 660, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rousseva, M.; Kontoudakis, N.; Schmidke, L.M.; Scollary, G.R.; Clark, A.C. Impact of wine production on the fractionation of copper and iron in Chardonnay wine: Implications for oxygen consumption. Food Chem. 2016, 203, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK; Waltham, MA, USA, 1997; ISBN 0-08-037941-9. [Google Scholar]
- Kierkegaard, P.; Nyberg, B. The crystal structure of Cu2SO3·CuSO3·2H2O. Acta Chem. Scand. 1965, 19, 2189–2199. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Smith, M.E.; Smith, P.A.; Wilkes, E.N. Formation of Hydrogen Sulfide in Wine: Interactions between Copper and Sulfur Dioxide. Molecules 2016, 21, 1214. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 25, 858. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, N.; Piano, F.; Davidson, S.J.; Larcher, R.; Fredrizzi, B.; Barker, D. Synthesis of alkyl sulfonic acid aldehydes and alcohols, putative precursors to important wine aroma thiols. Tetrahedron Lett. 2015, 56, 1728–1731. [Google Scholar] [CrossRef]
- Chevreul, M.E. Propriétés du sulfite de cuivre. Anal. Chim. 1812, 83, 187–190. [Google Scholar]
- Bobet, R.A.; Noble, A.C.; Boulton, R.B. Kinetics of the ethanethiol and diethyl disulfide interconversion in wine like solutions. J. Agric. Food Chem. 1990, 38, 449–452. [Google Scholar] [CrossRef]
- Ferreira, V.; Franco-Luesma, E. Understanding and Managing Reduction Problems. Int. J. Enol. Vitic. 2016, 2, 1–13. [Google Scholar]
- Franco-Luesma, E.; Ferreira, V. Quantitative analysis of free and bonded forms of volatile sulfur compounds in wine. Basic methodologies and evidences showing the existence of reversible cation-complexed forms. J. Chromatogr. A 2014, 1359, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luesma, E.; Ferreira, V. Reductive off-odours in wine: Formation and release of H2S and methanethiol during the accelerated anoxic storage of wines. Food Chem. 2016, 199, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bekker, M.Z.; Mierczynska-Vasilev, A.; Smith, P.; Wilkes, E.N. The effects of pH and copper on the formation of volatile sulfur compounds in Chardonnay and Shiraz wines post-bottling. Food Chem. 2016, 207. [Google Scholar] [CrossRef] [PubMed]
- Bekker, M.Z.; Wilkes, E.N.; Smith, P.A. Evaluation of putative precursors of key ‘reductive’ compounds in wines post-bottling. Food Chem. 2018, 245, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Ganguli, D.; Kaur, J.; Kasturia, N.; Thakur, A.; Kaur, H.; Kumar, A.; Yadav, A. Glutathione production in yeast. In Yeast Biotechnology: Diversity and Applications; Satyanarayana, T., Kunze, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; Chapter 13; pp. 259–278. [Google Scholar]
- Penninckx, M.A. Short review on the role of glutathione in the response of yeasts to nutritonal, environmental, and oxidative stresses. Enzyme Microb. Technol. 2000, 26, 737–742. [Google Scholar] [CrossRef]
| Type of Compound | Formula | (n *) | (n +) | Relevance in Wine |
|---|---|---|---|---|
| Sulfur homocycles | Sn | 6–20 | −80 | Added as fungicide |
| (Poly-)sulfane | H-Sn-H | 1–8 | 35 | Component (H2S3) [23] Me2S3 [24,25,26,27,28] |
| Diorganopoly sulfane | R-Sn-R | 1–7 | 35 | Component disulfides (n = 2) [2] |
| Organopoly sulfides | R-Sn-H | 1 | 2 | Most likely i.e., persulfides (n = 2) |
| Polythionates | -O3S-Sn-SO3- | 1–4 | 22 | Most likely: SO2 + H2S [29] |
| “Bunte salts” | R-Sn-SO3- | 1 | 2–13 | Most likely |
| Polythionates | -S-Sn-SO3- | 0 | 1 | Salts, i.e., sodium thiosulfate (n = 0) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, N.; Rauhut, D. Recent Developments on the Origin and Nature of Reductive Sulfurous Off-Odours in Wine. Fermentation 2018, 4, 62. https://doi.org/10.3390/fermentation4030062
Müller N, Rauhut D. Recent Developments on the Origin and Nature of Reductive Sulfurous Off-Odours in Wine. Fermentation. 2018; 4(3):62. https://doi.org/10.3390/fermentation4030062
Chicago/Turabian StyleMüller, Nikolaus, and Doris Rauhut. 2018. "Recent Developments on the Origin and Nature of Reductive Sulfurous Off-Odours in Wine" Fermentation 4, no. 3: 62. https://doi.org/10.3390/fermentation4030062
APA StyleMüller, N., & Rauhut, D. (2018). Recent Developments on the Origin and Nature of Reductive Sulfurous Off-Odours in Wine. Fermentation, 4(3), 62. https://doi.org/10.3390/fermentation4030062




