Abstract
Reductive sulfurous off-odors are still one of the main reasons for rejecting wines by consumers. In 2008 at the International Wine Challenge in London, approximately 6% of the more than 10,000 wines presented were described as faulty. Twenty-eight percent were described as faulty because they presented “reduced characters” similar to those presented by “cork taint” and in nearly the same portion. Reductive off-odors are caused by low volatile sulfurous compounds. Their origin may be traced back to the metabolism of the microorganisms (yeasts and lactic acid bacteria) involved in the fermentation steps during wine making, often followed by chemical conversions. The main source of volatile sulfur compounds (VSCs) are precursors from the sulfate assimilation pathway (SAP, sometimes named as the “sulfate reduction pathway” SRP), used by yeast to assimilate sulfur from the environment and incorporate it into the essential sulfur-containing amino acids methionine and cysteine. Reductive off-odors became of increasing interest within the last few years, and the method to remove them by treatment with copper (II) salts (sulfate or citrate) is more and more questioned: The effectiveness is doubted, and after prolonged bottle storage, they reappear quite often. Numerous reports within the last few years and an ongoing flood of publications dealing with this matter reflect the importance of this problem. In a recent detailed review, almost all relevant aspects were discussed on a scientific data basis, and a “decision tree” was formulated to support winemakers handling this problem. Since we are dealing with a very complicated matter with a multitude of black spots still remaining, these advices can only be realized using specific equipment and special chemicals, not necessarily found in small wineries. The main problem in dealing with sulfurous compounds arises from the high variability of their reactivities. Sulfur is a metalloid with a large valence span across eight electron transformations from S (−II) up to S (+VI). This allows it to participate in an array of oxidation, reduction and disproportionation reactions, both abiotic and linked to microbial metabolism. In addition, sulfur is the element with the most allotropes and a high tendency to form chains and rings, with different stabilities of defined species and a high interconvertibility among each other. We suppose, there is simply a lack of knowledge of what is transferred during filling into bottles after fermentation and fining procedures. The treatment with copper (II) salts to remove sulfurous off-odors before filling rather increases instead of solving the problem. This paper picks up the abundant knowledge from recent literature and tries to add some aspects and observations, based on the assumption that the formation of polythionates, hitherto not taken into consideration, may explain some of the mystery of the re-appearance of reductive off-odors.
1. Introduction
Reductive off-odor problems are mostly caused by volatile sulfur compounds (VSCs) and equal to cork taint are responsible for an important proportion (30%) of faulty wines [1]. H2S is the most frequently found of these compounds, followed by methanethiol (MeSH) and ethanethiol (EtSH) [2]. Hydrogen sulfide can be generated by Saccharomyces cerevisiae in the course of sulfur assimilation during fermentation. Inorganic sources used in this “sulfate assimilation pathway” (SAP) are mainly sulfate, naturally occurring in the grape must, sulfite, usually added as an antioxidant and microbial agent [3] to control microbial spoilage of must and wine, and elemental sulfur, which is used as a fungicide in the vineyard [4,5,6]. To date, yeast genetic mechanisms of H2S liberation during wine fermentation are well understood, and yeast strains producing low levels of H2S have been developed [7,8]. As outlined later on in the case of low nitrogen status in must, there is a lack of the activated precursors of the sulfur-containing amino acids cysteine and methionine (O-acetylserine and -homoserine). The surplus of sulfide then is excreted as H2S, which can react biochemically or chemically with other VSCs.
Other sources of VSCs are the enzymatic or chemical degradation of cysteine and methionine.
Chemical degradation takes place via a Strecker reaction [9] and partly via Ehrlich degradation [10]. Strecker degradation leads to products of reactions between α-dicarbonyl compounds like diacetyl or o-quinones, easily formed from wine polyphenols by oxidation, and amino acids present in wine. In the case of methionine, methional is formed, which itself is considered as an aroma component of wine, but also easily cleaves off MeSH. During Ehrlich degradation, amino acids are transformed by a transamination reaction, followed by decarboxylation to methional, as well.
On the other hand, H2S does not only serve as a reaction partner in the formation of sulfur-containing amino acids.
More and more attention is given to other functions of H2S. It is now not merely recognized as intermediate in the biosynthesis of the sulfur-containing amino acids, but it has important functions as a potent biological effector, i.e., in detoxification, population, signaling and extending the life-span of microorganisms and mammalian cells [7,11,12].
Winemakers commonly add copper (II) salts to wine before bottling to remove these unpleasant reductive aromas. Those allowed are copper sulfate [13] or copper citrate [14]. Due to increasing problems due to the reappearance of sulfurous (reductive) off-flavors during storage, OIV (Organisation Internationale de la Vigne et du Vin) recently allowed the use of silver chloride as an alternative [15], although little is known about the side reactions of this treatment.
Indeed, the reappearance of reductive off-odors has been one of the main topics in recent wine research. Numerous literature reports on the increases of free H2S and MeSH during wine storage have been published. A survey is given in [2].
In many of these articles, the addition of copper (II) salt to remove reduced aromas is made responsible for this phenomenon, and some striking theories have been developed. Nevertheless the factors for the appearance of reductive off-flavors in the bottle still remain arguably the most mysterious [16]. The main problem seems to be the lack of knowledge of what is transferred after a long wine processing storage in the bottles. In addition, it has to be kept in mind that the two chemical elements dealt with in the context of the reappearance of reductive off-odors belong simultaneously to those with a very “difficult” chemistry: the transition metal copper and the non-metal element sulfur. Therefore, some of the mystery might be elucidated by going back to the basic chemistry of these elements.
2. Origin of Reductive Off-Odors
In this article, according to the recent review of Kreitman et al. [2], the latent precursors and pathways likely to be responsible for the loss and formation of these sulfhydryls during wine storage based on the existing enology literature, as well as studies from food chemistry, geochemistry, biochemistry and synthetic chemistry have been evaluated and their findings have been expanded to substance classes, hitherto not taken into consideration.
In addition to these recently-proposed precursor classes [2], which have a sufficient concentration and metastability to serve as latent sulfhydryl precursors in wine, other VSCs forming precursor classes are considered:
- (1)
- Elemental sulfur [4,5] has been used as a pesticide since antiquity and is still used for the purpose of fighting fungal diseases in grapes. Elemental sulfur in all its allotropes and modifications is not by far inert and participates considerably in the formation of precursors like polysulfanes and polythionates.
- (2)
- Copper-sulfhydryl complexes formed by the addition of copper (II) salts. These release through an unknown mechanism of VSCs under reductive conditions [2,17].
- (3)
- Disulfides (symmetrical and asymmetrical), polysulfur compounds with chains and rings as listed in Table 1 (polysulfanes and (di)organopolysulfanes), cleaved by thiolysis or sulfitolysis reactions [2].
 Table 1. Presently-known sulfur rings and chains (n represents the chain length or ring size).
Table 1. Presently-known sulfur rings and chains (n represents the chain length or ring size). - (4)
- From polythionates formed by the reaction of yeast-borne hydrogen sulfide with added sulfur dioxide (‘Wackenrodersche Flüssigkeit”, from here on called “Wackenroder solution” (WS)).
- (5)
- By hydrolysis of S-alkylthioacetates, by-products of alcoholic fermentation [2,18,19].
- (6)
- Some evidence also exists for S-amino acids serving as precursors, as described below:
- (a)
- Enzymatically by lyase activity of C-S bond-cleaving enzymes, dependent on the cofactor pyridoxal phosphate [20].
- (b)
- Chemically by Strecker degradation of sulfur-containing amino acids [9].
- (c)
- Pyrophosphate-catalyzed decarboxylation tartly enzymatically starting with Ehrlich degradation (transamination, followed by followed by thiaminpyrophosphate catalyzed decarboxylation to aldehydes). The latter may decompose by chemical cleavage of an unstable intermediate [10,20].
These sulfur compounds of unknown structure are highly instable and interconvert easily. This results in a lack of knowledge, to what extent these VSC-forming compounds are transferred when bottling the wine. The common addition of copper (II) salts to remove reductive (sulfidic) off-odors may even make the matter worse [17,21]. The influence of oxygen, whose ingress is highly dependent on the kind of closure, creates additional complications [22].
Appropriate strategies for managing wines with sulfurous off-aromas have been proposed [2]. The practicability of its application in “normal”-sized wine producing units has to be questioned.
This article is focused on those factors involved in the formation of polythionates and their reactions to elemental sulfur in different modifications, sulfur dioxide and H2S. The effect of addition of copper (II) salts is also discussed. A later section will try to interpret contradicting and non-explainable findings under the assumption of polythionates being responsible for these effects.
3. The Role of Elemental Sulfur
Although sulfur is one of the oldest known elements and in use since ancient times, the elucidation of its chemistry remains an ongoing task [30,31]. Among all elements, sulfur is the one with the most allotropes. The oxidation states of sulfur span eight numbers, from −2 in sulfides to +6 in sulfates. Elemental sulfur, often considered as chemically rather inert, reacts with many wine components. Sulfur is attacked by nucleophiles like thiols (thiolysis) or hydrogen sulfite (SO2, sulfitolysis) due to the electrophilic character of -S-S-bonds [2]. It has been known for a long time that wine compounds with S-S groups could be formed from elemental sulfur residues used as pesticides [4,5,6]. The addition of sulfur prior to fermentation increases the formation of H2S and even of the aroma thiol 3-mercaptohexanol (3-MH) [32]. Recent work has demonstrated that wines fermented in the presence of sulfur continue developing H2S during storage. It was argued that these latent precursors could hardly arise from sulfur residues, due to its poor solubility in aqueous systems (5 µg L−1 in water at 25 °C) and the fact that settling and racking can remove 95%.
Some speculations make the formation of polysulfanes responsible for the abovementioned effect. Indeed, homogenous elemental sulfur suspensions in aqueous organic mixtures on standing contain after some time polythionates, as outlined in the following section. Additionally, it should not be forgotten that elemental sulfur not only occurs in a water-insoluble form, but also as hydrophobic and hydrophilic nanoparticles [30,31,33]. The reactivity of these elemental sulfur nanoparticles among other forms of elemental sulfur is quite different from the crystalline modification. The variation of their surface areas, character and coatings reflect their analytical and physical-chemical properties and play an important role in geo- and bio-chemical processes involved in sulfur recycling.
Elemental sulfur is found as a component in must and wine, either as a residue from fungicidal treatment or produced by yeasts or bacteria (“biosulfur”). The latter has distinctly different properties from crystalline elemental sulfur. Its hydrophilic properties are the most striking of these differences, making it dispersible or even soluble in water [34], whereas crystalline inorganic sulfur is hydrophobic and will not be wetted by an aqueous solution. Nanoparticulate sulfur passes filters with mesh sizes of a few microns and less, usually used in wine processing.
Explanations for the hydrophilicity of extracellularly-stored sulfur globules, i.e., those produced by Acidithiobacillus ferrooxidans, can probably be given by the vesicle structure consisting mainly of polythionates (−O3S-Sn-SO3−). Due to the small particle size and hydrophilic surface, biologically-produced sulfur has advantages over sulfur flower in bioleaching and fertilizer applications. In a model proposed by Steudel et al. [35], the globules consist of a nucleus of sulfur rings (S8) with water around it and long-chain sulfur compounds such as polysulfides or polythionates as amphiphilic compounds at the surface (Figure 1). Therefore, the following section deals with the chemistry of polythionates.
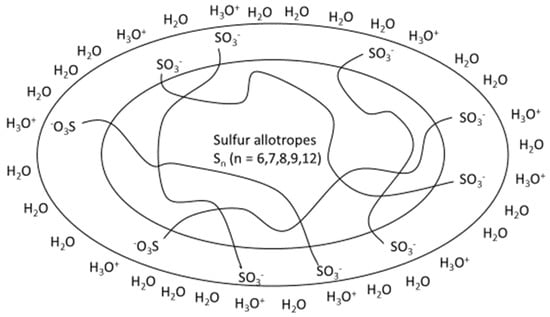
Figure 1.
Schematic model for the composition of the particles in hydrophilic sulfur sols consisting of long-chain polythionates Sn(SO3−)2 and sulfur homocycles Sn (according to [30,35]).
4. Polythionates and Polydithionates
The previous section about the role of elemental sulfur supports the theory that polythionates may play an important role in the chemistry of sulfur and its compounds in wine and must.
This class of sulfur chain-containing compounds may have been overlooked. They are closely related to polysulfanes and organopolysulfanes, which in some recent publications were made responsible for the reappearance of reductive off-odors [2,36,37]. In a broader context, polythionates may be considered as a subclass of polysulfanes due to their common structure with sulfur-sulfur bonds. Elemental sulfur, as well, should be treated in this connection, also because of its role in forming these compounds and their similar chemical reactivity due to the -S-S-bonds. In Table 1, the different types of compounds with sulfur rings and chains and their relation to wine chemistry are summarized.
5. History
The existence of polythionic acids (polythionates) dates back to the studies of John Dalton in 1808, devoted to the behavior of H2S in aqueous solutions of SO2. He found out that when introducing gaseous hydrogen sulfide into a freshly-prepared aqueous solution of SO2, the smell of both gases vanishes and a milky liquid is formed [38]. Later on, this liquid was named “Wackenroder solution” after Heinrich Wilhelm Ferdinand Wackenroder, who conducted a systematic study (1846) [39]. Over the next 60–80 years, numerous studies showed the presence of ions, in particular tetrathionate and pentathionate anion (S4O62− and S5O62−) respectively [40].
The mechanism of the formation of the “Wackenroder solution” still is not fully understood [41,42]. The reaction of hydrogen sulfide with sulfur dioxide in an aqueous solution yields as the primary product sulfoxylic acid (H2S2O2), which reacts with further SO2 to trithionate (H2S3O4). By incorporation of sulfur, polythionates (SxO62−: x = 4, 5 and 6) and thiosulfate are formed [43]. Due to sulfitolysis, an equilibrium between these polythionates is formed. According to Schmidt [44], elemental sulfur reacts with SO2 under sulfitolysis of the sulfur ring, which is finally degraded to thiosulfuric acid. Since free thiosulfuric acid is instable and decomposes to elemental sulfur, SO2 and H2S, the whole procedure will start de novo, but also, polythionates of a higher stability are formed.
5.1. Polythionates in Wine as Precursors
Recently, a polythionate (tetrathionate) was detected in wine [29]. Although this appearance is a first proof for speculations that organopolysulfanes and polysulfides could undergo two sulfitolysis steps to form polythionates, the reason why higher polythionates could not be detected remains unclear. To support this theory, tetrathionate could only be detected in sulfur-treated wine, but not in non-fermented model wine.
The majority of H2S produced by yeast during wine fermentation is from the sulfate assimilation pathway (SAP), where sulfate is progressively reduced to sulfide, the precursor of the sulfur-containing amino acids cysteine and methionine, which are required for yeast growth, as outlined in Figure 2 [45].
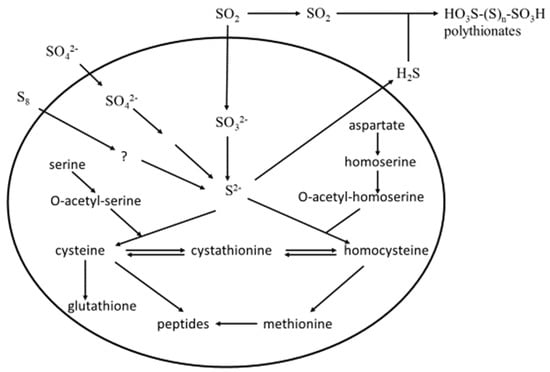
Figure 2.
Sulfate assimilation pathway (SAP) in Saccharomyces cerevisiae. Top right: the formation of polythionates from the yeast metabolites H2S and SO2 ([20], modified).
Grape juice usually contains plenty of sulfate (~160–700 mg L−1), but very low concentrations of cysteine and methionine (<20 mg L−1), and therefore, the SAP is triggered during alcoholic fermentation to support yeast growth [5,46]. The mechanisms by which H2S is released from the SAP are well studied and reviewed [5,47,48,49].
Handbooks for enology normally claim that excess H2S is purged by the developing carbon dioxide (about 90%). Residues of high volatile sulfur-containing compounds (MeSH, EtSH) could also be removed by purging with inert gases like nitrogen or argon. However, what if these off-odor gases are trapped by SO2 (employed for microbial must stabilization) or elemental sulfur as non-volatile, polar polythionates? These remain in wine and are transferred during bottling as latent sources for the reappearance of reductive off-odors.
Now, back to “real wine”: As outlined above, elemental sulfur is used as a fungicide and may be transferred to the must. Must is stabilized by the addition of SO2. Therefore, the formation of a “Wackenroder solution” (WS) is very likely. During fermentation, yeast produces H2S in order to fulfil the need for the sulfur-containing amino acids methionine and cysteine via the sulfate assimilation pathway, simulating the original conditions for the formation of the “WS”, containing polysulfanmono and disulfonic acids (polythionates). The experiments cited in [29] ran with 50 mg potassium bisulfite K2S2O5 (which equals 33.3 mg SO2) and 0–100 mg addition of elemental sulfur. In these experiments, up to 50 μg of total H2S was formed (most in TECP = triscarboxyethyl phosphine releasable form), which is far over its odor threshold of 1 μg L−1. This clearly demonstrates that if only 0.1% of the added sulfur reacts to polythionates, there is enough latent sources to form reductive off-odors.
Therefore, polythionates fulfil the criteria for the until now undefined precursors for reductive off-odors by Kreitman [2]: “They are metastabile during typical bottle storage conditions, i.e., the conversion of precursor to free sulfhydryl should occur on the order of several weeks to a couple of years at room temperature and their concentration should be large enough to generate concentrations of H2S and MeSH (up to 1–2 μM L−1)”. In addition, they are polar compounds, highly soluble in aqueous solutions like wine, passing every filter system used in wine making.
5.2. The Role of Copper
A main topic in recent literature about the reappearance of reductive off-odors deals with the application of copper (II) salts [17,21,50,51,52,53]. The reason why copper fining removes or reduces sulfur-containing off-odor components is based on the very low solubility of copper sulfides and mercaptides in aqueous solutions, leading to their precipitation and the possibility of physical separation from the rest of the wine.
Copper plays an important role in winemaking practice, but its application is more and more questioned. Presently, trends try to minimize the amount of copper; even a total ban for applications of copper in vineyards is intended [54,55]. Many investigations in recent time showed that the common practice to add copper (II), either as sulfate or citrate, to remove sulfidic off-odors even may result in an elevated concentration of VSCs in the finished (bottled) wine.
Residual copper in white wine has been linked to oxidative and reductive spoilage processes, in haze formation (copper casse) and protein instability. More recent concerns include the coexistence of residual copper and hydrogen sulfide in wine stored under low oxygen conditions.
Copper-(II) salts (added in order to remove sulfidic off-odors) may react in a manifold way with all kinds of wine components, as outlined in Figure 3.
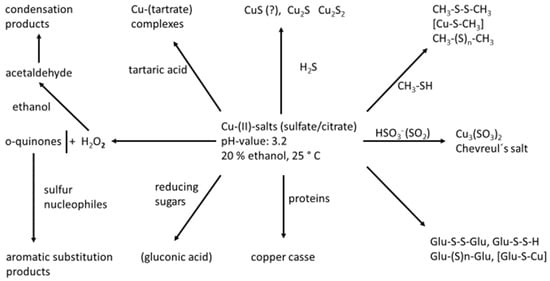
Figure 3.
Reactions of wine components with copper ([56], modified).
The chemistry of copper in white and red wine does not differ considerably. There are some overlapping issues, especially considering sulfidic off-odors. However, due to the quite different phenolic status of red wine, this review deals with the state of knowledge of copper in white wine.
Explanations of the reaction scheme (Figure 3) [56] are given below:
- (1)
- On reaction with H2S, no simple precipitation of insoluble CuS takes place; the “copper sulfide” consists of a mixture of Cu2S and CuS2, better described as [Cu3S3]. In addition, di- and poly-sulfanes are formed [29,53].
- (2)
- Reaction with “off-odor” VSCs MeSH and EtSH leads to the formation of disulfides, mixed disulfides [51], including those with H2S, which leads to hydropersulfides (R-S-S-H), the formation of dialkylpolysulfanes and alkylpolysulfanes, as well as mixed forms with organyl residues (R1-(S) n-R2); the formation of copper-(I)-mercaptides and soluble complexed forms of these [53].
- (3)
- If wines are treated with copper (II) salts alone, almost the total disappearance of thiols like 3-MH and the concomitant formation of disulfides were observed [51].
- (4)
- Reaction with sulfur dioxide (rather, bisulfite): There are ideal reaction conditions to form “Chevreul’s salt” [Cu3(SO3)2], a highly stable mixed valence sulfite compound [57,58]. Because of its high insolubility, the precipitation of Chevreul’s salt is used in hydrometallurgy.
- (5)
- Glutathione behaves like a normal thiol in forming the di- and polysulfanes and the corresponding mercaptide. This has to be considered in the light of using glutathione in enology (OIV) [59,60].
- (6)
- Glucose (reducing aldoses): Copper (II) sulfate is used for the quantitative determination of reducible sugars in wine analytics according to Rebelein. Although this method works in strong alkaline solution (the aldehyde group is oxidized to a carboxyl group; glucose is converted to gluconic acid), there still might be some evidence that copper (II) is converted to Cu (I) at normal wine pH of around 3.0–3.5.
- (7)
- Copper addition also induces the formation of hazes in wine by reaction with proteins.
- (8)
- Copper, especially in combination with iron ions, catalyzes the oxidation of wine o-diphenols and other phenolics like caftaric acid, cyanidin, catechin, epicatechin, gallic acid and its derivatives, to form highly reactive o-quinones. The impact on sulfur-containing aroma active compounds lies in their high nucleophilicity, giving rise to irreversible 1,4-Michael additions [61].
- (9)
- o-Quinones are also made responsible for the development of volatile sulfur compounds by the Strecker reaction with cysteine and methionine [9].
- (10)
- Copper forms stable complexes with tartaric acid [17,21,62].
Copper may be present in grape juice due to its application as a fungicide in the vineyard. Copper concentrations in must and wine range from 0.2 mg/L–7.3 mg/L. In a recent survey of 100 Chardonnay juices during the 2009 Australian vintage, the median Cu concentration was around 1 mg/L with the lowest at 0.2 and the highest at 7.0 mg/L. In a recent publication, different forms of metals in wine were claimed. Thus, a fractionation of Cu and Fe compounds into hydrophobic, cationic and residual forms was described [61]. Considering wine components, which react with copper ions, forming insoluble compounds like sulfidic species or the mixed valence “Chevreul’s salt” with sulfur dioxide, the question may be raised about how these species will contribute to the different fractions.
5.3. Some Remarks on the Oxidation States of Copper/Sulfur Compounds
The bonding in copper sulfides cannot be correctly described in terms of a simple oxidation state formalism because the Cu-S bonds are somewhat covalent rather than ionic in character and have a high degree of delocalization, resulting in complicated electronic band structures. Many textbooks (e.g., [63]) give preference to the mixed valence formula (Cu+)2(Cu2+)(S2−)(S2)2− For CuS, X-ray photoelectron spectroscopic data give strong evidence that, in terms of the simple oxidation state formalism, all the known copper sulfides should be considered as purely monovalent copper compounds, and a more appropriate formulae would be (Cu+)3(S2−)(S2)− for CuS and (Cu+)(S2)− for CuS2, respectively. An explanation therefore is given by the fact that the highly polarizing Cu2+-ion removes an electron from the highly polarizable S2−-anion:
2 Cu2+ + 2 S2− → 2 Cu+ + S−S2−( = Cu2S2)
Copper (II) reacts with thiols by reduction to copper (I) and the formation of disulfides and copper (I)-mercaptides or related complexes (Equation 2). Both are suspected to be sources for the reappearance of reductive off-odors during bottle-aging [17,21,53].
4 Cu2+ + 4 R-S-H → 2 Cu+ + R–S-S-R 2 [R-S-Cu]
Compounds with the co-existence of divalent Cu2+-ions in sulfidic compounds with oxidation state −2 simply do not exist.
The same conclusions may be drawn for the co-existence of copper (II) and SO2 in wine and must. Hydrogen sulfite ions, the most probable species at wine pH, react with copper (II) salts to form Chevreul’s salt, a mixed valence sulfite of copper Cu3(SO3)2 better represented by the formula [64]:
CuSO3 × Cu2SO3
In Chevreul’s salt crystals, there are two environments for copper. The +1 oxidation state copper is in a distorted tetrahedral space surrounded by three oxygens and a sulfur atom. The +2 oxidation state copper is in a distorted octahedral coordination surrounded by four oxygen atoms and two water molecules, which stresses the above statement [65]. Supposing must or wine contain average concentrations of 32 mg (0.5 mMol) free SO2 and 5 mg (about 0.1 mMol) copper (II) ions, the formation of Chevreul’s salt is very likely.
5.4. The Role of Sulfur Dioxide
Sulfur dioxide SO2 is the most widely-used additive in winemaking. Its antimicrobial effect, antioxidant properties and its capability to protect wine from various unwanted reactions makes it an unavoidable additive in winemaking [59]. The legal limit for total SO2 concentration according to the OIV is 150–200 mg L−1 for dry wines, while in exceptional cases, it can reach up to 400 mg L−1.
Sulfur dioxide participates in the formation of reductive sulfurous off-odors (Figure 4).
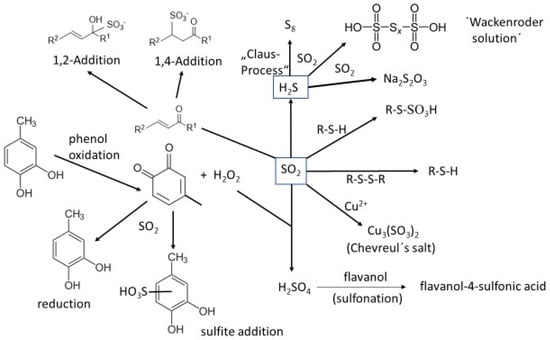
Figure 4.
Reactions of SO2 with wine components (top right: the formation of polythionates from the yeast metabolites H2S and SO2).
Besides the direct enzymatic reduction to hydrogen sulfide in the sulfate assimilation pathway (Figure 2), numerous different chemical pathways exist in a very complex system that makes it almost impossible to follow reductive odor compounds analytically.
In recent studies, SO2 in combination with copper (II) salts was cited as being responsible for the development of reductive off-odors [65]. Surprisingly, this effect was only observed in “real” wines, not in so-called model wines, usually employed in these trials. An explanation for this effect may easily be found in the circumstance that model wine has not passed a fermentation step. Therefore, all yeast-born “sulfurous compounds” (including polythionates) are simply not present and thus cannot result various VSCs by treatment with copper.
The following list refers to Figure 4, explaining some of the represented reactions.
- (1)
- The oxidation of wine o-diphenols leads to o-quinones and hydrogen peroxide, which oxidize sulfite to sulfuric acid. Highly reactive aromatic compounds like phenols, indoles, etc., may undergo electrophilic aromatic substitution (sulfonation) [66].
- (2)
- SO2 (as HSO3–) itself is a strong nucleophile, which reacts with o-quinones in a Michael addition-type reaction under the irreversible formation of aromatic sulfonic acids [61].
- (3)
- SO2 reacts with various carbonyl compounds forming bisulfite-addition products (“bound SO2”). α,β-unsaturated ketones (i.e., the wine-relevant aroma compound β-damascenone) form 1,4-addition products [67].
- (4)
- At elevated temperatures, H2S and SO2 comproportionate to elemental sulfur. This reaction is used in the technical “Claus-process” for desulfuration of crude gas.
- (5)
- At ambient temperature, H2S and SO2 react to the so-called “Wackenroder solution”, a mixture of polythionates, H2S, SO2 and elemental sulfur (see Section 5).
- (6)
- The formation of (at wine pH) instable thiosulfuric acid takes also part in the formation of “Wackenroder solution”.
- (7)
- Thiols are powerful reducing agents that may be oxidized by sulfur (IV) compounds to mixtures of polysulfanes. The first step of this reaction is the formation of S-sulfonated thiols.
- (8)
- Recently, various sulfonated compounds were observed in wine for the first time (e.g., S-sulfonated cysteine and glutathione) [66].
- (9)
- The addition of SO2 to anthocyanins results in colorless 4-sulfonic acids.
- (10)
- The addition of copper (II) to sulfite-containing solutions leads to the formation of a mixed valence copper sulfite (“Chevreul’s salt”) [68].
- (11)
- SO2 may cleave disulfide bonds under the formation of thiols (“sulfitolysis”) [69].
- (12)
- The so-called “sulfite degradation” of sulfur chains in elemental sulfur, polysulfanes and polythionates is part of the very complicated sulfur chemistry in aqueous systems (including wine).
6. Discussion of Polydithionates: The Answer to Some Unsolved Questions?
This section starts with the assumption that polythionates are part of the non-volatile and odorless forms in wine, acting as precursors for the redevelopment of reductive sulfurous off-odors. The following findings and statements mainly made by Ferreira and Franco-Luesma in the literature [70] may then be seen in a clearer light.
- (1)
- Wine contains H2S and MeSH in non-volatile and odorless forms. An analytical method has been developed to detect H2S and MeSH present in bonded forms [71]. We claim polythionates to be part of the non-volatile and odorless forms.
- (2)
- Ferreira and Franco-Luesma [70] pointed out that each wine has a specific ability to bind reversibly H2S and mercaptans. “If free H2S and MeSH are added to different wines, some of these molecules remain volatile, and hence can be easily smelled, while there are some others in which the same molecules are so strongly bonded that they cannot be measured (and hence smelled) as free forms” [70] (bonded forms include polythionates; → see Daltons’ experiment [38]). The reversible binding of H2S in polythionates lies in the labile character of these compounds. They easily decay into H2S, SO2 and elemental sulfur.
- (3)
- Oxygen only reacts in converting H2S and mercaptans into their free forms. Bound or complexed forms (including polythionates) require longer periods of treatment to release the free forms [37].
- (4)
- Even under strict oxygen-free conditions, H2S is able to slowly, but irreversibly (?) react to something (we claim polythionates) in some wine.
- (5)
- The increments of free H2S observed when wine is stored in anoxia are mostly the release of complexed forms: This is valid for polythionates and other components of the “Wackenroder solution”, if considered as part of the “complexed forms” [29].
- (6)
- Analytical methods to detect H2S, MeSH and other VSCs in the headspace do not work, if they suffer interactions in wines occasionally so strong that in some wines, they are not released to the wine headspace. In contrast, a previously-developed method in which the wine is strongly diluted with brine revealed that such interactions are reversible, demonstrating that wines contain a large pool of reversible complexed (as polythionates) non-volatile forms of H2S and mercaptans [72].
- (7)
- TCEP (Tris (2-carboxyethyl) phosphine) was evaluated for its ability to release complexed H2S from wine. Surprisingly, even though TCEP was much less effective than brine at releasing H2S from copper sulfide complexes in the recovery experiments, higher concentrations of H2S released by TCEP were observed in six of the seven wines (up to 25.3 µg/L). These results suggest the presence of additional TCEP-releasable sources (polythionates) of H2S (or thiol interferences) in wine [48]. Since TCEP acts specifically to break -S-S-bonds, polythionates will also be attacked with the release of H2S.
- (8)
- Elemental sulfur pesticide residues on grapes can not only produce H2S during fermentation, but are also able to form precursors capable of generating additional H2S after bottle storage. Among these precursors, glutathione tri- and polysulfanes (Glu-S-Sn-S-Glu) have been detected, but also for the first time, tetrathionate (S4O62−). These precursors are polar, wine-soluble intermediates that can be converted into H2S following TCEP (triscarboxyethyl phosphine) addition [29]. The identity of these intermediates was unclear, but could potentially involve polysulfide (polythionates) adducts. The authors claim sulfitolysis of polysulfanes as the reason; the classical formation by the “Wackenroder reaction” from the wine components H2S and SO2 is not mentioned. These findings come closest to our “polythionate theory” made in (6).
- (9)
- Wine producers know that if a wine has a tendency to develop or had reductive off-odors, it is possible that the off-flavor is reoccurring during the storage of the bottle, in particular if closures with low permeability to oxygen are used. This indicates that the oenological treatments affect the odor-active compounds, but not their diverse precursors [56,70]. Polythionates might belong to this type of precursor.
- (10)
- Up to this date, these precursors have not been identified. One reason why the progress in research has been so difficult lies in the complicated chemistry of sulfur. This element can be present in different redox states, has the ability to concatenate (polysulfanes and polythionic acids), making bonds with itself, and can form a large number of combinations [16]. These statements support our polythionates theory.
- (11)
- Copper, acting on unknown precursors, was associated with large increases of H2S in Shiraz wines [73]. The unknown precursors may be polythionates born in the fermentation step. There is no remarkable increase in model wine when treated with copper, due to the lack of these precursors.
- (12)
- This suggests that a pool of yet to be identified precursor compounds (polythionates) is also involved in modulating final concentrations in wine through copper-catalyzed reactions [74] (since copper salts are active in cleaving -S-S-bonds, these bonds will be cleaved in polythionates, as well).
The comparison of experimental results obtained in real wine with those obtained in model wine should be taken with care. Since model wine has not passed a fermentation step, it lacks all the yeast-born precursors (i.e., polythionates).
7. Conclusions
Still other latent sources of H2S and MeSH may exist, like the potential contribution of non-volatile asymmetric disulfides, trisulfides or even polysulfanes (e.g., adducts with other thiol compounds), which have not been well explored. Since the natural tripeptide glutathione [75,76] represents the thiol with the highest concentration in must and wine (up to 120 mg), this sulfanyl compound is strongly involved in these processes of reductive off-odor formation [16,29]. This topic would need its own section.
Among these compounds with molecules containing sulfur chains, polythionates have not been taken into consideration, although their existence has been known since the early development of modern chemistry. The combination of added or biologically-produced SO2 and H2S in must and wine form an ideal basis for their formation. Although it seems that the existence of these species can give more explanations for some strange findings in the matter of the reappearance of reductive odors in wine, these elucidations neither facilitate the whole complex, nor give a clear mode of action for how to quantify and remove these off-odors. Polythionates are part of the mystery that still exists about sulfur chemistry. Their instability, decay and de novo formation, as well as their interconvertibility make it almost impossible to establish a reliable analytical method.
The addition of copper to remove reductive off-odors even worsens the situation, since little is known about the interactions of copper in both oxidation states with these species. The presence of copper may lead to an immediate decrease of smelling free forms of H2S and MeSH, but the danger from a reservoir of unknown precursors for reductive off-odors is transferred into the bottles, with a high likelihood of reappearance during storing.
Numerous different chemical pathways exist in a very complex system that would make it almost impossible to follow reductive odor compounds analytically. The suspicious mixture of unknown instable sulfur intermediates may now give an explanation for the reappearance of sulfur-caused reductive off-odors, but hardly can recommend a mode of action. Furthermore, it is clearly demonstrated that the addition of copper (II) rather creates more confusion than helping to solve the problem.
We hope to have given part of an answer to the question and the statement made by Waterhouse et al. in his recently published textbook on wine chemistry [16]: “Finally, other potent (and common) contributors to reduced aromas may have been overlooked. If the standing hypotheses are not valid, then these questions are founded on unknown chemical processes; that is, what “reducing” agents or reactions are participating or occurring? Wine storage is a rare situation of very long lifetimes of reactive solutions where kinetically slow processes, perhaps those not commonly observed otherwise, have time to occur.
Determining the likely latent sources of H2S, CH3SH, or other causes of reduced aromas is more than interesting chemistry—it is also a crucial step in eliminating an increasingly vexing problem. Determining their identity should lead to better prevention, remediation, and detection strategies for reduced aroma formation in bottle. Currently, some winemakers will add ascorbic acid to predict the appearance of reduced aromas, but the rationale for this test is based on disulfides as latent precursors, and these tests have not been validated. In recent years, winemakers have benefited from accelerated aging tests, for example, contact tests to predict the likelihood of potassium bitartrate instability and it is expected that a validated accelerated reduction assay would be equally beneficial” [16].
Author Contributions
N.M. outlined the review and wrote the biggest part of the review. He realized by intensive literature study, that the formation of polythionates, hitherto not taken into consideration, could be the reason for the reappearance of sulfurous off-odours. D.R. gave intensive support in designing the review and by numerous technical discussions. Both authors contributed equally in revision and final version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goode, J.; Harrop, S. Wine faults and their prevalence: Data from the world’s largest blind tasting. In Proceedings of the Les XXes Entretiens Scientifiques Lallemand, Horsens, Denmark, 15 May 2008; pp. 7–9. [Google Scholar]
- Kreitman, G.Y.; Elias, R.J.; Jeffery, D.W.; Sacks, G.L. Loss and formation of malodourous volatile sulfhydryl compounds during wine storage. Crit. Rev. Food Sci Nutr. 2018, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Regulation of hydrogen sulfide liberation in wine producing. Saccharomyces cerevisiae by assimilable nitrogen. Appl. Environ. Microbiol. 1995, 61, 461–467. [Google Scholar] [PubMed]
- Rauhut, D.; Kürbel, H. The production from H2S from elemental sulfur residues during fermentation and its influence on the formation of sulfur metabolites causing off-flavours in wine. Vitic. Enol. Sci. 1994, 49, 27–36. [Google Scholar]
- Rauhut, D. Usage and formation of sulfur compounds. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 255–291. [Google Scholar]
- Rauhut, D. Yeast-Production of sulfur compounds. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 183–223. [Google Scholar]
- Huang, C.-W.; Walker, M.E.; Fredrizzi, B.; Gardner, J.V. Hydrogen sulfide and its roles in Saccharomyces cerevisiae in wine making context. FEMS Yeast Res. 2017, 17, fox058. [Google Scholar] [CrossRef] [PubMed]
- Rauhut, D.; Kürbel, H.; Dittrich, H.H.; Grossmann, M. Properties and differences of commercial yeast strains with respect to their formation of sulfur compounds. Vitic. Enol. Sci. 1996, 51, 187–192. [Google Scholar]
- Pripis-Nicolau, L.; de Revel, G.; Bertrand, A.; Maujean, A. Formation of Flavor Components by the Reaction of Amino Acid and Carbonyl Compounds under Mild Conditions. J. Agric. Food Chem. 2000, 48, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. Thiaminpyrophosphat-ein natürlich vorkommendes Iminiumsalz. Z. Naturforsch. B 2014, 69, 489–500. [Google Scholar] [CrossRef]
- Lloyd, D. Hydrogen sulfide: Clandestine microbial messenger? TRENDS Microbiol. 2006, 14, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Murray, D.B. The temporal architecture of eukaryotic growth. FEBS Lett. 2006, 580, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Reschke, S.; Tran, T.; Bekker, M.; Wilkes, E.; Johnson, D. Using copper more effectively in winemaking. Wine Vitic. J. 2015, 9, 35–39. [Google Scholar]
- Görtges, S. Böckserbeseitigung mit Kupfercitrat. Der Deutsche Weinba 2009, 5, 24–25. [Google Scholar]
- Steidl, R. Der Einsatz von Silberchlorid zur Böckserbekämpfung. Mitt. Klosterneubg. 2010, 3, 92–95. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. (Eds.) Appearance of Reduced Aromas during Bottle Storage. In Understanding Wine Chemistry; John Wiley & Sons: Chichester, UK, 2016; pp. 397–399. [Google Scholar]
- Clark, A.C.; Wilkes, E.N.; Scollary, G.R. Chemistry of Copper in white wine: A. review. Aust. J. Grape Wine Res. 2015, 21, 339–350. [Google Scholar] [CrossRef]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Evolution of Volatile Sulfur Compounds during Wine Fermentation. J. Agric. Food Chem. 2015, 63, 8017–8024. [Google Scholar] [CrossRef] [PubMed]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Hydrogen sulfide production during yeast fermentation causes the accumulation of ethanethiol, S-ethyl thioacetate and diethyl disulfide. Food Chem. 2016, 209, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. Iminiumsalz-Strukturen bei der durch Pyridoxalphosphat (Vitamin B6) katalysierten Bildung von Aromastoffen und Fehlaromen im Wein. Z. Naturforsch. B 2018, 73, 521–533. [Google Scholar] [CrossRef]
- Clark, A.C.; Grant-Peerce, P.; Cleghorn, N.; Scollary, G.R. Copper (II) addition to white wines containing hydrogen sulfide: Residual copper concentration and activity. Aust. J. Grape Wine Res. 2015, 21, 30–39. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Day, M.P.; Holt, H.; Wilkes, E.; Smith, P.A. Effect of oxygen exposure during fermentation on volatile sulfur compounds in Shiraz wine and a comparison of strategies for remediation of reductive character. Aust. J. Grape Wine Res. 2016, 22, 24–35. [Google Scholar] [CrossRef]
- Starkenmann, C.; Chappuis, C.J.-F.; Niclass, Y.; Deneulin, P. Identification of Hydrogen Disulfanes and Hydrogen Trisulfanes in H2S Bottle, in Flint, and in Dry Mineral White Wine. J. Agric. Food Chem. 2016, 64, 9033–9044. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, N.; Kuroda, H.; Yamada, O.; Goto-Yamamoto, N. Factors Affecting Dimethyl Trisulfide Formation in Wine. Food Sci. Technol. Res. 2017, 23, 241–248. [Google Scholar] [CrossRef]
- Gijs, L.; Perpète, P.; Timmermans, A.; Collin, S. 3-Methylthiopropionaldehyde as Precursor of Dimethyl Trisulfide in Aged Beers. J. Agric. Food Chem. 2000, 48, 6196–6199. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y. Characteristic Aroma Compounds of Chinese Dry Rice Wine by Gas-Chromatography-Olfactometry and Gas-Chromatography-Mass Spectroscopy. In Flavor Chemistry of Wine and Other Alcoholic Beverages; ACS Symposium Series 2012; American Chemical Society: Washington, DC, USA, 2012; Chapter 16; pp. 277–301. [Google Scholar]
- Isogai, I.; Utsonomiya, H.; Kanda, R.; Iwata, H. Changes in Aroma Compounds of Sake during Aging. J. Agric. Food Chem. 2005, 53, 4118–4123. [Google Scholar] [CrossRef] [PubMed]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulphur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Jastrembski, J.A.; Allison, R.B.; Friedberg, E.; Sacks, G.L. Role of Elemental Sulfur in Forming Latent Precursors of H2S in Wine. J. Agric. Food Chem. 2017, 65, 10542–10549. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, F.C. On the Reactivity of Nanoparticulate Elemental Sulfur: Experimentation and Field Observations. Ph.D. Thesis, Indiana University, Bloomington, IN, USA, December 2017. [Google Scholar]
- Steudel, R.; Eckert, B. Solid sulfur allotropes. In Elemental Sulfur and Sulfur-Rich Compounds; Topics in Current Chemistry Book Series; Springer: Berlin/Heidelberg, Germany, 2003; Volume 230, 79p. [Google Scholar]
- Araujo, L.D.; Vannevel, S.; Buica, A.; Callerot, S.; Fredrizzi, B.; Kilmartin, P.A.; Du Toit, W.J. Indications of the prominent role of elemental sulfur in the formation of the varietal thiol 3-mercapto- hexanol in Sauvignon blanc. Food Res. Int. 2017, 98, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Krahn, R. Mechanismen der Schwefelwasserstofferzeugung durch Saccharomyces Cerevisiae für die Biotechnologische Immobilisierung von Schwermetallen; Inaugural-Dissertation: Düsseldorf, Germany, 2000. [Google Scholar]
- Kleinjan, W.E.; Keizer, A.; Janssen, A.J.H. Biologically Produced Sulfur. Top. Curr. Chem. 2003, 230, 167–188. [Google Scholar]
- Steudel, R. Aqueous Sulfur Sols. Top. Curr. Chem. 2003, 230, 153–166. [Google Scholar]
- Nedjma, M.; Hoffmann, N. Hydrogen sulfide reactivity with thiols in the presence of copper (II) in hydroalcoholic solutions. J. Agric. Food Chem. 1996, 44, 3935–3938. [Google Scholar] [CrossRef]
- Vela, E.; Purificíon, H.-O.; Franco-Luesma, E.; Ferreira, V. Micro-oxygenation does not eliminate hydrogen sulfide and mercaptans from wine; it simply shifts redox and complex-related equilibria to reversible oxidized species and complexed forms. Food Chem. 2018, 243, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.; Wolff, F. Ein neues System des Chemischen Theils der Naturwissenschaft; Verlag Eduard Hitzig: Berlin, Germany, 1813; Volume 2, pp. 190–196. [Google Scholar]
- Wackenroder, H. Über eine neue Säure des Schwefels. Arch. Pharm. 1846, 97, 272–288. [Google Scholar] [CrossRef]
- Tomozo, K. Analytical Chemistry of Polythionates and Thiosulfate. A. Review. Anal. Sci. 1990, 6, 3–14. [Google Scholar] [CrossRef]
- Blasius, E.; Burmeister, W. Untersuchung der Wackenroderschen Reaktion mit Hilfe der radiopapierchromatographischen Methode. Fresenius’ Z. Anal. Chem. 1959, 168, 1–15. [Google Scholar] [CrossRef]
- Stamm, H.; Seipold, O.; Goehring, M. Zur Kenntnis der Polythionsäuren und ihrer Bildung. 4. Mitteilung. Die Reaktionen zwischen Polythionsäuren und schwefliger Säure bzw. Thioschwefelsäure. Z. Allg. Anorg. Chem. 1941, 247, 93–98. [Google Scholar] [CrossRef]
- Drozdova, Y.; Steudel, R. The Reaction of H2S with SO2: Molecular Structures, Energies, and Vibrational Data of Seven Isomeric Forms of H2SO3. Chem. A Eur. J. 1995, 1, 193–198. [Google Scholar] [CrossRef]
- Schmidt, M.; Heinrich, H. Beitrag zur Lösung des Problems der Wackenroderschen Flüssigkeit. Über Säuren des Schwefels, XII. Angew. Chem. 1958, 70, 572–573. [Google Scholar]
- Thomas, D.; Surdin-Kerjan, Y. Metabolism of Sulfur Amino Acids in Saccharomyces cerevisiae. Mol. Biol. Rev. 1997, 61, 503–532. [Google Scholar]
- Ugliano, M.; Henschke, P.A. Yeasts and wine flavour. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 313–392. [Google Scholar]
- Swiegers, J.H.; Pretorius, I.S. Modulation of volatile sulfur compounds by wine yeast. Appl. Microbiol. Biol. 2007, 74, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jastrzembski, J.A.; Sacks, G.L. Copper-Complexed Hydrogen Sulfide in Wine. Measurement by Gas Detection Tubes and Comparison of Release Approaches. Am. J. Enol. Vitic. 2017, 68, 191–199. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.-B.; Aagard, O.; Waters, E.J. Evolution of 3-mercaptohexanol, hydrogen sulfide, and methyl mercaptan during bottle storage of Sauvignon blanc wines. Effect of glutathione, copper, oxygen exposure, and closure-derived oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Vela, E.; Hernández-Orte, P.; Franco-Luesma, E.; Ferreira, V. The effects of copper fining on wine content in sulfur off-odors and on their evolution during accelerated anoxic storage. Food Chem. 2017, 231, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Roland, A.; Delpech, S.; Dagan, L.; Ducasse, M.-A.; Cavelier, F.; Schneider, R. Innovative Analysis of 3-mercaptohexan-1-ol, 3-mercaptohexylacetate and their corresponding disulfides in wine by stable isotope dilution assay and nano-liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1468, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Reaction Mechanisms of Metals with Hydrogen Sulfide and Thiols in Model Wine. Part 1: Copper-Catalyzed Oxidation. J. Agric. Food Chem. 2016, 64, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Copper (II)-Mediated Hydrogen Sulfide and Thiol Oxidation to Disulfides and Organic Polysulfanes and Their Reductive Cleavage in in Wine. Mechanistic Elucidation and Potential Applications. J. Agric. Food Chem. 2017, 65, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R. Künftig ohne Kupfer; Heft 11/18; Der Deutsche Weinbau: Neustadt an der Weinstraße, Germany, 2018; pp. 16–18. [Google Scholar]
- Altmayer, B. Genehmigung auf der Kippe; Heft 5/18; Das Deutsche Weinmagazin: Neustadt an der Weinstraße, Germany, 2018; pp. 32–35. [Google Scholar]
- Müller, N.; Rauhut, D. Neuere Erkenntnisse zur Behandlung von reduktiven Noten und Böcksern im Wein. In Deutsches Weinbau Jahrbuch; Eugen Ulmer: Stuttgart, Germany, 2018; pp. 83–94. [Google Scholar]
- Calban, T.; Sevim, F.; Lacin, O. Investigation of Precipitation Conditions of Chevreul’s Salt. Int. J. Chem. Mol. Eng. 2016, 10, 1021–1024. [Google Scholar]
- Pietsch, E.H.E. Gmelin’s Handbook; Verlag Chemie: Weinheim, Germany, 1958; Volume 8, p. 484. [Google Scholar]
- OIV. International Code of Oenological Practices; International Organisation of Vine and Wine: Pairs, France, 2015. [Google Scholar]
- Ribéreau-Gayon, G.Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine Stabization and Treatment; John Wiley & Sons: Chichester, UK, 2006; Volume 2. [Google Scholar]
- Nikolantonaki, M.; Chichuc, J.; Teissedre, P.-L.; Darriet, P. Reactivity of volatile thiols with polyphenols in a wine-model medium: Impact of oxygen, iron, and sulfur dioxide. Anal. Chim. Acta 2010, 660, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rousseva, M.; Kontoudakis, N.; Schmidke, L.M.; Scollary, G.R.; Clark, A.C. Impact of wine production on the fractionation of copper and iron in Chardonnay wine: Implications for oxygen consumption. Food Chem. 2016, 203, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK; Waltham, MA, USA, 1997; ISBN 0-08-037941-9. [Google Scholar]
- Kierkegaard, P.; Nyberg, B. The crystal structure of Cu2SO3·CuSO3·2H2O. Acta Chem. Scand. 1965, 19, 2189–2199. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Smith, M.E.; Smith, P.A.; Wilkes, E.N. Formation of Hydrogen Sulfide in Wine: Interactions between Copper and Sulfur Dioxide. Molecules 2016, 21, 1214. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 25, 858. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, N.; Piano, F.; Davidson, S.J.; Larcher, R.; Fredrizzi, B.; Barker, D. Synthesis of alkyl sulfonic acid aldehydes and alcohols, putative precursors to important wine aroma thiols. Tetrahedron Lett. 2015, 56, 1728–1731. [Google Scholar] [CrossRef]
- Chevreul, M.E. Propriétés du sulfite de cuivre. Anal. Chim. 1812, 83, 187–190. [Google Scholar]
- Bobet, R.A.; Noble, A.C.; Boulton, R.B. Kinetics of the ethanethiol and diethyl disulfide interconversion in wine like solutions. J. Agric. Food Chem. 1990, 38, 449–452. [Google Scholar] [CrossRef]
- Ferreira, V.; Franco-Luesma, E. Understanding and Managing Reduction Problems. Int. J. Enol. Vitic. 2016, 2, 1–13. [Google Scholar]
- Franco-Luesma, E.; Ferreira, V. Quantitative analysis of free and bonded forms of volatile sulfur compounds in wine. Basic methodologies and evidences showing the existence of reversible cation-complexed forms. J. Chromatogr. A 2014, 1359, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luesma, E.; Ferreira, V. Reductive off-odours in wine: Formation and release of H2S and methanethiol during the accelerated anoxic storage of wines. Food Chem. 2016, 199, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bekker, M.Z.; Mierczynska-Vasilev, A.; Smith, P.; Wilkes, E.N. The effects of pH and copper on the formation of volatile sulfur compounds in Chardonnay and Shiraz wines post-bottling. Food Chem. 2016, 207. [Google Scholar] [CrossRef] [PubMed]
- Bekker, M.Z.; Wilkes, E.N.; Smith, P.A. Evaluation of putative precursors of key ‘reductive’ compounds in wines post-bottling. Food Chem. 2018, 245, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Ganguli, D.; Kaur, J.; Kasturia, N.; Thakur, A.; Kaur, H.; Kumar, A.; Yadav, A. Glutathione production in yeast. In Yeast Biotechnology: Diversity and Applications; Satyanarayana, T., Kunze, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; Chapter 13; pp. 259–278. [Google Scholar]
- Penninckx, M.A. Short review on the role of glutathione in the response of yeasts to nutritonal, environmental, and oxidative stresses. Enzyme Microb. Technol. 2000, 26, 737–742. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).