Enhancement of the Efficiency of Bioethanol Production by Saccharomyces cerevisiae via Gradually Batch-Wise and Fed-Batch Increasing the Glucose Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cultivation
2.2. Media and Fermentations
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
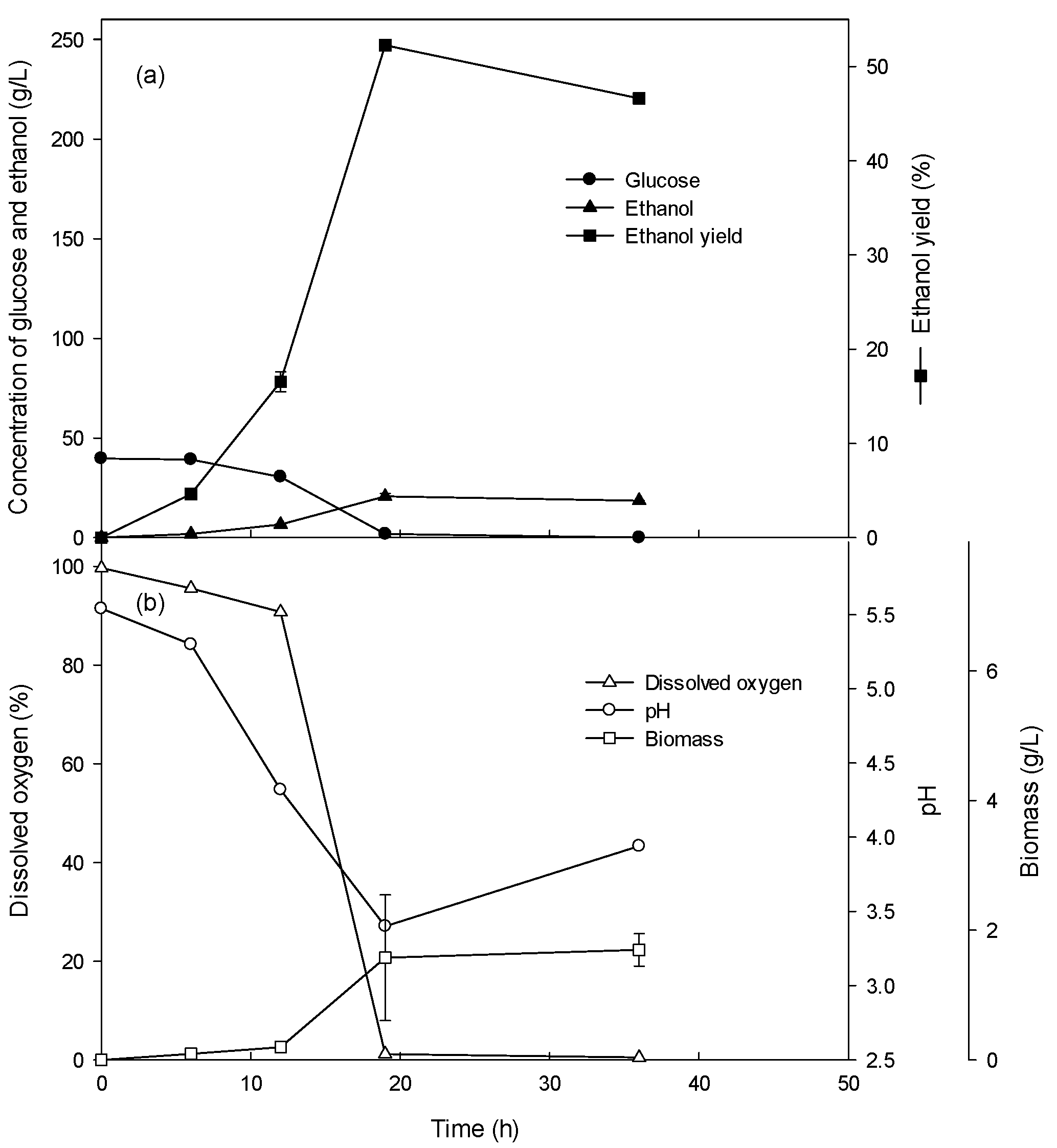
3.1. Ethanol Production of the Batch Culture with 4–10% Glucose
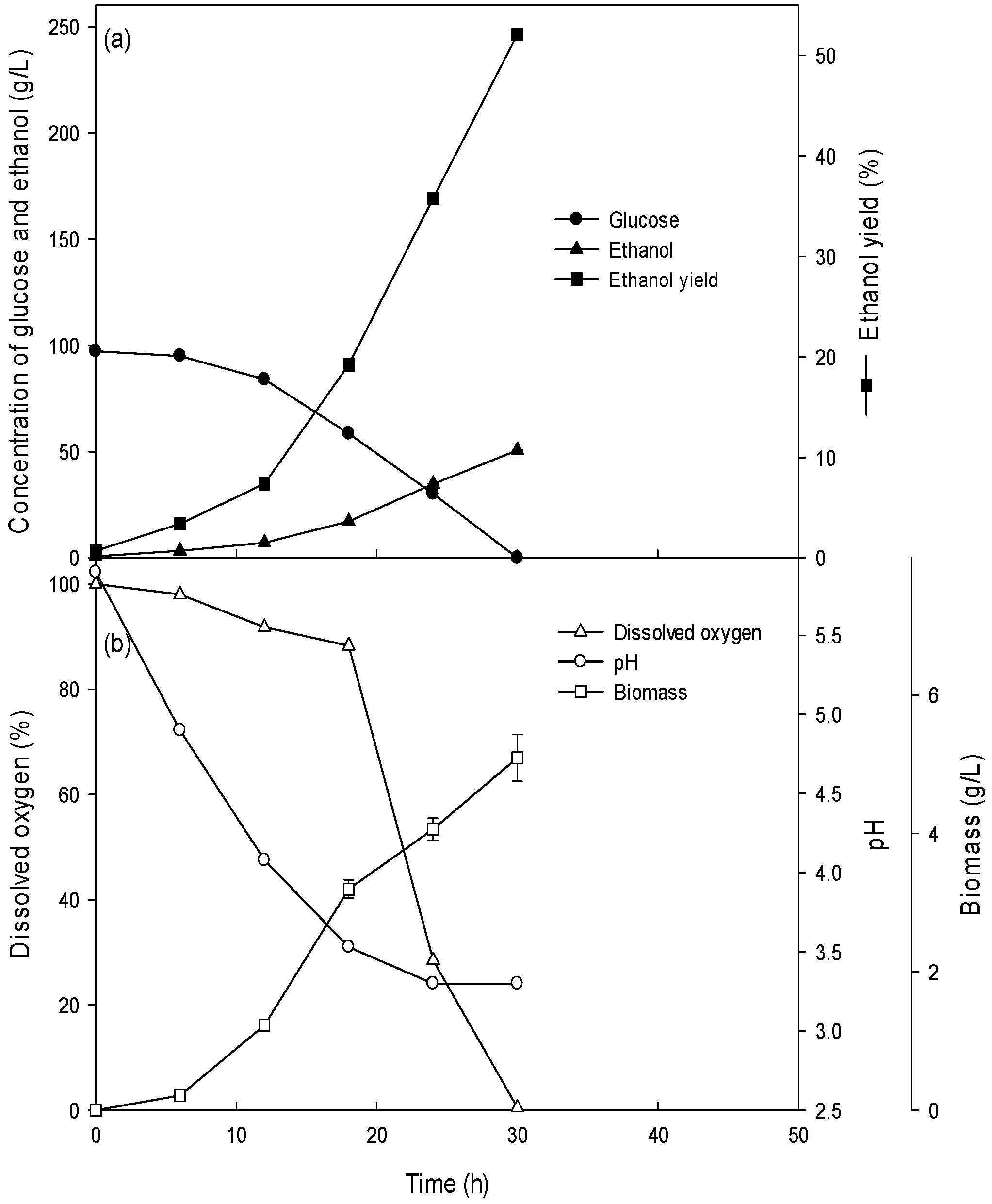
3.2. Ethanol Production of the Batch Culture with High Concentrations (15–26%) of Glucose
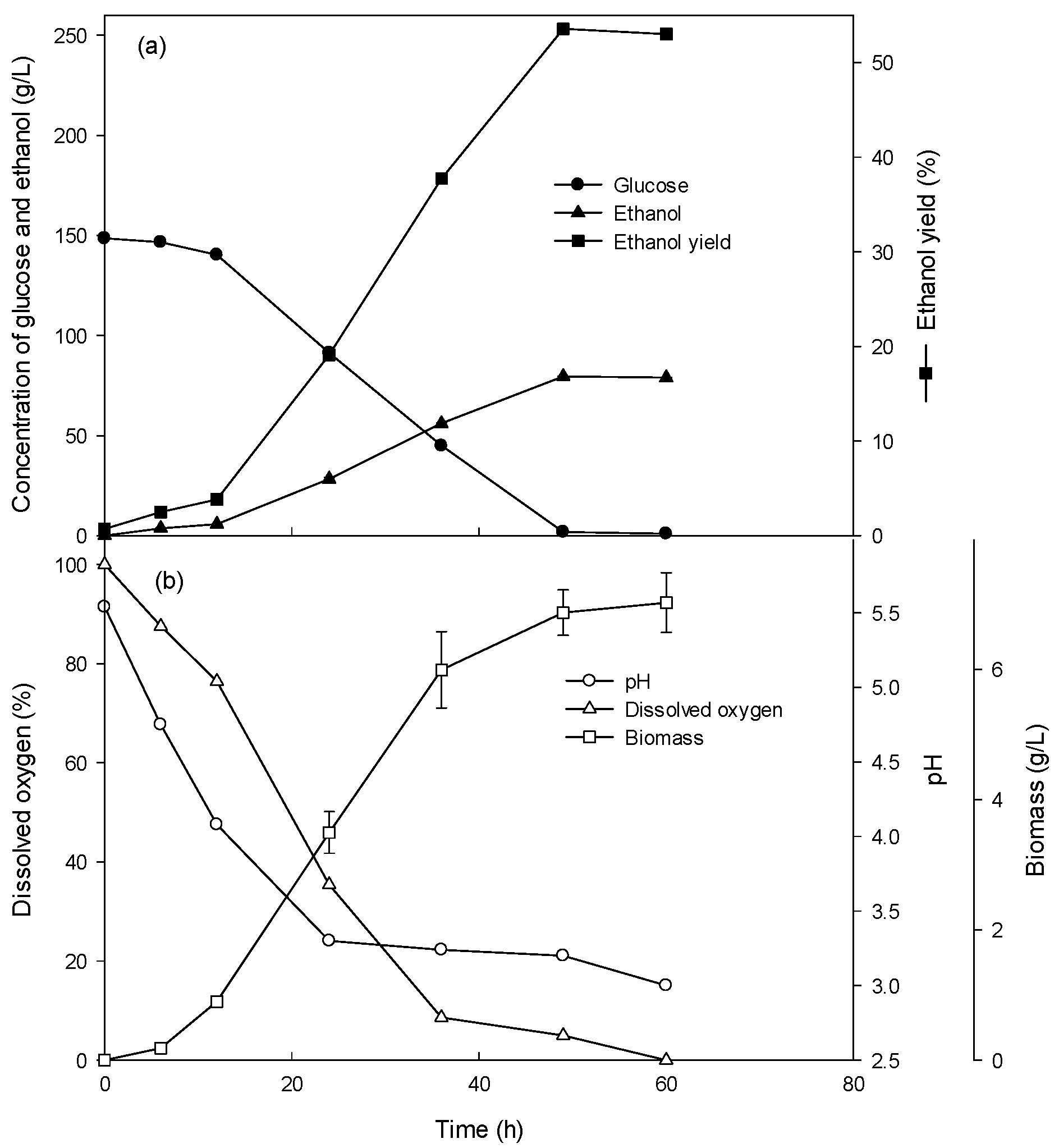
3.3. Ethanol Production at High Glucose Concentration in the Fed-Batch Culture
3.4. Comparison of Batch and Fed-Batch Culture for Ethanol Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailey, B.K. Performance of ethanol as a transportation fuel. In Handbook on Bioethanol: Production and Utilization; Wayman, C.E., Ed.; Taylor & Francis: Washington, DC, USA, 1996; pp. 37–60. [Google Scholar]
- Prasertwasu, S.; Khumsupan, D.; Komolwanich, T.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Efficient process for ethanol production from Thai Mission grass (Pennisetum polystachion). Bioresour. Technol. 2014, 163, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, W.; Li, C.; Sakakibara, K.; Tanaka, S.; Kong, H. Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass Bioenergy 2012, 47, 395–401. [Google Scholar] [CrossRef]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Cell Physiol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Younesi, H.; Ghasempouri, S.M.; Zinatizadeh, A.A.; Amini, M.; Daneshi, A. Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomyces cerevisiae. Chem. Eng. J. 2008, 45, 267–275. [Google Scholar] [CrossRef]
- Djekrif-Dakhmouche, S.; Gheribi-Aoulmi, Z.; Meraihi, Z.; Bennamoun, L. Application of a statistical design to the optimization of culture medium for alpha-amylase production by Aspergillus niger ATCC 16404 grown on orange waste powder. J. Food Eng. 2006, 73, 190–197. [Google Scholar] [CrossRef]
- Gangadharan, D.; Sivaramakrishnan, S.; Nampoothiri, K.M.; Sukumaran, R.K.; Pandey, A. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresour. Technol. 2008, 99, 4597–4602. [Google Scholar] [CrossRef] [PubMed]
- Ingledew, W.M. Alcohol Production by Saccharomyces Cerevisiae: A Yeast Primer, in the Alcohol Textbook, 3rd ed.; Nottingham University Press: Nottingham, UK, 1999. [Google Scholar]
- Bafrnacová, P.; Smogrovicova, D.; Slavikova, I.; Atkova, J.; Domeny, Z. Improvement of very high gravity ethanol fermentation by media supplementation using Saccharomyces cerevisiae. Biotechnol. Lett. 1999, 21, 337–341. [Google Scholar] [CrossRef]
- Casey, G.P.; Ingledew, W.M. Ethanol tolerance in yeast. Crit. Rev. Microbiol. 1986, 13, 219–280. [Google Scholar] [CrossRef] [PubMed]
- Ivorra, C.; Pérez-Ortin, J.; Del Olmo, M. An inverse correlation between stress resistance and stuck fermentation in wine yeast: A molecular study. Biotechnol. Bioeng. 1999, 64, 698–708. [Google Scholar] [CrossRef]
- Xu, P.; Thomas, A.; Gilson, C.D. Combined use of three methods for high concentration ethanol production by Saccharomyces cerevisiae. Biotechnol. Lett. 1996, 18, 1439–1440. [Google Scholar] [CrossRef]
- Yamaoka, C.; Kurita, O.; Kubo, T. Improved ethanol tolerance of Saccharomyces cerevisiae in mixed cultures with Kluyveromyces lactis on high-sugar fermentation. Microbiol. Res. 2014, 169, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, W.; Zhang, C.; Yan, P.; Jia, S.; Xu, Z.; Zhang, C. Vitalized yeast with high ethanol productivity. RSC Adv. 2014, 4, 52299–52306. [Google Scholar] [CrossRef]
- Thomas, K.; Hynes, S.H.; Ingledew, W.M. Effect of nitrogen limitation on synthesis of enzymes in Saccharomyces cerevisiae during fermentation of high concentration of carbohydrates. Biotechnol. Lett. 1996, 18, 1165–1168. [Google Scholar] [CrossRef]
- Mauricio, J.C.; Salmon, J.M. Apparent loss of sugar transport activity in Saccharomyces cerevisiae may mainly account for maximum ethanol production during alcoholic fermentation. Biotechnol. Lett. 1992, 14, 577–582. [Google Scholar] [CrossRef]
- Salmon, J.M.; Vincent, O.; Mauricio, J.M.; Bely, M.; Barre, P. Sugar transport inhibition and apparent loss of activity in Saccharomyces cerevisiae as a major limiting factor of enological fermentations. Am. J. Enol. Vitic. 1993, 44, 56–64. [Google Scholar]
- Ciriacy, M.; Reifenberger, M. Hexose transport. In Yeast Sugar Metabolism; Zimmermann, F.K., Entian, K., Eds.; Technomic Publishing Company: Lancaster, PA, USA, 1997. [Google Scholar]
- Bisson, L.F.; Neigeborn, L.; Carlson, M.; Fraenkel, D.G. The SNF3 gene is required for high-affinity glucose transport in Saccharomyces cerevisiae. J. Bacteriol. 1987, 169, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Caylak, B.; Vardar, S.F. Comparison of different production processes for bioethanol. Turk. J. Chem. 1996, 22, 351–359. [Google Scholar]
- Alfenore, S.; Molina-Jouve, C.; Guillouet, S.E.; Uribelarrea, J.L.; Goma, G.; Benbadis, L. Improving ethanol production and viability of Saccharomyces cerevisiae by vitamin feeding strategy during fed batch process. Appl. Environ. Microbiol. 2002, 60, 67–72. [Google Scholar]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology, 2nd ed.; Pergamon Press: Oxford, UK, 1995. [Google Scholar]
- Alfenore, S.; Cameleyre, X.; Benbadis, L.; Bideaux, C.; Uribelarrea, J.L.; Goma, G.; Molina-Jourve, C.; Guillouet, S.E. Aeration strategy: A need for very high ethanol performance in Saccharomyces cerevisiae fed-batch process. Appl. Microbiol. Biotechnol. 2004, 63, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chang, K.S.; Huang, C.W.; Hsu, C.L.; Jang, H.D. Comparison of batch and fed-batch fermentations using corncob hydrolysate for bioethanol production. Fuel 2012, 97, 166–173. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Govindaswamy, S.; Vane, L.M. Multi-stage continuous culture fermentation of glucose-xylose mixtures to fuel ethanol using genetically engineered Saccharomyces cerevisiae 424S. Bioresour. Technol. 2010, 101, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Shankar, T.; Anandapandian, K.T.K. Characterization of alcohol resistant yeast Saccharomyces cerevisiae isolated from Toddy. Int. Res. J. Microbiol. 2011, 2, 339–405. [Google Scholar]
- Cheng, N.G.; Hasan, M.; Kumoro, A.C.; Ling, C.F.; Tham, M. Production of ethanol by fed-batch fermentation. Pertanika J. Sci. Technol. 2009, 17, 399–408. [Google Scholar]
- Bonin, S.; Skwira, J. Effect of continuous fermentation of high-sugar fruit must on the viability and morphology of immobilized yeast on white foam glass. Food Technol. Biotechnol. 2008, 46, 164–170. [Google Scholar]
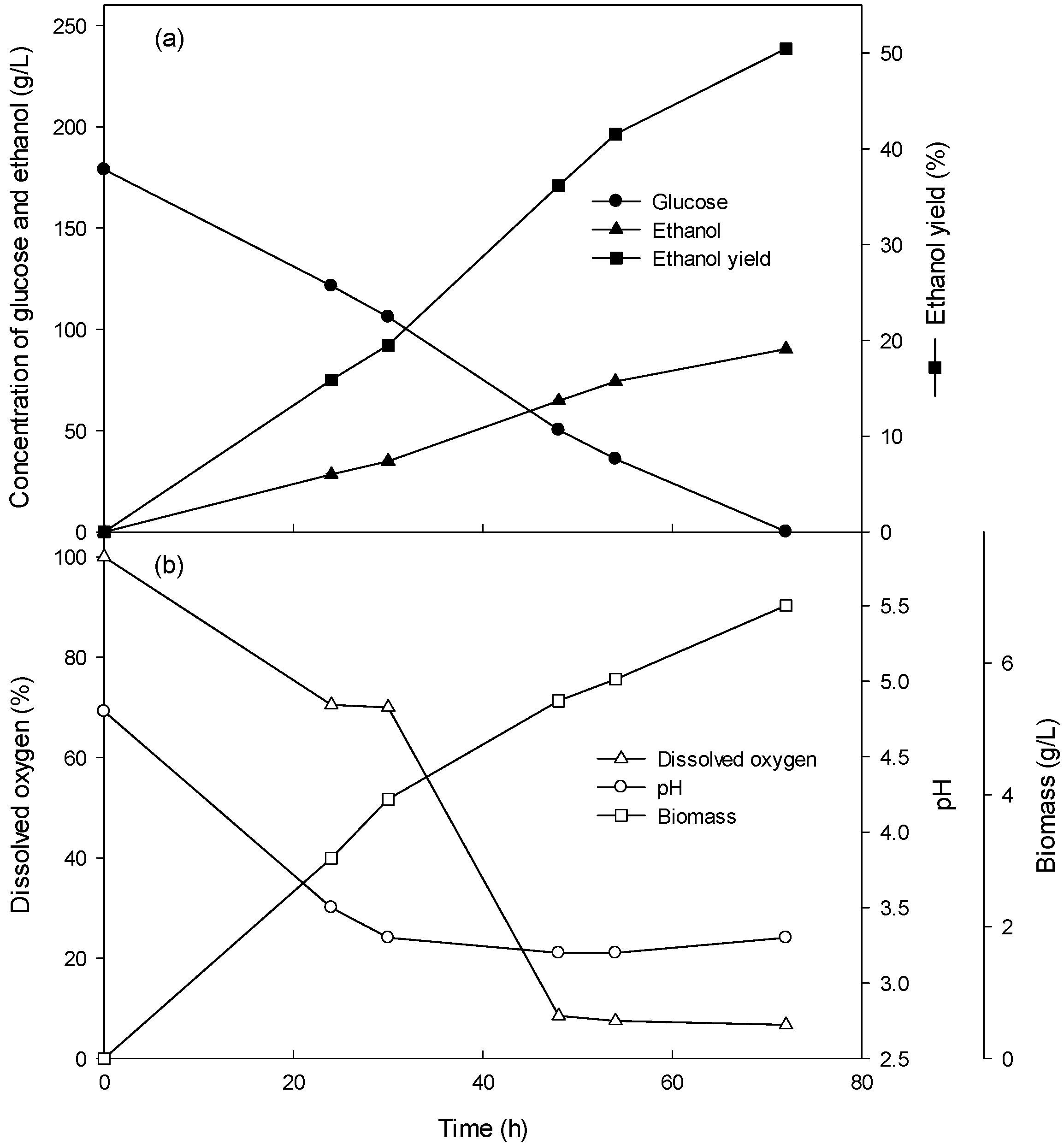
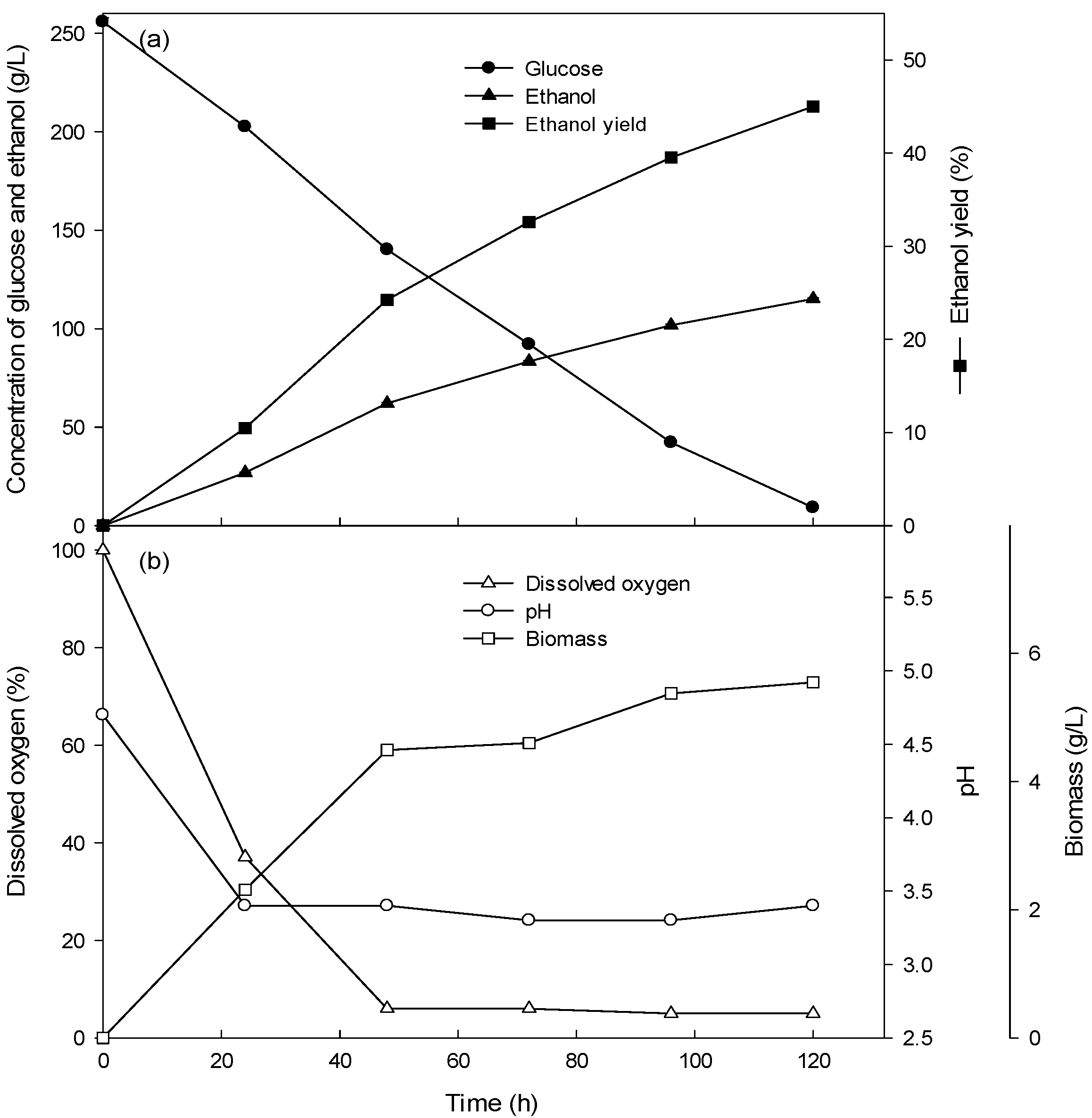
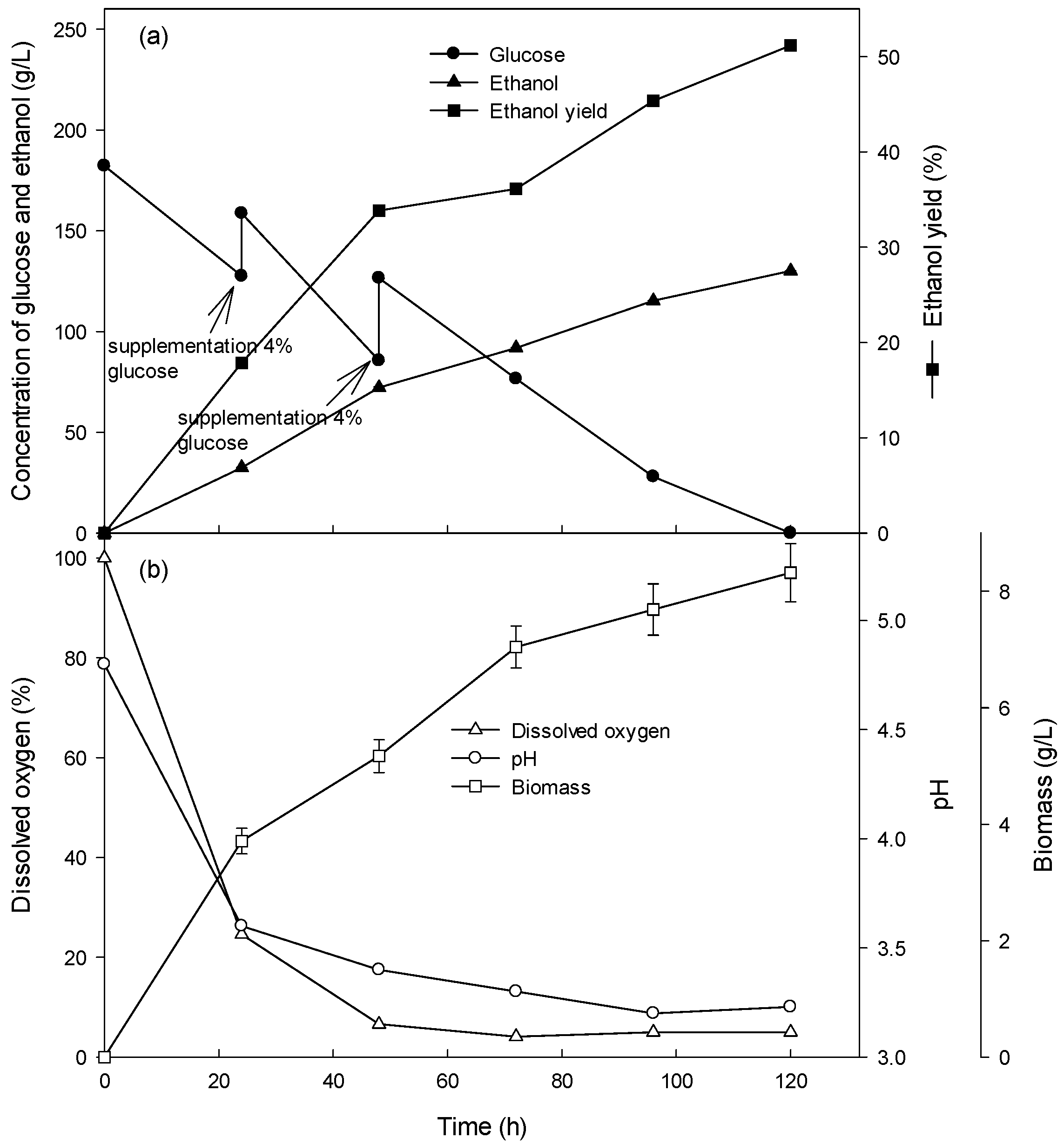
| Fermentation Type | Glucose Concentration (g/L) | Glucose Consumption Rate (g/L/h) | Volumetric Cell Mass Production Rate (g/L/h) | Specific Growth Rate (/h) | Ethanol Production Rate (g/L/h) |
|---|---|---|---|---|---|
| Batch fermentation | 10 | 0.71 | 0.04 | 0.01 | 1.02 |
| 40 | 1.99 | 0.09 | 0.05 | 1.12 | |
| 100 | 3.06 | 0.17 | 0.18 | 1.62 | |
| 150 | 3.13 | 0.12 | 0.14 | 1.61 | |
| 180 | 2.5 | 0.10 | 0.09 | 1.25 | |
| 200 | 2.08 | 0.07 | 0.07 | 1.08 | |
| 260 | 2.09 | 0.05 | 0.04 | 0.96 | |
| Fed-batch fermentation | 180 + 80 | 2.17 | 0.07 | 0.07 | 1.08 |
| Fermentation Type | Glucose Concentration (g/L) | Residual Glucose Concentration (g/L) | Maximal Cell Biomass (g/L) | Ethanol Concentration (g/L) | Theoretical Ethanol Yield (%) |
|---|---|---|---|---|---|
| Batch fermentation | 10 | ND | 0.2 ± 0.0 | 5.1 ± 0.0 | 98.4 ± 0.1 |
| 40 | ND | 1.7 ± 0.1 | 20.1 ± 0.6 | 98.2 ± 0.2 | |
| 100 | ND | 5.1 ± 0.2 | 48.7 ± 0.1 | 99.6 ± 0.4 | |
| 150 | ND | 7.0 ± 0.4 | 77.5 ± 0.2 | 100.0 ± 0.4 | |
| 180 | 0.2 ± 0.0 | 6.9 ± 0.4 | 90.3 ± 0.1 | 98.2 ± 0.3 | |
| 200 | 0.2 ± 0.0 | 7.0 ± 0.5 | 101 ± 0.1 | 97.6 ± 0.4 | |
| 260 | 9.1 ± 0.1 | 5.6 ± 0.2 | 115 ± 0.5 | 87.6 ± 0.6 | |
| Fed-batch fermentation | 180 + 80 | ND | 8.3 ± 0.8 | 130 ± 0.1 | 100.0 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Chang, K.-S.; Chen, C.-Y.; Hsu, C.-L.; Chang, T.-C.; Jang, H.-D. Enhancement of the Efficiency of Bioethanol Production by Saccharomyces cerevisiae via Gradually Batch-Wise and Fed-Batch Increasing the Glucose Concentration. Fermentation 2018, 4, 45. https://doi.org/10.3390/fermentation4020045
Chang Y-H, Chang K-S, Chen C-Y, Hsu C-L, Chang T-C, Jang H-D. Enhancement of the Efficiency of Bioethanol Production by Saccharomyces cerevisiae via Gradually Batch-Wise and Fed-Batch Increasing the Glucose Concentration. Fermentation. 2018; 4(2):45. https://doi.org/10.3390/fermentation4020045
Chicago/Turabian StyleChang, Yi-Huang, Ku-Shang Chang, Chien-Yu Chen, Chuan-Liang Hsu, Tsan-Chang Chang, and Hung-Der Jang. 2018. "Enhancement of the Efficiency of Bioethanol Production by Saccharomyces cerevisiae via Gradually Batch-Wise and Fed-Batch Increasing the Glucose Concentration" Fermentation 4, no. 2: 45. https://doi.org/10.3390/fermentation4020045
APA StyleChang, Y.-H., Chang, K.-S., Chen, C.-Y., Hsu, C.-L., Chang, T.-C., & Jang, H.-D. (2018). Enhancement of the Efficiency of Bioethanol Production by Saccharomyces cerevisiae via Gradually Batch-Wise and Fed-Batch Increasing the Glucose Concentration. Fermentation, 4(2), 45. https://doi.org/10.3390/fermentation4020045