Fumaric Acid Production: A Biorefinery Perspective
Abstract
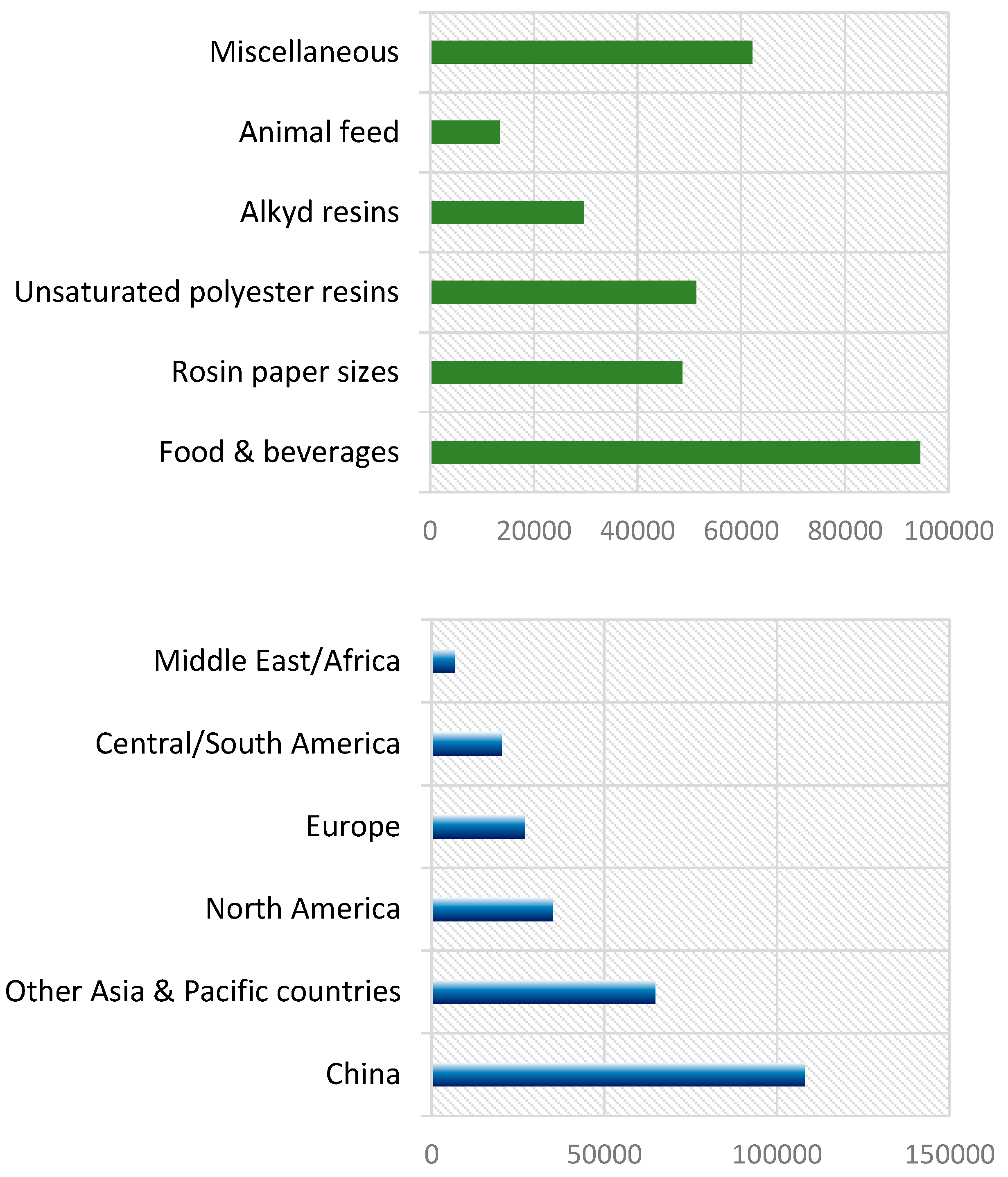
1. Introduction
2. Production Processes
2.1. Enzymatic Processes
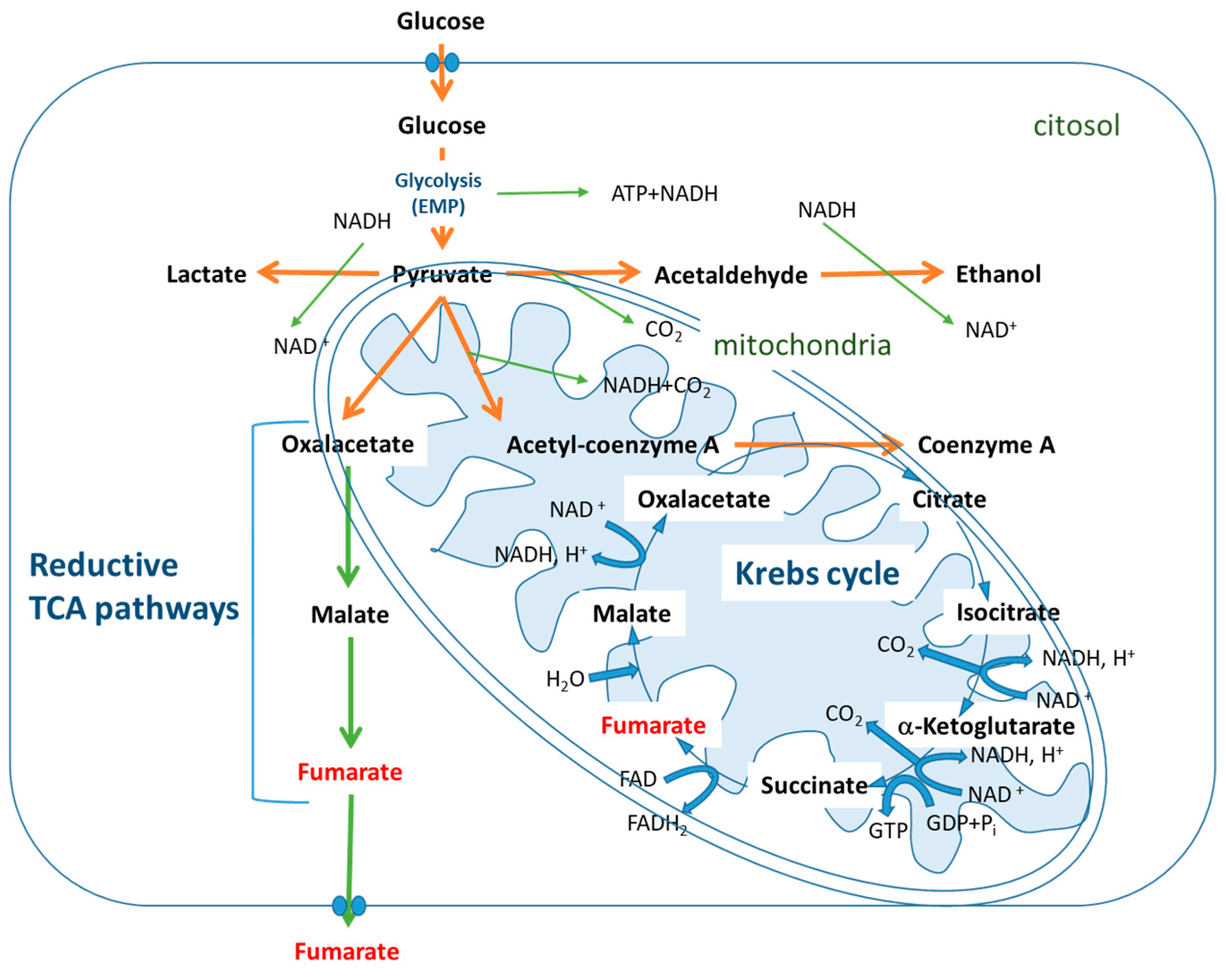
2.2. Fermentative Pathways
3. Microorganisms
3.1. Mutagenesis
3.2. Directed Evolution
4. Production Conditions
4.1. pH
4.2. Morphology
4.3. Alternative Substrates
4.3.1. Xylose
4.3.2. Glycerol
4.3.3. Apple Wastes
4.3.4. Brewery Wastewater
4.3.5. Food Wastes
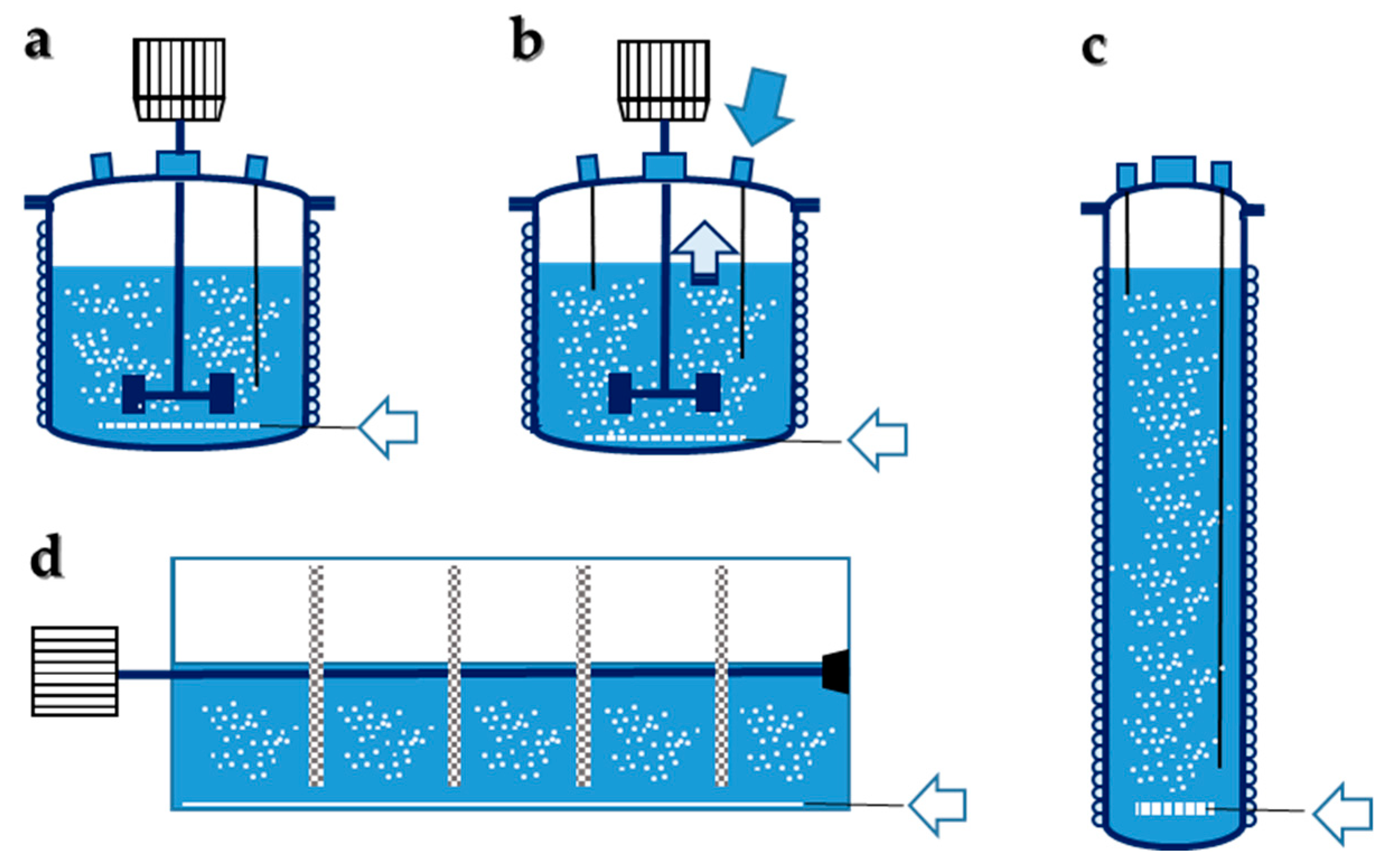
5. Bioreactors
5.1. Stirred Tanks
5.2. Bubble Column
5.3. Immobilized Biomass
5.3.1. Stirred Tank with Immobilized Biomass
5.3.2. Rotary Biofilm Contactor
5.3.3. Fluidized Bed
5.4. Comparison
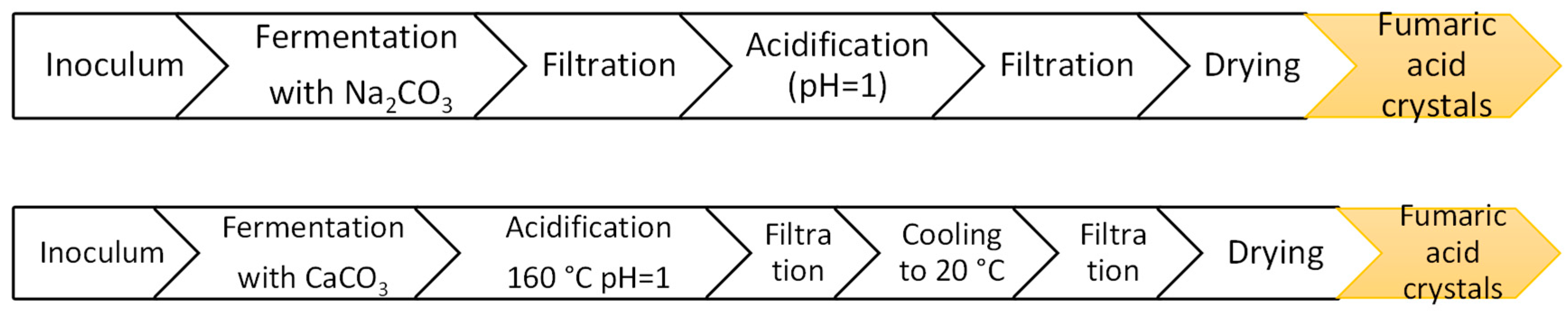
6. Downstream Processing: Fumaric Acid Purification
7. Conclusions and Future Prospects
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Safdel, M.; Anbaz, M.A.; Daryasafar, A.; Jamialahmadi, M. Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew. Sustain. Energy Rev. 2017, 74, 159–172. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Delucchi, M.A.; Bauer, Z.A.F.; Goodman, S.C.; Chapman, W.E.; Cameron, M.A.; Bozonnat, C.; Chobadi, L.; Clonts, H.A.; Enevoldsen, P.; et al. 100% Clean and Renewable Wind, Water, and Sunlight All-Sector Energy Roadmaps for 139 Countries of the World. Joule 2017, 1, 108–121. [Google Scholar] [CrossRef]
- Manevski, K.; Lærke, P.E.; Jiao, X.; Santhome, S.; Jørgensen, U. Biomass productivity and radiation utilisation of innovative cropping systems for biorefinery. Agric. For. Meteorol. 2017, 233, 250–264. [Google Scholar] [CrossRef]
- Corona, A.; Ambye-Jensen, M.; Vega, G.C.; Hauschild, M.Z.; Birkved, M. Techno-environmental assessment of the green biorefinery concept: Combining process simulation and life cycle assessment at an early design stage. Sci. Total Environ. 2018, 635, 100–111. [Google Scholar] [CrossRef] [PubMed]
- El-Halwagi, M.M. Sustainable Design Through Process Integration: Fundamentals and Applications to Industrial Pollution Prevention, Resource Conservation, and Profitability Enhancement; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Choi, S.; Song, C.W.; Shin, J.H.; Lee, S.Y. Biorefineries for the production of top building block chemicals and their derivatives. Metab. Eng. 2015, 28, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A Review and Techno-economical Evaluation. ChemBioEng Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- Lidén, G. Carboxylic Acid Production. Fermentation 2017, 3, 46. [Google Scholar] [CrossRef]
- Straathof, A.J.J. Transformation of Biomass into Commodity Chemicals Using Enzymes or Cells. Chem. Rev. 2014, 114, 1871–1908. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Chapter 8—Fumaric acid: Production and application aspects. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 133–157. [Google Scholar] [CrossRef]
- The National Institute of Standards and Technology (NIST). Fumaric Acid Properties. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=110-17-8 (accessed on 5 March 2018).
- Insight, R. Fumaric Acid Market Size, Price Trends, Research Report 2020. Available online: https://www.radiantinsights.com/research/fumaric-acid-market (accessed on 5 March 2018).
- Markit, I. Fumaric Acid: Chemical Economics Handbook. Available online: https://ihsmarkit.com/products/fumaric-acid-chemical-economics-handbook.html (accessed on 5 March 2018).
- Research, G.V. Malic Acid Market Size, Share, Trend Analysis Report By End-use (Beverage, Confectionery, Personal Care & Cosmetics), By Region, Competitive Landscape, and Segment Forecasts, 2018–2024. Available online: https://www.grandviewresearch.com/industry-analysis/malic-acid-market (accessed on 5 March 2018).
- Research, G.V. Aspartic Acid Market Analysis by Application (Feed Supplements, Medicine, Polyaspartic Acid, Aspartame, L-Alanine) and Segment Forecasts to 2022. Available online: https://www.grandviewresearch.com/industry-analysis/aspartic-acid-market (accessed on 5 March 2018).
- Farmer, T.; Castle, R.; Clark, J.; Macquarrie, D. Synthesis of Unsaturated Polyester Resins from Various Bio-Derived Platform Molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A. Tissue Engineering Bionanocomposites Based on Poly(propylene fumarate). Polymers 2017, 9, 260. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, S.; Oh, D.-H. Chitosan grafted monomethyl fumaric acid as a potential food preservative. Carbohydr. Polym. 2016, 152, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, D.-G. Preparation and Performance of Poly(butyl fumarate)-Based Material for Potential Application in LED Encapsulation. Materials 2017, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Roa Engel, C.A.; Straathof, A.J.J.; Zijlmans, T.W.; van Gulik, W.M.; van der Wielen, L.A.M. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kamra, D. Production of Methane by the Livestock and its Mitigation Techniques. Agric. Clim. Chang. Threats Strateg. Polic. 2017, 1, 261. [Google Scholar]
- Li, Z.; Liu, N.; Cao, Y.; Jin, C.; Li, F.; Cai, C.; Yao, J. Effects of fumaric acid supplementation on methane production and rumen fermentation in goats fed diets varying in forage and concentrate particle size. J. Anim. Sci. Biotechnol. 2018, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Recent advances in the biomedical applications of fumaric acid and its ester derivatives: The multifaceted alternative therapeutics. Pharmacol. Rep. 2016, 68, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Smith, D. Fumaric acid esters for psoriasis: A systematic review. Ir. J. Med. Sci. 2017, 186, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Luciani, S.; Esposti, E.D.; Cortelli, C.; Leanza, R. Surface Dynamics of A Vanadyl Pyrophosphate Catalyst for n-Butane Oxidation to Maleic Anhydride: An In Situ Raman and Reactivity Study of the Effect of the P/V Atomic Ratio. Chemi. A Eur. J. 2010, 16, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Liao, J.C. Frontiers in microbial 1-butanol and isobutanol production. FEMS Microbiol. Lett. 2016, 363, fnw020. [Google Scholar] [CrossRef] [PubMed]
- Pavarelli, G.; Ochoa, J.V.; Caldarelli, A.; Puzzo, F.; Cavani, F.; Dubois, J.-L. A New Process for Maleic Anhydride Synthesis from a Renewable Building Block: The Gas-Phase Oxidehydration of Bio-1-butanol. ChemSusChem 2015, 8, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Yamagishi, J.; Asano, Y. Maleate cis-trans isomerase from Arthrobacter sp. TPU 5446. J. Ferment. Bioeng. 1995, 80, 610–612. [Google Scholar] [CrossRef]
- Ichikawa, S.; Iino, T.; Sato, S.; Nakahara, T.; Mukataka, S. Improvement of production rate and yield of fumaric acid from maleic acid by heat treatment of Pseudomonas alcaligenes strain XD-1. Biochem. Eng. J. 2003, 13, 7–13. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lübeck, M.; Lübeck, P.S. Aspergillus as a versatile cell factory for organic acid production. Fungal Biol. Rev. 2017, 31, 33–49. [Google Scholar] [CrossRef]
- Jiménez-Quero, A.; Pollet, E.; Zhao, M.; Marchioni, E.; Averous, L.; Phalip, V. Fungal Fermentation of Lignocellulosic Biomass for Itaconic and Fumaric Acid Production. J. Microbiol. Biotechnol. 2017, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Z.; Deng, L.; Tan, T.; Wang, F.; Yan, Y. Pull-in urea cycle for the production of fumaric acid in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 5033–5044. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Lee, H.; Kim, D.; Lee, D.; Kim, S.; Chun, G.-T.; Lee, J.; Kim, S.W.; Park, C. Strain development and medium optimization for fumaric acid production. Biotechnol. Bioprocess Eng. 2010, 15, 761–769. [Google Scholar] [CrossRef]
- Yu, S.; Huang, D.; Wen, J.; Li, S.; Chen, Y.; Jia, X. Metabolic profiling of a Rhizopus oryzae fumaric acid production mutant generated by femtosecond laser irradiation. Bioresour. Technol. 2012, 114, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-Q.; Xu, Q.; Li, S.; Chen, Y.; Huang, H. Strain improvement of Rhizopus oryzae for over-production of fumaric acid by reducing ethanol synthesis pathway. Korean J. Chem. Eng. 2010, 27, 183–186. [Google Scholar] [CrossRef]
- Deng, Y.; Li, S.; Xu, Q.; Gao, M.; Huang, H. Production of fumaric acid by simultaneous saccharification and fermentation of starchy materials with 2-deoxyglucose-resistant mutant strains of Rhizopus oryzae. Bioresour. Technol. 2012, 107, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, S.-T. Metabolic engineering of Rhizopus oryzae: Effects of overexpressing fumR gene on cell growth and fumaric acid biosynthesis from glucose. Process Biochem. 2012, 47, 2159–2165. [Google Scholar] [CrossRef]
- Wen, S.; Liu, L.; Nie, K.L.; Deng, L.; Tan, T.W.; Wang, F. Enhanced Fumaric Acid Production by Fermentation of Xylose Using a Modified Strain of Rhizopus Arrhizus. BioResources 2013, 8, 2186–2194. [Google Scholar] [CrossRef]
- Huang, L.; Wei, P.; Zang, R.; Xu, Z.; Cen, P. High-throughput screening of high-yield colonies of Rhizopus oryzae for enhanced production of fumaric acid. Ann. Microbiol. 2010, 60, 287–292. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, C.; Xu, Q.; Li, S.; Huang, H.; Ouyang, P. Enhanced acid tolerance of Rhizopus oryzae during fumaric acid production. Bioprocess Biosyst. Eng. 2015, 38, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Q.; Lv, C.; Yan, C.; Li, S.; Jiang, L.; Huang, H.; Ouyang, P. Study of Metabolic Profile of Rhizopus oryzae to Enhance Fumaric Acid Production Under Low pH Condition. Appl. Biochem. Biotechnol. 2015, 177, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Application of calcium carbonate nanoparticles and microwave irradiation in submerged fermentation production and recovery of fumaric acid: A novel approach. RSC Adv. 2016, 6, 25829–25836. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, C.; Yang, S.-T. Effects of soybean meal hydrolysate as the nitrogen source on seed culture morphology and fumaric acid production by Rhizopus oryzae. Process Biochem. 2015, 50, 173–179. [Google Scholar] [CrossRef]
- Papadaki, A.; Androutsopoulos, N.; Patsalou, M.; Koutinas, M.; Kopsahelis, N.; Castro, A.; Papanikolaou, S.; Koutinas, A. Biotechnological Production of Fumaric Acid: The Effect of Morphology of Rhizopus arrhizus NRRL 2582. Fermentation 2017, 3, 33. [Google Scholar] [CrossRef]
- Ding, Y.; Li, S.; Dou, C.; Yu, Y.; Huang, H. Production of Fumaric Acid by Rhizopus oryzae: Role of Carbon–Nitrogen Ratio. Appl. Biochem. Biotechnol. 2011, 164, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Riscaldati, E.; Moresi, M.; Federici, F.; Petruccioli, M. Direct ammonium fumarate production by Rhizopus arrhizus under phosphorous limitation. Biotechnol. Lett. 2000, 22, 1043–1047. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Effects of Different Metallic Nanoparticles on Germination and Morphology of the Fungus Rhizopus oryzae 1526 and Changes in the Production of Fumaric Acid. BioNanoScience 2015, 5, 217–226. [Google Scholar] [CrossRef]
- Wang, G.; Huang, D.; Qi, H.; Wen, J.; Jia, X.; Chen, Y. Rational medium optimization based on comparative metabolic profiling analysis to improve fumaric acid production. Bioresour. Technol. 2013, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Hakim, S.H.; Zhou, S.; Won, W.; Hosseinaei, O.; Tao, J.; Garcia-Negron, V.; Motagamwala, A.H.; Mellmer, M.A.; Huang, K. Increasing the revenue from lignocellulosic biomass: Maximizing feedstock utilization. Sci. Adv. 2017, 3, e1603301. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet Explosion: A Universal and Efficient Pretreatment Process for Lignocellulosic Biorefineries. BioEnergy Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Li, C.; Zhang, D.; Xiao, Y.; Guan, G.; Zhu, W. Modification of chitosan with monomethyl fumaric acid in an ionic liquid solution. Carbohydr. Polym. 2015, 117, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.D.V.d.; Mancilha, I.M.d.; Silva, S.S.d.; Felipe, M.d.G.d.A. Improvement of biotechnological xylitol production by glucose during cultive of Candida guilliermondii in sugarcane bagasse hydrolysate. Braz. Arch. Biol. Technol. 2007, 50, 207–215. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Deng, L.; Wang, F.; Tan, T. High production of fumaric acid from xylose by newly selected strain Rhizopus arrhizus RH 7-13-9#. Bioresour. Technol. 2015, 186, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yue, X.; Jin, Y.; Wang, M.; Deng, L.; Wang, F.; Tan, T. Preparation of hydrolytic liquid from dried distiller’s grains with solubles and fumaric acid fermentation by Rhizopus arrhizus RH 7-13. J. Environ. Manag. 2017, 201, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, Y.; Li, S.; Jiang, L.; Huang, H.; Wen, J. Transcriptome analysis of Rhizopus oryzae in response to xylose during fumaric acid production. Bioprocess Biosyst. Eng. 2016, 39, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Monte, M.C.; Fuente, E.; Blanco, A.; Negro, C. Waste management from pulp and paper production in the European Union. Waste Manag. 2009, 29, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Potential use of pulp and paper solid waste for the bio-production of fumaric acid through submerged and solid state fermentation. J. Clean. Prod. 2016, 112, 4435–4444. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Ouyang, S.; Ouyang, J.; Yong, Q. Fumaric Acid Production from Alkali-Pretreated Corncob by Fed-Batch Simultaneous Saccharification and Fermentation Combined with Separated Hydrolysis and Fermentation at High Solids Loading. Appl. Biochem. Biotechnol. 2017, 181, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Quero, A.; Pollet, E.; Zhao, M.; Marchioni, E.; Averous, L.; Phalip, V. Itaconic and fumaric acid production from biomass hydrolysates by Aspergillus strains. J. Microbiol. Biotechnol. 2016, 26, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nie, K.; Zhang, X.; Liu, S.; Wang, M.; Deng, L.; Wang, F.; Tan, T. Production of fumaric acid from biodiesel-derived crude glycerol by Rhizopus arrhizus. Bioresour. Technol. 2014, 163, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, R.; Du, W.; Wang, G.; Xia, M. Activation of glycerol metabolic pathway by evolutionary engineering of Rhizopus oryzae to strengthen the fumaric acid biosynthesis from crude glycerol. Bioresour. Technol. 2015, 196, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, B.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Engineering Escherichia coli for fumaric acid production from glycerol. Bioresour. Technol. 2014, 174, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. A fermentative approach towards optimizing directed biosynthesis of fumaric acid by Rhizopus oryzae 1526 utilizing apple industry waste biomass. Fungal Biol. 2015, 119, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K. Enhanced Fumaric Acid Production from Brewery Wastewater and Insight into the Morphology of Rhizopus oryzae 1526. Appl. Biochem. Biotechnol. 2014, 172, 2974–2988. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Ladero, M. Food waste as a source of value-added chemicals and materials: A biorefinery perspective. Int. J. Food Sci. Technol. 2018, 53, 1095–1108. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Wang, M.; Wang, W.; Deng, L.; Nie, K.; Yue, X.; Wang, F.; Tan, T. Food Waste Fermentation to Fumaric Acid by Rhizopus arrhizus RH7-13. Appl. Biochem. Biotechnol. 2016, 180, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, S.; Jin, Y.; Yue, X.; Deng, L.; Wang, F.; Tan, T. Production of fumaric acid by immobilized Rhizopus arrhizus RH 7-13-9# on loofah fiber in a stirred-tank reactor. Bioresour. Technol. 2017, 244, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-Q.; Li, S.; Chen, Y.; Xu, Q.; Huang, H.; Sheng, X.-Y. Enhancement of Fumaric Acid Production by Rhizopus oryzae Using a Two-stage Dissolved Oxygen Control Strategy. Appl. Biochem. Biotechnol. 2010, 162, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.B.; Ng, T.K. Fermentation Process for Carboxylic Acids. U.S. Patent US4877731A, 31 October 1989. [Google Scholar]
- Zhou, Y.; Du, J.; Tsao, G. Comparison of fumaric acid production by Rhizopus oryzae using different neutralizing agents. Bioprocess Biosyst. Eng. 2002, 25, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Du, J.; Gong, C.S.; Tsao, G.T. Simultaneous Production and Recovery of Fumaric Acid from Immobilized Rhizopus oryzae with a Rotary Biofilm Contactor and an Adsorption Column. Appl. Environ. Microbiol. 1996, 62, 2926–2931. [Google Scholar] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Valorization of Egg Shell Biowaste and Brewery Wastewater for the Enhanced Production of Fumaric Acid. Waste Biomass Valoriz. 2015, 6, 535–546. [Google Scholar] [CrossRef]
- Gu, C.; Zhou, Y.; Liu, L.; Tan, T.; Deng, L. Production of fumaric acid by immobilized Rhizopus arrhizus on net. Bioresour. Technol. 2013, 131, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Petruccioli, M.; Angiani, E.; Federici, F. Semi-continuous fumaric acid production by Rhizopus arrhizus immobilized in polyurethane sponge. Process Biochem. 1996, 31, 463–469. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S. Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material – dairy manure – using a pelletized filamentous fungus Rhizopus oryzae ATCC 20344. Bioresour. Technol. 2008, 99, 5859–5866. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.H.; Bright, M.M.; Scott, W.E. Mechanism of fumaric acid accumulation in Rhizopus nigricans. J. Bacteriol. 1967, 93, 600–604. [Google Scholar] [PubMed]
- Lubowitz, H.R.; La, R.E.G. Fumaric Acid Fermentation Process. U.S. Patent US2861922A, 25 November 1958. [Google Scholar]
- Xu, Q.; Li, S.; Fu, Y.; Tai, C.; Huang, H. Two-stage utilization of corn straw by Rhizopus oryzae for fumaric acid production. Bioresour. Technol. 2010, 101, 6262–6264. [Google Scholar] [CrossRef] [PubMed]
- Tejayadi, S.; Cheryan, M. Lactic acid from cheese whey permeate. Productivity and economics of a continuous membrane bioreactor. Appl. Microbiol. Biotechnol. 1995, 43, 242–248. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Wang, D.; Dong, L.; Chen, R. Enhanced succinic acid productivity by expression of mgtCB gene in Escherichia coli mutant. J. Ind. Microbiol. Biotechnol. 2016, 43, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Yang, S.-T. Fumaric Acid Recovery and Purification from Fermentation Broth by Activated Carbon Adsorption Followed with Desorption by Acetone. Ind. Eng. Chem. Res. 2014, 53, 12802–12808. [Google Scholar] [CrossRef]
- Gemici, A.; Uslu, H.; Gök, A.; Kirbaşlar, Ş.İ. Effect of Diluents on the Extraction of Fumaric Acid by Tridodecyl Amine (TDA). J. Chem. Eng. Data 2015, 60, 919–924. [Google Scholar] [CrossRef]
- Figueira, D.; Cavalheiro, J.; Ferreira, B. Purification of Polymer-Grade Fumaric Acid from Fermented Spent Sulfite Liquor. Fermentation 2017, 3, 13. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, S.-T. In situ recovery of fumaric acid by intermittent adsorption with IRA-900 ion exchange resin for enhanced fumaric acid production by Rhizopus oryzae. Biochem. Eng. J. 2015, 96, 38–45. [Google Scholar] [CrossRef]
- Uslu, H.; Gemici, A.; Gök, A.; Kırbaşlar, Ş.İ. Reactive Extraction of (E)-Butenedioic Acid (Fumaric Acid) by Nontoxic Diluents. J. Chem. Eng. Data 2014, 59, 3767–3772. [Google Scholar] [CrossRef]
- Prochaska, K.; Staszak, K.; Woźniak-Budych, M.J.; Regel-Rosocka, M.; Adamczak, M.; Wiśniewski, M.; Staniewski, J. Nanofiltration, bipolar electrodialysis and reactive extraction hybrid system for separation of fumaric acid from fermentation broth. Bioresour. Technol. 2014, 167, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kang, M.-S.; Lee, C.-G.; Wang, N.-H.L.; Mun, S. Design of simulated moving bed for separation of fumaric acid with a little fronting phenomenon. J. Chromatogr. A 2017, 1491, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Gangl, I.C.; Weigand, W.A.; Keller, F.A. Economic comparison of calcium fumarate and sodium fumarate production by Rhizopus arrhizus. Appl. Biochem. Biotechnol. 1990, 24, 663–677. [Google Scholar] [CrossRef]
| Raw Material | Species | Broth Composition (ref) | Operational Conditions | Main Results |
|---|---|---|---|---|
| Glucose | Rhizopus arrhizus | 130 g·L−1 glucose 80 g·L−1 Ca2+ 1.8 g·L−1 NH4+ 0.3 g·L−1 KH2PO4 0.4 g·L−1 MgSO4·7 H2O 0.044 g·L−1 ZnSO4·7 H2O 0.0075 g·L−1 FeCl3·6 H2O | Strain NRRL 1526 Changes in stirring speed V = 3 L T = 34 °C N = 200–800 rpm pH = 5–7 Qair = 0.5 L·L−1·min−1 t = 6 day | Titer = 121 g·L−1 Productivity = 1.02 g·L−1·h−1 Yield = 0.37 gprod·gsust−1 Reference [72] |
| Several C/N ratios (140–200 gC·gN−1) 130 g·L−1 glucose 0.4 g·L−1 Yeast extract 0.4 g·L−1 MgSO4·7 H2O 0.044 g·L−1 ZnSO4·7 H2O 0.01 g·L−1 Fe tartrate 100 g·L−1 CaCO3 15 mL CH3OH 0.5 mL corn oil | Strain NRRL 1526 Phosphorus stress V = 3 L T = 32 °C N = 800 rpm pH = 5.5 Qair = 1 L·L−1·min−1 t = 6–10 day | Titer = 40 g·L−1 Productivity = 0.46 g·L−1·h−1 Yield = 0.33 gprod·gsust−1 Reference [48] | ||
| Rhizopus oryzae | 10% glucose 0.1% KH2PO4 0.05% MgSO4·7 H2O 0.002% ZnSO4·7 H2O 2.0% CaCO3 0.5% corn steep liquor Varied nitrogen source | Strain RUR709 V = 5 L T = 35 °C N = 400 rpm Qair = 1 L·L−1·min−1 t = 4 day | Titer = 32.1 g·L−1 Productivity = 0.32 g·L−1·h−1 Yield = 0.45 gprod·gsust−1 Reference [35] | |
| Rhizopus oryzae | 100 g·L−1 glucose 0.2 g·L−1 urea 0.6 g·L−1 KH2PO4 0.5 g·L−1 MgSO4·7 H2O 0.11 g·L−1 ZnSO4·7 H2O 0.0088 g·L−1 FeSO4·7 H2O 50 g·L−1 CaCO3 | Strain ME-F01 GMO (ME-UN-8) V = 5 L T = 35 °C N = 400 rpm pH = 5.5 Qair = 0.5 L·L−1·min−1 t = 4 day | Titer = 52.7 g·L−1 Productivity = 0.54 g·L−1·h−1 Reference [37] | |
| 100 g·L−1 glucose 2.0 g·L−1 urea 0.6 g·L−1 KH2PO4 0.5 g·L−1 MgSO4·7 H2O 0.11 g·L−1 ZnSO4·7 H2O 0.0088 g·L−1 FeSO4·7 H2O 50 g·L−1 CaCO3 | Strain ME-F12 Oxygen control (stage 1: 80% OD; stage 2: 30% OD) V = 7 L T = 35 °C t = 5 day | Titer = 56.2 g·L−1 Productivity = 0.7 g·L−1·h−1 Yield = 0.54 gprod·gsust−1 Reference [71] | ||
| Glucose | Rhizopus oryzae | 10 g·L−1 glucose 2.0 g·L−1 Urea 0.6 g·L−1 KH2PO4 0.25 g·L−1 MgSO4·7 H2O 0.088 g·L−1 FeSO4·7 H2O | Strain ATCC 20344 O2 and pH control V = 2 L T = 35 °C N = 600 rpm pH = 2.8–6.3 (pH0 = 3.2) Qair = 0.5 L·L−1·min−1 t = 7 day | Titer = 30.2 g·L−1 Productivity = 0.18 g·L−1·h−1 Yield = 0.28 gprod·gsust−1 Reference [21] |
| Manure | Rhizopus oryzae | 100 g·L−1 glucose 0.6 g·L−1 KH2PO4 0.25 g·L−1 MgSO4·7 H2O 0.088 g·L−1 ZnSO4·7 H2O Fiber hydrolases | Strain ATCC 20344 pH control V = 1 L T = 30 °C N = 200 rpm pH = 5 Qair = 1 L·L−1·min−1 t = 4 day | Titer = 31 g·L−1 Productivity = 0.31 g·L−1·h−1 Yield = 0.31 gprod·gsust−1 Reference [78] |
| Raw Material | Species | Broth Composition | Operational Conditions | Main Results |
|---|---|---|---|---|
| Glucose | Rhizopus nigricans | 50 g·L−1 glucose 2.0 g·L−1 (NH4)2SO4 0.5 g·L−1 KH2PO4 0.5 g·L−1 MgSO4·7 H2O 0.01 g·L−1 CaCl2 0.01 g·L−1 Fe2(SO4)3 | Strain 45 Isocitrate lyase extraction V = 0.25 L T = 28 °C pH = 7.0 t = 3.5 day | Titer = 20 g·L−1 Productivity = 0.25 g·L−1·h−1 Yield = 0.66 gprod·gsust−1 Reference [79] |
| Glycerol | Rhizopus arrhizus | 0–40 g·L−1 glucose 40–80 g·L−1 glycerol 0.3 g·L−1 peptone 1.55 g·L−1 KH2PO4 1.0 g·L−1 MgSO4·7 H2O 0.0176 g·L−1 ZnSO4·7 H2O 0.0005 g·L−1 FeSO4·7 H2O 50 g·L−1 CaCO3 | Strain RH-07-13 Crude glicerol alone (80 g·L−1) or Crude glicerol-glucose mixture (40 g·L−1 each) V = 0.25 L T = 30 °C N = 200 rpm pH = 5.5 t = 8 day | Titer = 22.8 g·L−1 Productivity = 0.16 g·L−1·h−1 Yield = 0.35 gprod·gsust−1 Reference [62] |
| Hydrolyzed molasses | Rhizopus oryzae | 107.1 g·L−1 sucrose 69.6 g·L−1 CaCO3 1.34 g·L−1 (NH4)2SO4 0.26 g·L−1 MgSO4·7 H2O 0.53 g·L−1 KH2PO4 0.042 g·L−1 ZnSO4·7 H2O 0.086 g·L−1 Fe2(SO4)3·H2O 0.01 g·L−1 MnSO4·H2O 0–150 mg·L−1 NiCl2·H2O | Invertase for hydrolysis Ni2+ effects T = 28 °C T = 6 day | Titer = 68 g·L−1 Productivity = 0.48 g·L−1·h−1 Yield = 0.64 gprod·gsust−1 Reference [80] |
| Corn stover | Rhizopus oryzae | 80 g·L−1 glu or xyl 0.2 g·L−1 urea 0.6 g·L−1 KH2PO4 0.5 g·L−1 MgSO4·7 H2O 0.11 g·L−1 ZnSO4·7 H2O 0.0088 g·L−1 FeSO4·7 H2O 50 g·L−1 CaCO3 | Strain ATC 20344 Diluted H2SO4 acid CS pretreatment: Liquid phase rich in xylose Enzymatic Hydrolysis: Liquid phase rich in glucose V = 0.25 L T = 35 °C N = 200 rpm pH = 3.0 (pH0 = 4.0) t = 3.5–4.5 day | Titer = 27.79 g·L−1 Productivity = 0.33 g·L−1·h−1 Yield = 0.35 gprod·gsust−1 Reference [81] |
| Xylose | Rhizopus arrhizus | 80–100 g·L−1 xylose 0.4 g·L−1 urea 0.6 g·L−1 KH2PO4 0.5 g·L−1 MgSO4·7 H2O 0.0018 g·L−1 ZnSO4·7 H2O 0.0005 g·L−1 FeSO4·7 H2O 45 g·L−1 CaCO3 | Strain RH-07-13 GMO Adapted to several xylose concentrations (50–100 g·L−1) V = 0.25 L T = 32 °C N = 220 rpm pH = 5.5 t = 7 day | Titer = 38.48 g·L−1 Productivity = 0.23 g·L−1·h−1 Yield = 0.43 gprod·gsust−1 Reference [40] |
| Hydrolyzed yucca bagasse and potato residue | Rhizopus formosa | Weight ratio 80:20 yucca bagasse: potato waste Weight C/N: 168 (C = cassava bagasse; N = KNO3) 0.15 g·L−1 KH2PO4 0.25 g·L−1 MgSO4·7 H2O 0.04 g·L−1 ZnSO4·7 H2O 20 g·L−1 CaCO3 10 g·L−1 biotin 15 mL·L−1 CH3OH | Strain MUCL 28422 Two stage raw material enzymatic hydrolysis T = 32 °C N = 200 rpm pH = 6.5 | Titer = 21.28 g·L−1 Yield = 0.23 gprod·gsust−1 Reference [38] |
| Raw Material | Species | Broth Composition | Reactor Type and Operational Conditions | Main Results |
|---|---|---|---|---|
| Glucose | Rhizopus oryzae | 95 g·L−1 glucose 0.3 g·L−1 urea 0.1 g·L−1 KH2PO4 0.05 g·L−1 MgSO4·7 H2O 0.01 g·L−1 ZnSO4·7 H2O | Bubble column Strain ATCC 20344 Effects of basic agents (CaCO3, Ca(OH)2 and NaHCO3) V = 10 L T = 32 °C pH = 5.5 Qair = 1.5 L·L−1·min−1 t = 1.5 day | Titer = 37.2 g·L−1 Productivity = 1.03 g·L−1·h−1 Yield = 0.53 gprod·gsust−1 Reference [73] |
| Rhizopus arrhizus | 80 g·L−1 glucose 0.044 g·L−1 urea 0.6 g·L−1 KH2PO4 0.5 g·L−1 MgSO4·7 H2O 0.002 g·L−1 ZnSO4·7 H2O 60 g CaCO3 6 mL soybean oil | Batch slurry reactor GMO strain RH-7-139# Effects of support (stainless steel, loofah fiber, sponge) V = 5 L, 5 g L−1 support T = 30 °C Qair = 2.0 L·L−1·min−1 N = 300–500 rpm t = 120 h | Best N = 400 rpm Titer = 30.3 g·L−1 Productivity = 0.63 g·L−1·h−1 Yield = 0.211 gprod·gsust−1 Reference [70] | |
| Hydrolyzed molasses | Rhizopus oryzae | 100 g·L−1 glucose 2.0 g·L−1 urea 0.6 g·L−1 KH2PO4 0.25 g·L−1 MgSO4·7 H2O 0.088 g·L−1 ZnSO4·7 H2O Base CaCO3 | Rotating biofilm reactor Strain ATCC 20344 Compares RBC with batch reactor V = 2 L Biodisc surface: 750 cm2 T = 35 °C pH = 5 Qair = 1.0 L·L−1·min−1 t = 20 h | Titer = 85.0 g·L−1 Productivity = 4.25 g·L−1·h−1 Theoretical yield = 0.91 gprod·gsust−1 Reference [74] |
| Corn stover | Rhizopus arrhizus | 50 g·L−1 glucose molasses 0.25 g·L−1 (NH4)2SO4 0.4 g·L−1 yeast extract 0.45 g·L−1 corn oil 0.3 g·L−1 KH2PO4 0.4 g·L−1 MgSO4·7 H2O 0.044 g·L−1 ZnSO4·7 H2O 0.01 g·L−1 Fe tartrate | Fluidized bed reactor Strain NRRL 1526 Polyurethane immobilized microorganisms V = 0.25 L T = 32 °C pH = 6.0 Qair = 3.0 L·L−1·min−1 t = 1–3 day | Titer = 17.5 g·L−1 Productivity = 0.36 g·L−1·h−1 Yield = 0.36 gprod·gsust−1 Reference [77] |
| Reactor | Final Concentration | Productivity | Yield | Production Costs |
|---|---|---|---|---|
| Bubble column | = | ++ | + | = |
| Biodisc | ++ | ++++ | ++ | − |
| Fluidized bed | − | − | = | − |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Dominguez, V.; Estevez, J.; Ojembarrena, F.D.B.; Santos, V.E.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 33. https://doi.org/10.3390/fermentation4020033
Martin-Dominguez V, Estevez J, Ojembarrena FDB, Santos VE, Ladero M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation. 2018; 4(2):33. https://doi.org/10.3390/fermentation4020033
Chicago/Turabian StyleMartin-Dominguez, Victor, Juliana Estevez, Francisco De Borja Ojembarrena, Victoria E. Santos, and Miguel Ladero. 2018. "Fumaric Acid Production: A Biorefinery Perspective" Fermentation 4, no. 2: 33. https://doi.org/10.3390/fermentation4020033
APA StyleMartin-Dominguez, V., Estevez, J., Ojembarrena, F. D. B., Santos, V. E., & Ladero, M. (2018). Fumaric Acid Production: A Biorefinery Perspective. Fermentation, 4(2), 33. https://doi.org/10.3390/fermentation4020033







