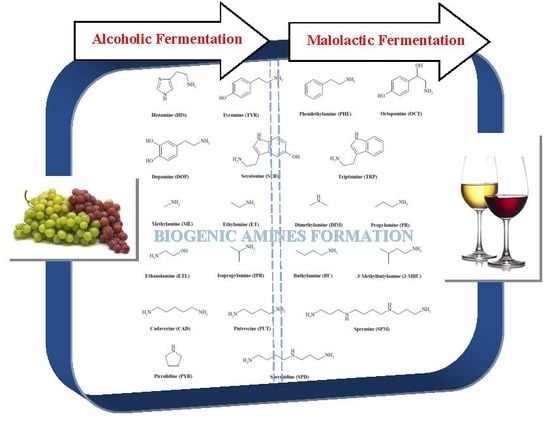
Accumulation of Biogenic Amines in Wine: Role of Alcoholic and Malolactic Fermentation
Abstract
1. Introduction
2. Analytical Determination of BAs in Wine
3. BAs and Alcoholic Fermentation
4. BAs and Malolactic Fermentation
5. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented food. Eur. J. Clin. Nutr. 2010, 64, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdan, T.; Martinez-Gil, A.M.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Implications of nitrogen compounds during alcoholic fermentation from some grape varieties at different maturation stages and cultivation systems. Food Chem. 2011, 124, 106–116. [Google Scholar] [CrossRef]
- Smit, A.Y.; du Toit, W.J.; du Toit, M. Biogenic amines in wine: Understanding the headache. S. Afr. J. Enol. Vitic. 2008, 29, 109–127. [Google Scholar] [CrossRef]
- Polo, L.; Ferrer, S.; Pea-Gallego, A.; Hernndez-Orte, P.; Pardo, I. Biogenic amine synthesis in high quality Tempranillo wines. Relationship with lactic acid bacteria and vinification conditions. Ann. Microbiol. 2010, 61, 191–198. [Google Scholar] [CrossRef]
- Martuscelli, M.; Arfelli, G.; Manetta, A.C.; Suzzi, G. Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chem. 2013, 140, 590–597. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No. 2073/2005 of November 2005 on Microbiological Criteria for Foodstuffs. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32005R2073:en:NOT (accessed on 18 July 2017).
- Ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huis in’t Veld, J.H.H. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Bremer, P.J.; Fletcher, G.C.; Osborne, C. Scombrotoxin in Seafood; New Zealand Institute for Crop and Food Research Limited: Christchurch, New Zealand; A Crown Research Institute: Wellington, New Zealand, 2003. Available online: www.crop.cri.nz/home/research/marine/pathogens/Scombrotoxin.pdf (accessed on 16 March 2011).
- Anonymous; Australian Food Standards Code 2001. Part D: Fish and fish products. In Standards D1 and D2; Version 18; ANSTAT: South Melbourne, VIC, Australia, 2001. [Google Scholar]
- Anonymous. Chapter 7: Scombrotoxin (histamine) formation (a chemical hazard). In Fish and Fisheries Products Hazards and Controls Guidance, 3rd ed.; Office Food Safety, Center for Food Safety and Applied Nutrition, Food and Drug Administration: Washington, DC, USA, 2001. Available online: http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/Seafood/FishandFisheriesProductsHazardsandControlsGuide/ucm089637.htm (accessed on 16 March 2011).
- Anli, R.E.; Vural, N.; Yilmaz, S.; Vural, Y.H. The determination of biogenic amines in Turkish red wines. J. Food Compos. Anal. 2004, 17, 53–62. [Google Scholar] [CrossRef]
- Onal, A.; Tekkeli, S.E.; Onal, C. A review of the liquid chromatographic methods for the determination of biogenic amines in foods. Food Chem. 2013, 138, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Anli, E.R.; Bayram, M. Biogenic Amines in Wines. Food Rev. Int. 2009, 25, 86–102. [Google Scholar] [CrossRef]
- Ancin-Azpilicueta, C.; Gonzalez-Marco, A.; Jimenez-Moreno, N. Current knowledge about the presence of amines in wine. Crit. Rev. Food Sci. Nutr. 2008, 48, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Mangani, S.; Geurrini, S.; Granchi, L.; Vincenzini, M. Putrescine accumulation in wine: Role of Oenococcus oeni. Curr. Microbiol. 2005, 51, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Beneduce, L.; Romano, A.; Capozzi, V.; Lucas, P.; Barnavon, L.; Bach, B.; Vuchot, P.; Grieco, F.; Spano, G. Biogenic amine in wines. Ann. Microbiol. 2010, 60, 573–578. [Google Scholar] [CrossRef]
- Cecchini, F.; Morassut, M. Effect of grap storage time on biogenic amines content in must. Food Chem. 2010, 123, 263–268. [Google Scholar] [CrossRef]
- Del Prete, V.; Costantini, A.; Cecchini, F.; Morassut, M.; Garcia-Moruno, E. Occurrence of biogenic amines in wine: The role of grapes. Food Chem. 2009, 112, 474–481. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Yang, Y.-P.; Peng, Q.; Han, Y. Biogenic amines in wine: A review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Önal, A. Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Coton, M.; Romano, A.; Spano, G.; Ziegler, K.; Vetrano, C.; Desmarias, C.; Lonvaud-Funel, A.; Lucas, P.; Coton, E. Occurence of biogenic amine forming lactic acid bacteria in wine and cider. Food Microbiol. 2010, 27, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- BediaErim, F. Recent analytical approaches to the analysis of biogenic amines in food samples. Trends Anal. Chem. 2013, 52, 239–247. [Google Scholar] [CrossRef]
- De Borba, B.M.; Rohrer, J.S. Determination of biogenic amines in alcoholic beverages by ion chromatography with suppressed conductivity detection and integrated pulsed amperometric detection. J. Chromatogr. A 2007, 115, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Piasta, A.M.; Jastrzebska, A.; Krzeminski, M.P.; Muzioł, T.M.; Szłyk, E. New procedure of selected biogenic amines determination in wine samples by HPLC. Anal. Chim. Acta 2014, 834, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; GarcíaCañas, V.; Simo, C.; Cifuentes, A. Recent advances in the application of capillary electromigration methods for food analysis and foodomics. Electrophoresis 2010, 31, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.O.; Ferreira, M.A. Combined ion-pair extraction and gas chromatography-mass spectrometry for the simultaneous determination of diamines, polyamines and aromatic amines in port wine and grape juice. J. Chromatogr. A 2000, 886, 183–195. [Google Scholar] [CrossRef]
- Lange, J.; Wittmann, C. Enzyme sensor array for the determination of biogenic amines in food samples. Anal. Bioanal. Chem. 2002, 372, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Peña-Gallego, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. High-Performance Liquid Chromatography Analysis of Amines in Must and Wine: A Review. Food Rev. Int. 2012, 28, 71–96. [Google Scholar] [CrossRef]
- Berbegal, C.; Pardo, I.; Ferrer, S. The use of core-shell high-performance liquid chromatography column technology to improve biogenic amine quantification in wine. J. Sci. Food Agric. 2016, 96, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cassou, S.; Saurina, J. Derivatization strategies for the determination of biogenic amines in wines by chromatographic and electrophoretic techniques. J. Chromatogr. B 2011, 879, 1270–1281. [Google Scholar]
- Romero, R.; Jönsson, J.Å.; Gázquez, D.; GraciaBagur, M.; Sánchez-Vinas, M. Multivariate optimization of supported liquid membrane extraction of biogenic amines from wine samples prior to liquid chromatography determination as dabsyl derivatives. J. Sep. Sci. 2002, 25, 584–592. [Google Scholar] [CrossRef]
- Pineda, A.; Carrasco, J.; Peña-Farfal, C.; Henríquez-Aedo, K.; Aranda, M. Preliminary evaluation of biogenic amines content in Chilean young varietal wines by HPLC. Food Control 2012, 23, 251–257. [Google Scholar] [CrossRef]
- Özdestan, Ö.; Üren, A. A method for benzoyl chloride derivatization of biogenic amines for high performance liquid chromatography. Talanta 2009, 78, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.; Santos, L.; Alves, A. Simultaneous quantification of primary, secondary amino acids and biogenic amines in musts and wines using OPA/3-MPA/FMOC-Cl fluorescent derivatives. J. Food Sci. 2001, 66, 1319–1325. [Google Scholar] [CrossRef]
- Garcıá-Marino, M.; Trigueros, Á.; Escribano-Bailon, T. Influence of oenological practices on the formation of biogenic amines in quality red wines. J. Food Compos. Anal. 2010, 23, 455–462. [Google Scholar] [CrossRef]
- Nouadje, G.; Simeon, N.; Dedieu, F.; Nertz, M.; Pulg, P.; Couderc, F. Determination of twenty eight biogenic amines and amino acids during wine aging by micellar electrokinetic chromatography and laser-induced fluorescence detection. J. Chromatogr. A 1997, 765, 337–343. [Google Scholar] [CrossRef]
- García-Villar, N.; Hernández-Cassou, S.; Saurina, J. Determination of biogenic amines in wines by pre-column derivatization and high-performance liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2009, 1216, 6387–6393. [Google Scholar] [CrossRef] [PubMed]
- Bach, B.; Le Quere, S.; Vuchot, P.; Grinbaum, M.; Barnavon, L. Validation of a method for the analysis of biogenic amines: Histamine instability during wine sample storage. Anal. Chim. Acta 2012, 732, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Callejon, R.M.; Troncoso, A.M.; Morales, M.L. Determination of amino acids in grape-derived products: A review. Talanta 2010, 81, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Kang, Y.P.; Park, J.H.; Lee, J.; Kwon, S.W. Determination of biogenic amines in Bokbunja (Rubus coreanus Miq.) wines using a novel ultra-performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry. Food Chem. 2012, 132, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; Schieberle, P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agric. Food Chem. 2012, 60, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Klebanowski, H.; La Guerche, S.; Beneduce, L.; Spano, G.; Murat, M.L.; Lucas, P. Determination of biogenic amines in wine by thin-layer chromatography/densitometry. Food Chem. 2012, 135, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, J.L.; Troncoso, A.M.; García-Parrilla, M.D.C.; Callejon, R.M. Recent trends in the determination of biogenic amines in fermented beverages—A review. Anal. Chim. Acta 2016, 939, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Faria, M.A.; Fernandes, J.O. Gas chromatography-mass spectrometry assessment of amines in port wine and grape juice after fast chloroformate extraction/derivatization. J. Agric. Food Chem. 2011, 59, 8742–8753. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.; Dos Santos, V.B.; Vidal, D.T.R.; do Lago, C.L. Determination of biogenic amines in beer and wine by capillary electrophoresis-tandem mass spectrometry. J. Chromatogr. A 2015, 1416, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Martín-Álvarez, P.J.; Marcobal, Á.; Polo, C.; Moreno-Arribas, M.V. Influence of technological practices on biogenic amine contents in red wines. Eur. Food Res. Technol. 2006, 222, 420–424. [Google Scholar] [CrossRef]
- Martìnez-Pinilla, O.; Guadalupe, Z.; Hernàndez, Z.; Ayestaràn, B. Amino acids and biogenic amines in red varietal wines: The role of grape variety, malolactic fermentation and vintage. Eur. Food Res. Technol. 2013, 237, 887–895. [Google Scholar] [CrossRef]
- Torrea, D.; Ancin, C. Influence of yeast strain on biogenic amines content in wines: Relationship with the utilization of amino acids during fermentation. Am. J. Enol. Vitic. 2001, 52, 185–190. [Google Scholar]
- Torrea, D.; Ancín, C. Content of biogenic amines in a Chardonnay wine obtained through spontaneous and inoculated fermentation. J. Agric. Food Chem. 2002, 50, 4895–4899. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Ferrer, S.; Pardo, I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control 2007, 18, 1569–1574. [Google Scholar] [CrossRef]
- Caruso, M.; Fiorel, C.; Contursi, M.; Salzano, G.; Paparella, A.; Romano, P. Formation of biogenic amines as criteria for the selection of wine yeasts. World J. Microbiol. Biotechnol. 2002, 18, 159–163. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Ye, D.-Q.; Zhu, B.-Q.; Wu, G.-F.; Duan, C.-Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, Á.; de las Rivas, B.; Moreno-Arribas, M.V.; Muñoz, R. Multiplex PCR method for the simultaneous detection of histamine, tyramine and putrescine producing lactic acid bacteria in foods. J. Food Prot. 2005, 68, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Carou, M.C.; Codony-Salcedo, R.; Mariné-Font, A. Histamine and tyramine in Spanish wines: Relationship with total sulfur dioxide level, volatile acidity and malolactic fermentation intensity. Food Chem. 1990, 35, 217–227. [Google Scholar] [CrossRef]
- López-Rituerto, E.; Avenoza, A.; Busto, J.H.; Peregrina, J.M. NMR Study of histidine metabolism during alcoholic and malolactic fermentations of wine and their influence on histamine production. J. Agric. Food Chem. 2013, 61, 9464–9469. [Google Scholar] [CrossRef] [PubMed]
- Granchi, L.; Romano, P.; Mangani, S.; Guerrini, S.; Vincenzini, M. Production of biogenicamines by winemicroorganisms. Bull. OIV 2005, 78, 595–609. [Google Scholar]
- Vigentini, I.; Romano, A.; Compagno, C.; Merico, A.; Molinari, F.; Tirelli, A.; Foschino, R.; Volonterio, G. Physiological and oenological traits of differentDekkera/Brettanomycesbruxellensisstrains under wine-model conditions. FEMS Yeast Res. 2008, 8, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.Y.; du Toit, W.J.; Stander, M.; du Toit, M. Evaluating the influence of maceration practices on biogenic amine formation in wine. LWT Food Sci. Technol. 2013, 53, 297–307. [Google Scholar] [CrossRef]
- Marques, A.P.; Leităo, M.C.; San Romăo, M.C. Biogenic amines in wines: Influence of oenological factors. Food Chem. 2008, 107, 853–860. [Google Scholar] [CrossRef]
- Tofalo, R.; Patrignani, F.; Lanciotti, R.; Perpetuini, G.; Schirone, M.; Di Gianvito, P.; Pizzoni, D.; Arfelli, G.; Suzzi, G. Aroma profile of Montepulciano d’Abruzzo wine fermented by single and co-culture starters of autochthonous Saccharomyces and non-saccharomyces yeasts. Front. Microbiol. 2016, 610, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Sicari, V.; Pellicanò, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Jeffares, D.; Palomero, F.; Calderón, F.; Bai, F.-Y.; Bähler, J.; Benito, S. Selected Schizosaccharomyces pombe strains have characteristics that are beneficial for winemaking. PLoS ONE 2016, 11, e0151102. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, M.W.; Rodriguez, S.B.; Honey, N.K.; Thornton, R.J. Purification and characterization of urease from Schizosaccharomyces pombe. Can. J. Microbiol. 1996, 42, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar]
- Gardini, F.; Zaccarelli, A.; Belletti, N.; Faustini, F.; Cavazza, A.; Martuscelli, M.; Mastrocola, D.; Suzzi, G. Factors influencing biogenic amine production by a strain of Oenococcus oeni in a model system. Food Control 2005, 16, 609–616. [Google Scholar] [CrossRef]
- Garai, G.; Dueñas, M.T.; Irastorza, A.; Moreno-Arribas, M.V. Biogenic amine production by lactic acid bacteria isolated from cider. Lett. Appl. Microbiol. 2007, 45, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.M.; Wolken, W.A.M.; Claisse, O.; Lolkema, J.S.; Lonvaud-Funel, A. Histamine-producing pathway encoded on an unstable plasmid in Lactobacillus hilgardii 0006. Appl. Environ. Microbiol. 2005, 71, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Garcia-Moruno, E.; Muñoz, R. Does Oenococcus oeni produce histamine? Int. J. Food Microbiol. 2012, 157, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Coton, E.; Rollan, G.; Bertrand, A.; Lonvaud-Funel, A. Histamine-producing lactic acid bacteria in wines: Early detection, frequency and distribution. Am. J. Enol. Vitic. 1998, 49, 199–204. [Google Scholar]
- Landete, J.M.; Ferrer, S.; Polo, L.; Pardo, I. Biogenic amines in wines from three Spanish regions. J. Agric. Food Chem. 2005, 53, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, V.; Polo, M.C.; Jorganes, F.; Muñoz, R. Screening of biogenic amine production by lactic acid bacteria isolated from grape must and wine. Int. J. Food Microbiol. 2003, 84, 117–123. [Google Scholar] [CrossRef]
- Berbegal, C.; Benavent-Gil, Y.; Navascués, E.; Pardo, I.; Ferrer, S. Lowering histamine formation in a red Ribera del Duero wine (Spain) by using an indigenous O. oeni strain as a malolactic starter. Int. J. Food Microbiol. 2017, 244, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, V.; Torlois, S.; Joyex, A.; Bertrand, A.; Lonvaud-Funel, A. Isolation, properties and behaviour of tyramineproducing lactic acid bacteria from wine. J. Appl. Microbiol. 2000, 88, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, Á.; de las Rivas, B.; Muñoz, R. Methods for the detection of bacteria producing biogenic amines on foods: A survey. J. Consum. Prot. Food Saf. 2006, 1, 187–196. [Google Scholar] [CrossRef]
- Manfroi, L.; Silva, P.H.A.; Rizzon, L.A.; Sabaini, P.S.; Gloria, M.B.A. Influence of alcoholic and malolactic starter cultures on bioactive amines in Merlot wines. Food Chem. 2009, 116, 208–213. [Google Scholar] [CrossRef]
- Arena, M.E.; Manca, de Nadra, M.C. Biogenic amine production by Lactobacillus. J. Appl. Microbiol. 2001, 90, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Aedo, K.; Duràn, D.; Garcia, A.; Hengst, M.B.; Aranda, M. Identification of biogenic amines-producing lactic acid bacteria isolated from spontaneous malolactic fermentation of chilean red wines. LWT Food Sci. Technol. 2016, 68, 183–189. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Biogenic amines in wines: Role of lactic acid bacteria. FEMS Microbiol. Lett. 2001, 199, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martín, F.; Seseña, S.; Izquierdo, P.M.; Palop, M.L. Are Enterococcus populations present during malolactic fermentation of red wine safe? Food Microbiol. 2014, 42, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Ladero, V.; Beneduce, L.; Fernández, M.; Alvarez, M.A.; Benoit, B.; Laurent, B.; Grieco, F.; Spano, G. Isolation and characterization of tyramine-producing Enterococcus faecium strain from red wine. Food Microbiol. 2011, 28, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Izquierdo, P.M.; Seseña, S.; Palop, M.L. Bacterial biodiversity and dynamics during malolactic fermentation of Tempranillo wines as determined by a culture-independent method (PCR-DGGE). Appl. Microbiol. Biotechnol. 2010, 86, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Martín, B.; Garriga, M.; Hugas, M.; Bover-Cid, S.; Veciana-Nogués, M.T.; Aymerich, T. Molecular, technological and safety characterization of gram-positive catalase-positive cocci from slightly fermented sausages. Int. J. Food Microbiol. 2006, 107, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Benavent-Gil, Y.; Berbegal, C.; Lucio, O.; Pardo, I.; Ferrer, S. A new fear in wine: Isolation of Staphylococcus epidermidis histamine producer. Food Control 2016, 62, 142–149. [Google Scholar] [CrossRef]
- Ruiz, P.; Izquierdo, P.M.; Seseña, S.; Palop, M.L. Selection of autochthonous Oenococcus oeni strains according to their oenological properties and vinification results. Int. J. Food Microbiol. 2010, 137, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Orte, P.; Lapeña, A.C.; Peña-Gallego, A.; Astrain, J.; Baron, C.; Pardo, I. Biogenic amine determination in wine fermented in oak barrels. Factors affecting formation. Food Res. Int. 2008, 41, 697–706. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.Y.; Engelbrecht, L.; duToit, M. Managing your wine fermentation to reduce the risk of biogenic amine formation. Front. Microbiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Buteau, C.; Duitschaever, C.L.; Ashton, G.C. A study of the biogenesis of amines in a Villard Noir Wine. Am. J. Enol. Vitic. 1984, 35, 228–236. [Google Scholar]
- Costantini, A.; Vaudano, E.; delPrete, V.; Danei, M.; García-Moruno, E. Biogenic amine production by contaminating bacteria found in starter preparations used in winemaking. J. Agric. Food Chem. 2009, 57, 10664–10669. [Google Scholar] [CrossRef] [PubMed]
| Sample | BAs | Microorganism/Spontaneous | Analytical Method | Total BAs Content (mg/L) (Harvest Year) | Reference |
|---|---|---|---|---|---|
| Tempranillo | HIS; MET; ETH; TYR; PHE; PUT; CAD | Spontaneous | Derivatization with o-phthaldialdehyde and separation with HPLC coupled to fluorescence detector. | 22.37 (2004) | [47] |
| Merlot | AGM; ETA; ETH; PUT; TYR | S. cerevisiae (106 cells/mL) | Derivatization with o-phthaldialdehyde and separation with HPLC coupled to fluorescence detector. | 6.69 (2004) 25.87 (2005) | [19] |
| Syrah | 9.70 (2004) 23.67 (2005) | ||||
| Cabernet Franc. | 9.65 (2004) 14.02 (2005) | ||||
| Montepulciano | 12.01 (2004) 21.31 (2005) | ||||
| Sangiovese | 10.96 (2004) 18.11 (2005) | ||||
| Carmenere | 8.94 (2004) 20.59 (2005) | ||||
| Cesanese d’Affile | 15.62 (2004) 27.53 (2005) | ||||
| Montepulciano d’Abruzzo | CAD; TRY; PHE; TYR; HIS; ETA; ETH; PUT | S. cerevisiae SRS1 (106 cells/mL) | Derivatization with dansyl chloride and separation by HPLC coupled with PDA detector. | 21–24 (2011) | [61] |
| Arvino | PHE; PUT; HIS; TYR; SPD; SPM | Spontaneous | Derivatization with dansyl chloride and separation with RP-LC-UV with gradient elution (solvents water and acetonitrile). | 23.7 (2016) | [62] |
| Gaglioppo | 41.9 (2016) | ||||
| Greco Nero | 44.0 (2016) | ||||
| Magliocco Canino | 63.1 (2016) | ||||
| Magliocco Dolce | 36.6 (2016) | ||||
| Nocera | 46.8 (2016) | ||||
| Tempranillo | HIS; AGM; SPD; TYR; PUT; TRY; CAD; PHE | Spontaneous | Derivatization with diethyl ethoxy methylene malonate and separation by RP-LC-UV with gradient elution. | 14.6 (2009) 6.9 (2010) | [48] |
| Monastel | 14.3 (2009) 10.2 (2010) | ||||
| Maturana Tinta de Navarrete | 13.9 (2009) 9.6 (2010) | ||||
| Aglianico of Vulture | ETA; MET; AGM; TRY; PHE; PUT; CAD; HIS | Dekkera/B. bruxellensis (5%) | Derivatization with dansyl chloride and separation with RP-LC-UV with gradient elution (solvents water and acetonitrile). | 15.01 (2000) | [52] |
| S. cerevisiae (5%) | 12.4 (2000) | ||||
| Kloeckeraapiculata (5%) | 6.21 (2000) | ||||
| Candida stellata (5%) | 6.73 (2000) | ||||
| Metschnikowiapulcherrima (5%) | 9.60 (2000) | ||||
| Italian red wine | PUT; CAD; SPM | Dekkera/B. bruxellensis (CBS2336 and CBS4601) | Derivatization with dabsyl-chloride and separation with separation with RP-LC-UV with gradient elution (Water and acetonitrile). | 0.40 (2006) | [58] |
| Tempranillo | HIS; TYR; PHE; PUT; CAD | Kluyveromycesthermotolerans/Schizosaccharomyces pombe V2 | UHPLC coupled to fluorescence detector and separation with separation gradient elution (A: methanol/acetonitrile—B: sodium acetate/tetrahydrofuran). | 2.89 (NR) | [63] |
| Selected S. pombe (JB899/Y470) | 1.47 (NR) | [64] | |||
| Selected S. pombe (JB917/CBS1057) | 1.55 (NR) | ||||
| Selected S. pombe (JB873/NCYC3422) | 1.39 (NR) | ||||
| Selected S. pombe V1 | 1.47 (NR) | ||||
| Non-Selected S. pombe (936/CECT12774) | 1.50 (NR) | ||||
| Non-Selected S. pombe (935/CECT12774) | 1.51 (NR) |
| Sample | BAs | Microorganisms | Analytical Method | Total BAs Content (mg/L) (Harvest Year) | Reference |
|---|---|---|---|---|---|
| Periquita | TYR; PUT; HIS | Spontaneous | Derivatization with o-phthaldialdehyde and separation with HPLC coupled to fluorescence detector. | 27.6 (2006) 7.0 (TYR) (2006) | [60] |
| CMS2 (inducer of MLF) | 2.0 (TYR) (2006) | ||||
| Merlot | AGM; ETA; ETH; PUT; TYR | O. oeni (5 × 106 cells/mL) | Derivatization with o-phthaldialdehyde and separation with HPLC coupled to fluorescence detector. | 6.59 (2004) 37.55 (2005) | [19] |
| Syrah | 8.73 (2004) 47.59 (2005) | ||||
| Cabernet Franc. | 6.88 (2004) 31.26 (2005) | ||||
| Montepulciano | 8.43 (2004) 33.85 (2005) | ||||
| Sangiovese | 8.16 (2004) 34.09 (2005) | ||||
| Carmenere | 8.63 (2004) 29.71 (2005) | ||||
| Cesanese d’Affile | 13.64 (2004) 37.80 (2005) | ||||
| Tempranillo | HIS; AGM; SPD; TYR; PUT; TRY; CAD; PHE | Spontaneous | Derivatization with diethyl ethoxy methylene malonate and separation by RP-LC-UV with gradient elution. | 2.227 (2009) 1.313 (2010) | [48] |
| Monastel | 3.772 (2009) 2.236 (2010) | ||||
| Maturana Tinta de Navarrete | 5.019 (2009) 1.646 (2010) | ||||
| Merlot | PUT; SPD; SPM; AGM; CAD; SRT; HIS; TYR; TRY; PHE | Spontaneous * | Derivatization with o-phthaldialdehyde and separation with HPLC coupled to fluorescence detector. | <0.40 (2008) | [79] |
| O. oeni DSM 7008 (6 mg/L) * | 1.93 | ||||
| O. oeni DSM 12923 (6 mg/L) * | 15.5 | ||||
| L. plantarum DSM 4361 (200 mg/L) * | 14.3 | ||||
| Yeast * | 7.94 | ||||
| Spontaneous ** | 12.4 | ||||
| O. oeni DSM 7008 (6 mg/L) ** | 7.4 | ||||
| O. oeni DSM 12923 (6 mg/L) ** | 7.7 | ||||
| L. plantarum DSM 4361 (200 mg/L) ** | 24.1 | ||||
| Yeast ** | 12.9 | ||||
| Spontaneous * | 6.88 | ||||
| O. oeni DSM 7008 (6 mg/L) *** | 9.08 | ||||
| O. oeni DSM 12923 (6 mg/L) *** | 6.23 | ||||
| L. plantarum DSM 4361 (200 mg/L) *** | 14.6 | ||||
| Yeast *** | 9.20 | ||||
| Spontaneous † | 6.43 | ||||
| O. oeni DSM 7008 (6 mg/L) † | 6.13 | ||||
| O. oeni DSM 12923 (6 mg/L) † | 9.81 | ||||
| L. plantarum DSM 4361 (200 mg/L) † | 17.7 | ||||
| Arvino | PHE; PUT; HIS; TYR; SPD; SPM | Spontaneous | Derivatization with dansyl chloride and separation with RP-LC-UV with gradient elution (solvents water and acetonitrile). | 30.0 (2015) | [62] |
| Gaglioppo | 50.3 (2015) | ||||
| Greco Nero | 54.4 (2015) | ||||
| Magliocco Canino | 74.1 (2015) | ||||
| Magliocco Dolce | 43.3 (2015) | ||||
| Nocera | 54.5 (2015) | ||||
| Tempranillo | HIS; MET; ETH; TYR; PHE; PUT; CAD | Spontaneous | Derivatization with o-phthaldialdehyde and separation with HPLC coupled to fluorescence detector. | 22.37 (2004) | [47] |
| Commercial malolacticbacteria | 14.75 (2004) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restuccia, D.; Loizzo, M.R.; Spizzirri, U.G. Accumulation of Biogenic Amines in Wine: Role of Alcoholic and Malolactic Fermentation. Fermentation 2018, 4, 6. https://doi.org/10.3390/fermentation4010006
Restuccia D, Loizzo MR, Spizzirri UG. Accumulation of Biogenic Amines in Wine: Role of Alcoholic and Malolactic Fermentation. Fermentation. 2018; 4(1):6. https://doi.org/10.3390/fermentation4010006
Chicago/Turabian StyleRestuccia, Donatella, Monica Rosa Loizzo, and Umile Gianfranco Spizzirri. 2018. "Accumulation of Biogenic Amines in Wine: Role of Alcoholic and Malolactic Fermentation" Fermentation 4, no. 1: 6. https://doi.org/10.3390/fermentation4010006
APA StyleRestuccia, D., Loizzo, M. R., & Spizzirri, U. G. (2018). Accumulation of Biogenic Amines in Wine: Role of Alcoholic and Malolactic Fermentation. Fermentation, 4(1), 6. https://doi.org/10.3390/fermentation4010006