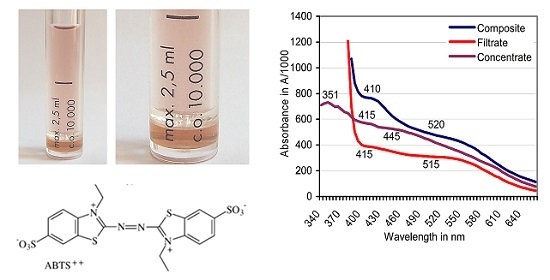
Reappraising a Controversy: Formation and Role of the Azodication (ABTS2+) in the Laccase-ABTS Catalyzed Breakdown of Lignin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sapwood Chips
2.2. Oxidation of ABTS in the Presence of Sapwood Chips
2.3. Molecular Size Separation of the ABTS Derivative by Ultrafiltration
2.4. Extraction of Peroxidase from the Outer Sapwood
2.5. Extraction of Total Phenol from the Outer Sapwood
2.6. Solvent Extraction of Potential Redox Mediator Substances from Sapwood of European Beech
2.7. Examination of ABTS Derivative by Liquid Chromatography
2.8. Oxidation of ABTS by Potassium Peroxodisulfate (K2O8S2)
2.9. Oxidative Discoloration of Remazol Brilliant Blue R (RBBR; M 626.5)
2.10. Data Processing
3. Results
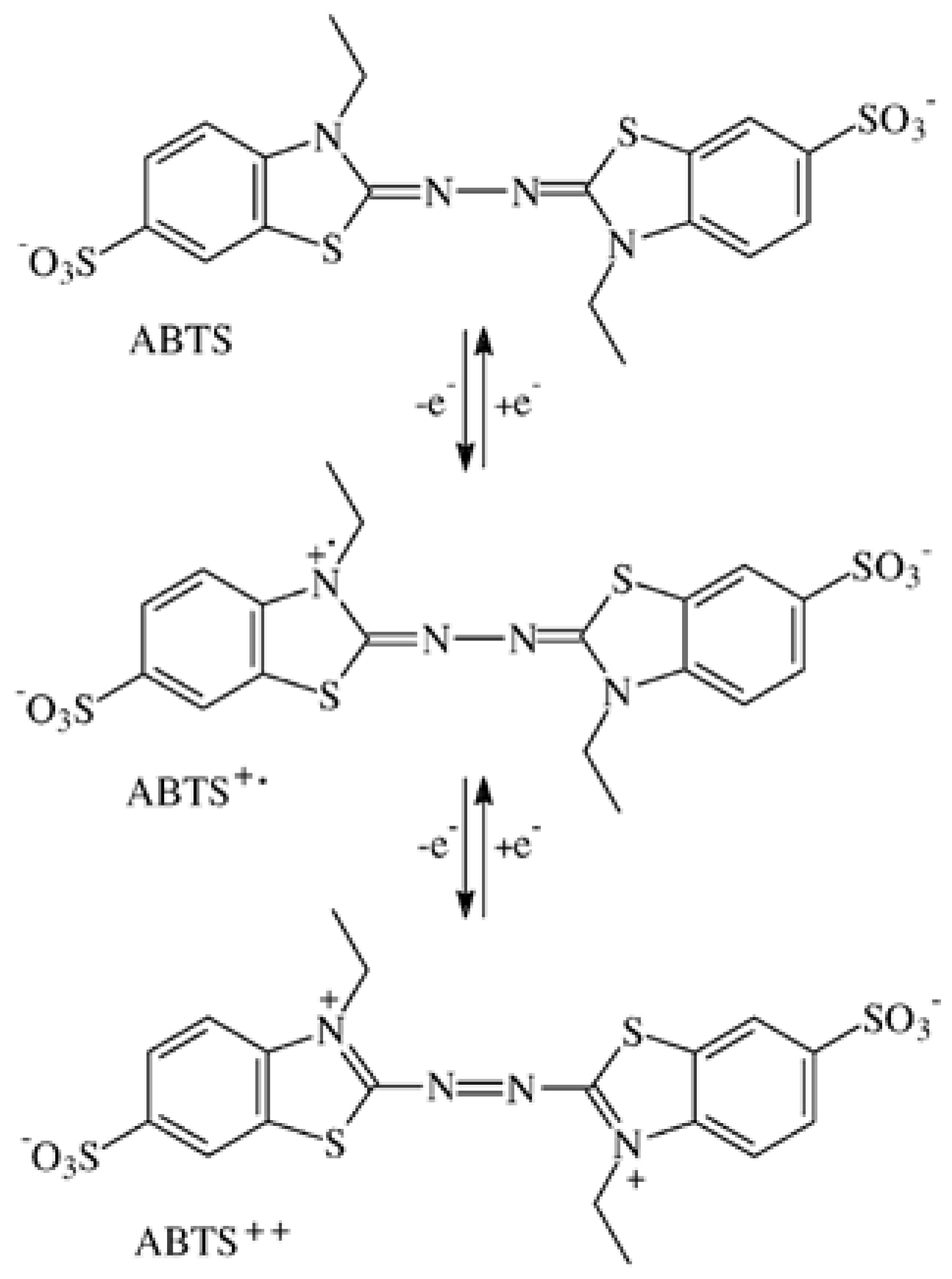
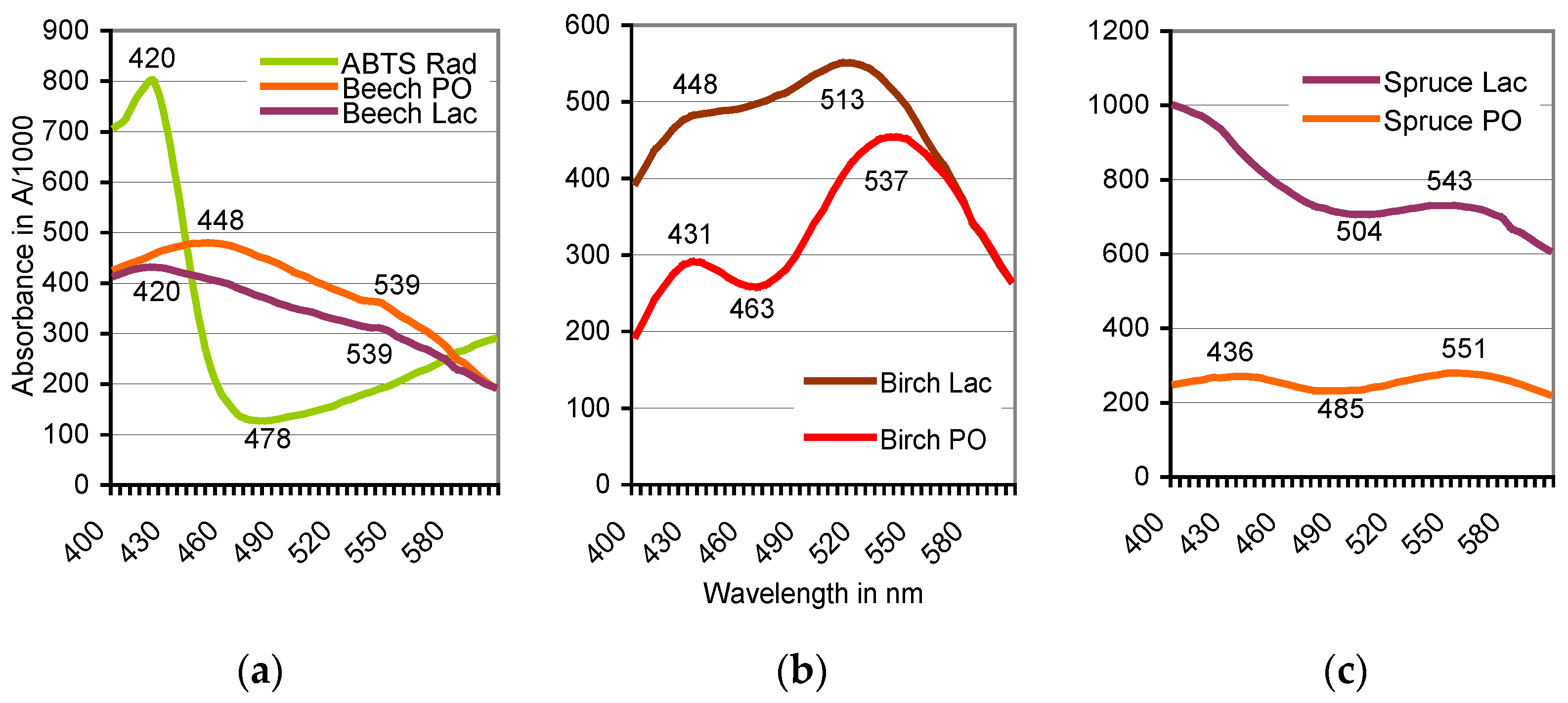
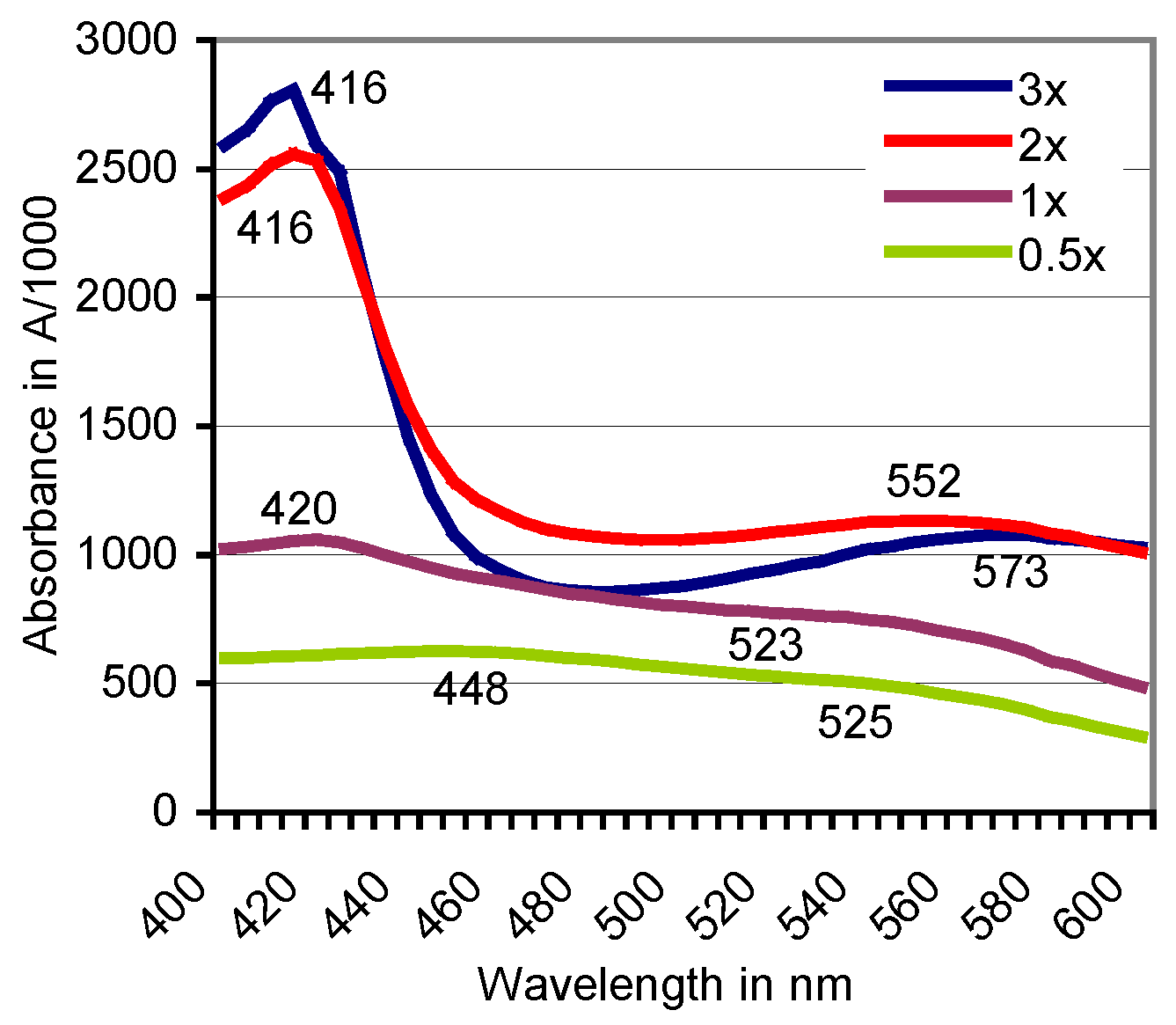
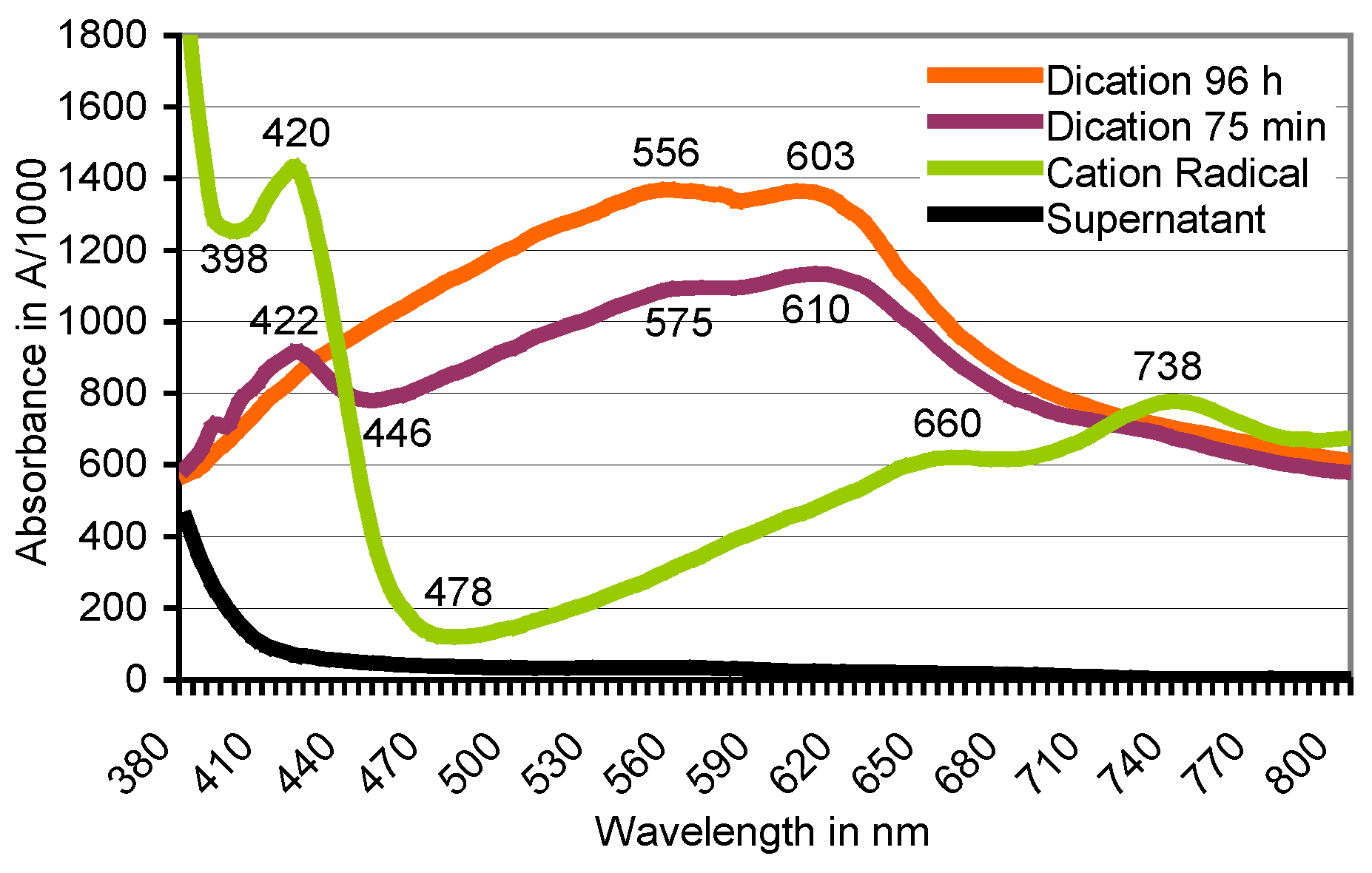
3.1. Formation of ABTS Derivatives
3.2. Transformation of ABTS•+ to the ABTS Derivative in the Absence of Oxidoreductases
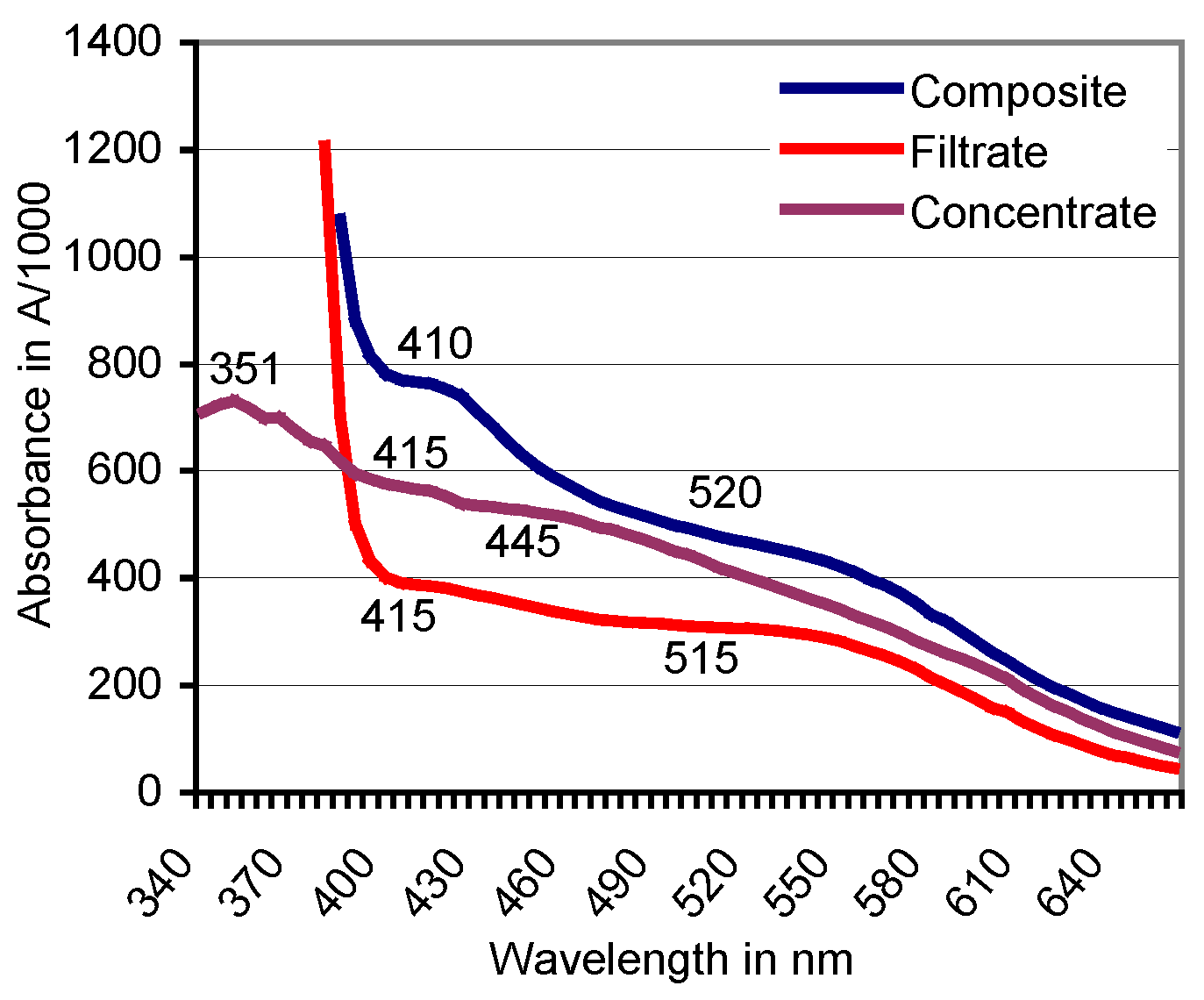
3.3. Filtration of the Red ABTS Derivative Against a 10-kDa Cutoff
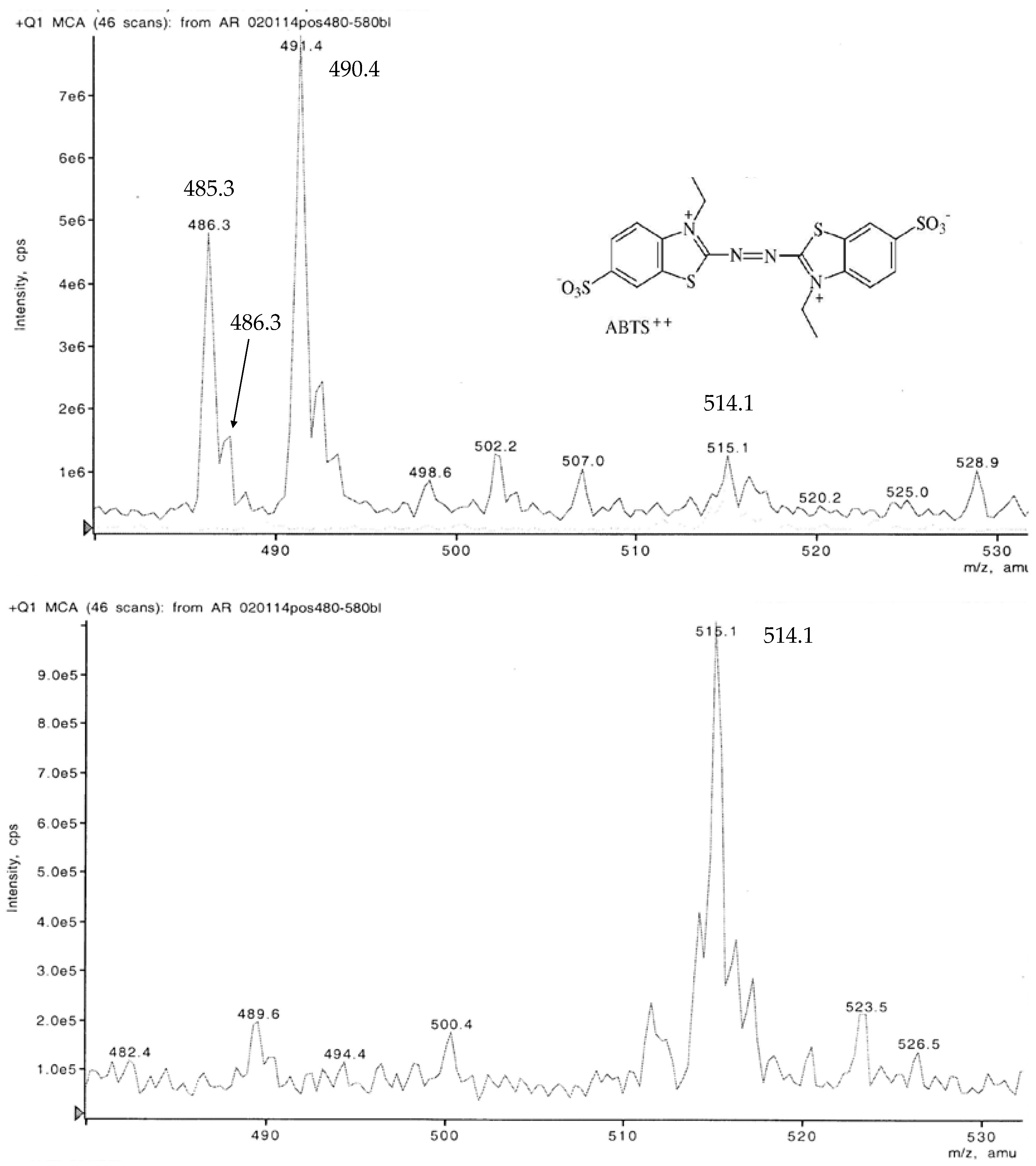
3.4. LC-MS Examination of a Non-Purified ABTS Derivative from White Mustard
3.5. Chemical ABTS Oxidation
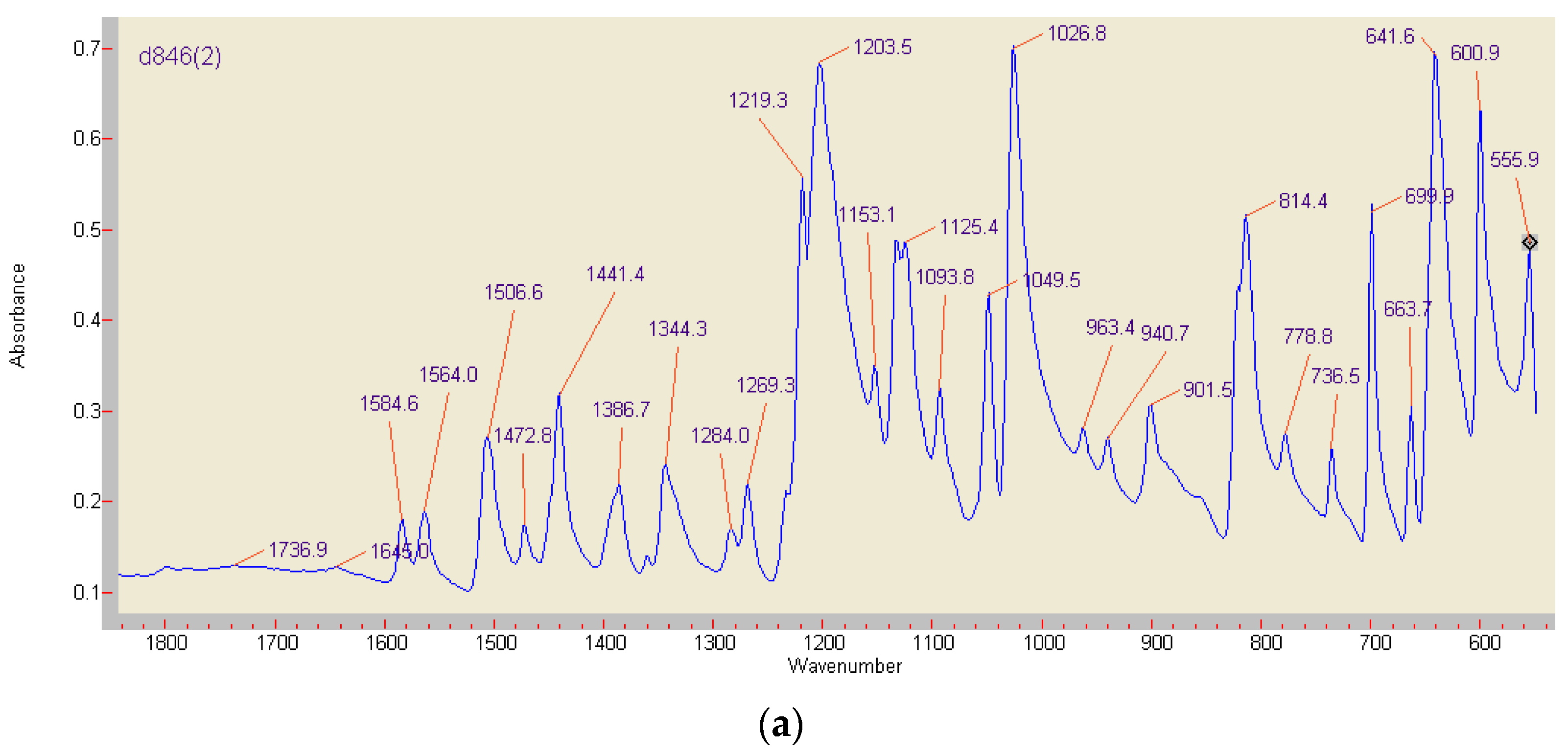
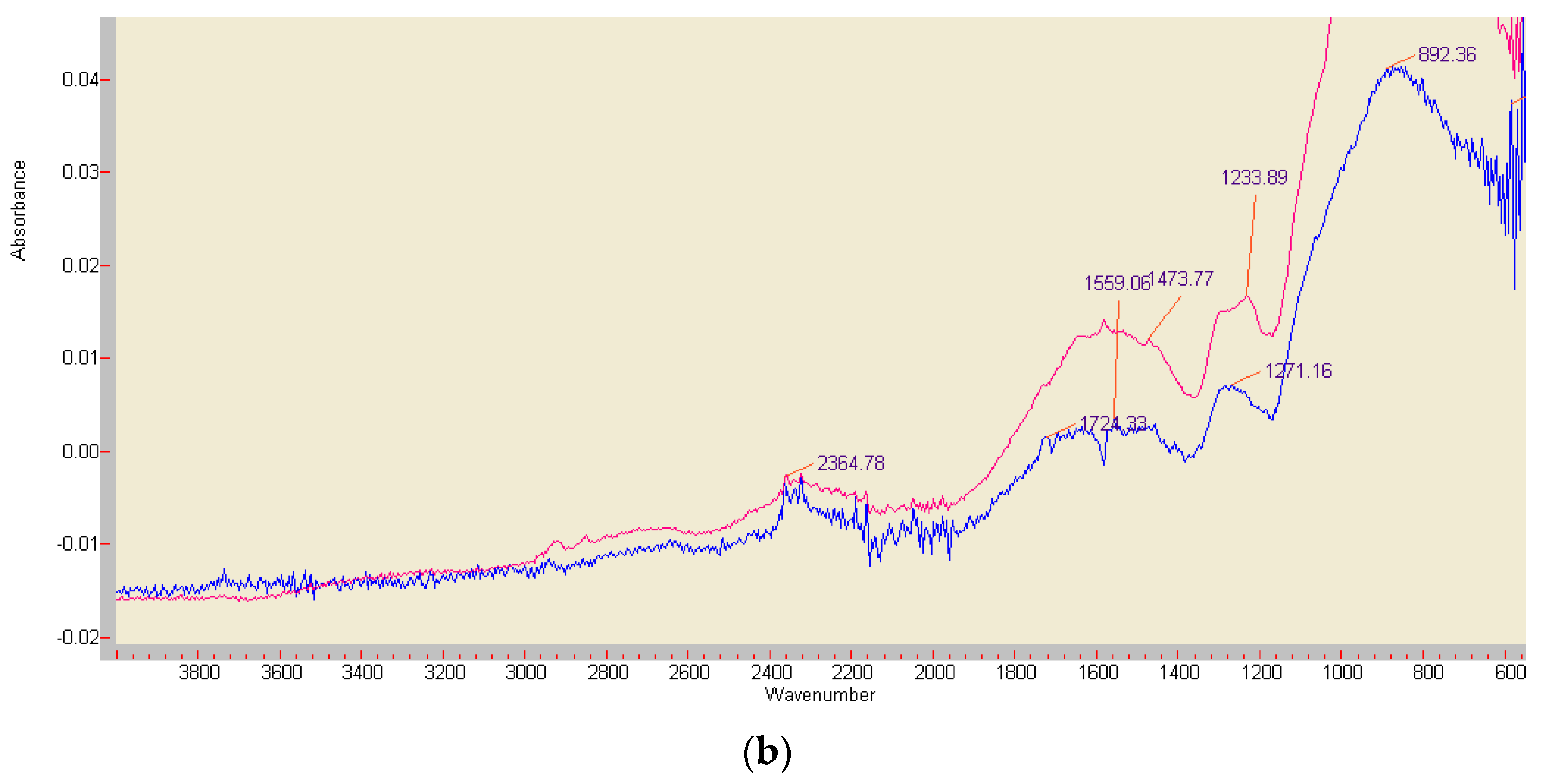
3.6. Comparison of ABTS Derivatives by Fourier Transform Infrared Spectroscopy (FT-IR) Analyses
3.7. Discoloration of Remazol Brilliant Blue R
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schachtschabel, P.; Blume, H.P.; Brümmer, G.; Hartge, K.H.; Schwertmann, U. Lehrbuch der Bodenkunde, 14th ed.; Enke: Stuttgart, Germany, 1998. [Google Scholar]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; De Gruyter: Berlin, Germany, 1984. [Google Scholar]
- Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Cabañas, A.; Manzanares, P.; Ballesteros, M. Ethanol production from steam-explosion pretreated wheat straw. Appl. Biochem. Biotechnol. 2006, 129–132, 496–508. [Google Scholar] [CrossRef]
- Han, L.; Feng, J.; Zhang, S.; Ma, Z.; Wang, Y.; Zhang, X. Alkali pretreated of wheat straw and its enzymatic hydrolysis. Braz. J. Microbiol. 2012, 43, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.S.; Mubeen, U. Wheat straw: A pragmatic overview. Curr. Res. J. Biol. Sci. 2012, 4, 673–675. [Google Scholar]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jørgensen, H.; Elder, T. Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Sharma, R.K. Ligninolytic fungal laccases and their biotechnological applications. Appl. Biochem. Biotechnol. 2010, 160, 1760–1788. [Google Scholar] [CrossRef] [PubMed]
- Kirk, T.K.; Farrell, R.L. Enzymatic “combustion”: The microbial degradation of lignin. Annu. Rev. Microbiol. 1987, 41, 465–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nie, Y.; Tang, Y.-Q.; Song, X.-M.; Cao, K.; Sun, L.-Z.; Wang, Z.-J.; Wu, X.-L. Diverse bacteria with lignin degrading potentials isolated from two ranks of coal. Front. Microbiol. 2016, 7, 1428. [Google Scholar] [CrossRef] [PubMed]
- Eggert, C.; Temp, U.; Dean, J.F.D.; Eriksson, K.-E.L. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996, 391, 144–148. [Google Scholar] [CrossRef]
- Gramss, G. Potential contributions of oxidoreductases from alfalfa plants to soil enzymology and biotechnology: A review. J. Nat. Sci. Sustain. Technol. 2012, 6, 169–223. [Google Scholar]
- Husain, Q. Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: A review. Rev. Environ. Sci. Biotechnol. 2010, 9, 117–140. [Google Scholar] [CrossRef]
- Johannes, C.; Majcherczyk, A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 2000, 66, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kaur, K.; Puri, S.; Sharma, P. Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl. Microbiol. Biotechnol. 2015, 99, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.E.; Negro, C.; Fuente, E.; Blanco, A. Enzymatic approaches in paper industry for pulp refining and biofilm control. Appl. Microbiol. Biotechnol. 2012, 96, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Kudanga, T.; Le Roes-Hill, M. Laccase applications in biofuels production: Current status and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 6525–6542. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.; Roman, R.; Vazquez-Duhalt, R. A catalytic approach to estimate the redox potential of heme-peroxidases. Biochem. Biophys. Res. Commun. 2007, 357, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.J.; Liers, C.; Lundell, T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Dolphin, D. The role of manganese in model systems related to lignin biodegradation. Holzforschung 1990, 44, 279–283. [Google Scholar] [CrossRef]
- Hammel, K.E.; Jensen, K.A., Jr.; Mozuch, M.D.; Landucci, L.L.; Tien, M.; Pease, E.A. Ligninolysis by a purified lignin peroxidase. J. Biol. Chem. 1993, 268, 12274–12281. [Google Scholar] [PubMed]
- Nousiainen, P.; Kontro, J.; Manner, H.; Hatakka, A.; Sipila, J. Phenolic mediators enhance the manganese peroxidase catalyzed oxidation of recalcitrant lignin model compounds and synthetic lignin. Fungal Genet. Biol. 2014, 72, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kersten, P.J.; Kalyanaraman, B.; Hammel, K.E.; Reinhammar, B. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem. J. 1990, 268, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Umezawa, T.; Higuchi, T. Degradation mechanisms of phenolic β-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch. Biochem. Biophys. 1988, 262, 99–110. [Google Scholar] [CrossRef]
- Kawai, S.; Umezawa, T.; Shimada, M.; Higuchi, T. Aromatic ring cleavage of 4,6-di(tert-butyl) guaiacol, a phenolic lignin model compound, by laccase of Coriolus versicolor. FEBS Lett. 1988, 236, 309–311. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G.; Freiermuth, B.; Bodie, E.; Borneman, S. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl. Environ. Microbiol. 1997, 63, 4627–4632. [Google Scholar] [PubMed]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Branchi, B.; Galli, C.; Gentili, P. Kinetics of oxidation of benzyl alcohols by the dication and radical cation of ABTS. Comparison with laccase-ABTS oxidations: An apparent paradox. Org. Biomol. Chem. 2005, 3, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Brijwani, K.; Rigdon, A.; Vadlani, P.V. Fungal laccases: Production, function, and applications in food processing. Enzyme Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Ibarra, D.; Martínez, M.J.; Martínez, A. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl. Environ. Microbiol. 2005, 71, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Cañas, A.I.; Nousiainen, P.; Record, E.; Lomascolo, A.; Martínez, M.J.; Martínez, A.T. p-Hydroxycinnamic acids as natural mediators for laccase oxidation of recalcitrant compounds. Environ. Sci. Technol. 2008, 42, 6703–6709. [Google Scholar] [CrossRef] [PubMed]
- Cañas, A.I.; Alcalde, M.; Plou, F.; Martínez, M.J.; Martínez, A.T.; Camarero, S. Transformation of polycyclic aromatic hydrocarbons by laccase is strongly enhanced by phenolic compounds present in soil. Environ. Sci. Technol. 2007, 41, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, M.; Vidal, T.; Tzanov, T. Phenolic compounds as enhancers in enzymatic and electrochemical oxidation of veratryl alcohol and lignins. Appl. Microbiol. Biotechnol. 2011, 89, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, T.N.; Dolgonosov, B.M. Modeling the biodegradation of multicomponent organic matter in an aquatic environment: 3. Analysis of lignin degradation mechanisms. Water Res. 2010, 37, 332–346. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G.; Reid, I.D.; Lanthier, P.; Yaguchi, M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 1995, 61, 1876–1880. [Google Scholar] [PubMed]
- Niladevi, K.N. Characterization of Laccase from Streptomyces Psammoticus: An Enzyme for Eco-Friendly Treatment of Environmental Pollutants. Ph.D. Thesis, Biotechnology Division, National Institute for Interdisciplinary Science and Technology (CSIR), Trivandrum 695 OI9, India, 2008. [Google Scholar]
- Masoudian, S.; Rasoulifard, M.H.; Pakravan, P. Degradation of acid red 14 in contaminated water by Ag-SiO2 nanocomposite. Ind. J. Chem. 2015, 54, 757–761. [Google Scholar]
- Kim, S.Y. Synthesis, Characterization, and Applications of Conducting Polymers with Functional Dopants. Ph.D. Thesis, The School of Engineering and the Division of Biology and Medicine at Brown University, Providence, RI, USA, 2011. [Google Scholar]
- Scott, S.L.; Chen, W.-J.; Jjakac, A.; Espenson, J.H. Spectroscopic parameters, electrode potentials, acid ionization constants, and electron exchange rates of the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) radicals and ions. J. Phys. Chem. 1993, 97, 6710–6714. [Google Scholar] [CrossRef]
- Bolis, C.; Huwig, A.K.; Grimm, R.; Rheinberger, V.M. Agents for Bleaching Teeth. U.S. Patent 20050158251, 21 July 2005. [Google Scholar]
- Garcia-Leis, A.; Jancura, D.; Antalik, M.; Garcia-Ramos, J.V.; Sanchez-Cortes, S.; Jurasekova, Z. Catalytic effects of silver plasmonic nanoparticles on the redox reaction leading to ABTS•+ formation studied using UV-visible and Raman spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 26562–26571. [Google Scholar] [CrossRef] [PubMed]
- Maruthamuthu, P.; Venkatasubramanian, L.; Dharmalingam, P. A fast kinetic study of formation and decay of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) radical cation in aqueous solution. Bull. Chem. Soc. Jpn. 1987, 60, 1113–1117. [Google Scholar] [CrossRef]
- Venkatasubramanian, L.; Maruthamuthu, P. Kinetics and mechanism of formation and decay of 2,2′-azinobis-(3-ethylbenzothiazole-6-sulphonate) radical cation in aqueous solution by inorganic peroxides. Int. J. Chem. Kinet. 1989, 21, 399–421. [Google Scholar] [CrossRef]
- Majcherczyk, A.; Johannes, C.; Hüttermann, A. Oxidation of aromatic alcohols by laccase from Trametes versicolor mediated by the 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) cation radical and dication. Appl. Microbiol. Biotechnol. 1999, 51, 267–276. [Google Scholar] [CrossRef]
- Ley, C.; Zengin Çekiç, S.; Kochius, S.; Mangold, K.-M.; Schwaneberg, U.; Schrader, J.; Holtmann, D. An electrochemical microtiter plate for parallel spectroelectrochemical measurements. Electrochim. Acta 2013, 89, 98–105. [Google Scholar] [CrossRef]
- Rodgers, C.J.; Blanford, C.F.; Giddens, S.R.; Skamnioti, P.; Armstrong, F.A.; Gurr, S.J. Designer laccases: A vogue for high-potential fungal enzymes? Trends Biotechnol. 2010, 28, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.Y.; Chen, C.; Gratzl, J.S.; Kirkman, A.G.; Jakob, H. Kinetic studies on oxidation of veratryl alcohol by laccase-mediator system. Part 1. Effects of mediator concentration. Holzforschung 2000, 54, 165–170. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Chen, C.; Gratzl, J.S.; Kirkman, A.G.; Jakob, H. Kinetic studies on oxidation of veratryl alcohol by laccase-mediator system. Part 2. The kinetics of dioxygen uptake. Holzforschung 2000, 54, 171–175. [Google Scholar] [CrossRef]
- Solís-Oba, M.; Ugalde-Saldívar, V.M.; González, I.; Viniegra-González, G. An electrochemical–spectrophotometrical study of the oxidized forms of the mediator 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) produced by immobilized laccase. J. Electroanal. Chem. 2005, 579, 59–66. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Fischer, K. Oxidation of benzyl alcohols by the laccase-mediator system (LMS)—A comprehensive kinetic description. Holzforschung 2001, 55, 47–56. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B Enzym. 2002, 16, 231–240. [Google Scholar] [CrossRef]
- Dong, S.; Xiao, H.; Huang, Q.; Zhang, J.; Mao, L.; Gao, S. Graphene facilitated removal of labetalol in laccase-ABTS system: Reaction efficiency, pathways and mechanism. Sci. Rep. 2016, 6, 21396. [Google Scholar] [CrossRef] [PubMed]
- Krainer, F.W.; Glieder, A. An updated view on horseradish peroxidases: Recombinant production and biotechnological applications. Appl. Microbiol. Biotechnol. 2015, 99, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Sterjiades, R.; Dean, J.F.D.; Eriksson, K.-E.L. Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiol. 1992, 99, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Dobson, A.D.W.; Field, J.A. Reduction of the 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) cation radical by physiological organic acids in the absence and presence of manganese. Appl. Environ. Microbiol. 1998, 64, 2026–2031. [Google Scholar] [PubMed]
- Ahmed, F.; Dewani, R.; Pervez, M.K.; Mahboob, S.J.; Soomro, S.A. Non-destructive FT-IR analysis of mono azo dyes. Bulg. Chem. Commun. 2016, 48, 71–77. [Google Scholar]
- Awale, A.G.; Gholse, S.B.; Utale, P.S. Synthesis, spectral properties and applications of some mordant and disperse mono azo dyes derived from 2-amino-1,3-benzothiazole. Res. J. Chem. Sci. 2013, 3, 81–87. [Google Scholar]
- Dinçalp, H.; Toker, F.; Durucasu, I.; Avcıbaşı, N.; Icli, S. New thiophene-based azo ligands containing azo methine group in the main chain for the determination of copper(II) ions. Dyes Pigments 2007, 75, 11–24. [Google Scholar] [CrossRef]
- Masoud, M.S.; Khalil, E.A.; Hindawya, A.M.; Ali, A.E.; Mohamed, E.F. Spectroscopic studies on some azo compounds and their cobalt, copper and nickel complexes. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2004, 60, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.W.M.; Ku, S.K.A.; Choi, P.S.R.; Kan, C.W.; Tsang, S.Y. Determining functional groups of commercially available ink-jet printing reactive dyes using Infrared Spectroscopy. Res. J. Text. Appar. 2005, 9, 26–38. [Google Scholar] [CrossRef]
- Xu, F.; Shin, W.; Brown, S.H.; Wahleithner, J.A.; Sundaram, U.A.; Solomon, E.I. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophys. Acta. 1996, 1292, 303–311. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, P.; Wu, X.; Sun, J.; Chen, S. Radical scavenging by acetone: A new perspective to understand Laccase/ABTS inactivation and to recover redox mediator. Molecules 2015, 20, 19907–19913. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, M.; Monreal, C.M.; Powell, E.E. Wheat straw biomass: A resource for high-value chemicals. J. Environ. Sci. Health. B 2014, 49, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Kenten, R.H.; Mann, P.J.G. The oxidation of manganese by peroxidase systems. Biochem. J. 1950, 46, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.J.; Kepp, K.P. Setting the stage for electron transfer: Molecular basis of ABTS-binding to four laccases from Trametes versicolor at variable pH and protein oxidation state. J. Mol. Catal. B Enzym. 2014, 100, 68–77. [Google Scholar] [CrossRef]
- Enguita, F.J.; Marçal, D.; Martins, L.O.; Grenha, R.; Henriques, A.O.; Lindley, P.F.; Carrondo, M.A. Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J. Biol. Chem. 2004, 279, 23472–23476. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Neumann, G.; Bott, S.; Ohler, M.A.; Mock, H.-P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa L.cv.Tizian) as affected by different soils. Front. Microbiol. 2014, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Li, W.-G.; Chen, L.-F.; Xiao, B.-K.; Yang, J.-Y.; Yang, L.; Zhang, C.-G.; Huang, R.-Q.; Dong, J.-X. ABTS+ scavenging potency of selected flavonols from Hypericum perforatum L. by HPLC-ESI/MS QQQ: Reaction observation, adduct characterization and scavenging activity determination. Food Res. Int. 2014, 58, 47–58. [Google Scholar] [CrossRef]
- Brausam, A.; Eigler, S.; Jux, N.; Van Eldik, R. Mechanistic investigations of the reaction of an iron(III) octa-anionic porphyrin complex with hydrogen peroxide and the catalyzed oxidation of diammonium-2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate). Inorg. Chem. 2009, 48, 7667–7678. [Google Scholar] [CrossRef] [PubMed]
- Labrinea, E.P.; Georgiou, C.A. Stopped-flow method for assessment of pH and timing effect on the ABTS total antioxidant capacity assay. Anal. Chim. Acta 2004, 526, 63–68. [Google Scholar] [CrossRef]

| Tree Species | Total Phenol a | Peak Absorbance (A) b | ||
|---|---|---|---|---|
| 12 °C Extract | 98 °C Extract | Peroxidase | Laccase | |
| European beech | 3309 ± 1107 | 9912 ± 653 | A539 = 0.356 | A539 = 0.312 |
| Sycamore | 180 ± 20 | 91 ± 0 | A560 = 0.284 | A566 = 0.190 |
| European birch | 98 ± 6 | 352 ± 7 | A537 = 0.455 | A513 = 0.551 |
| Common oak | 3227 ± 958 | 1915 ± 737 | A542 = 0.190 | A545 = 0.440 |
| Hornbeam | 231 ± 22 | 224 ± 4 | No peak | No peak |
| Norway spruce | 53 ± 3 | 66 ± 12 | A551 = 0.280 | A543 = 0.732 |
| Scots pine | 116 ± 32 | 64 ± 2 | A560 = 0.329 | A559 = 0.330 |
| Composition of the Reaction Mixture (mL) a | Active Oxidants | A595 Loss min−1 (±SD) |
|---|---|---|
| RBBR, 0.5; buffer, 0.5; enzyme, 0.1 | Lac | 0.0147 ± 0.0021 |
| RBBR, 0.5; buffer, 0.3; H2O2, 0.2; enzyme, 0.1 | Lac; MnP | 0.0193 ± 0.0018 |
| RBBR, 0.5; buffer, 0.1; H2O2, 0.2; enzyme, 0.1; ABTS derivative, 0.2 | Lac; MnP; ABTS derivative | 0.0486 ± 0.0037 |
| Composition of the Reaction Mixture (mL) a | Active Oxidants | A595 Loss min−1 (±SD) after | |
|---|---|---|---|
| 5 h | 50 h | ||
| RBBR, 1; H2O2, 0.5; buffer, 0.2 | PO | 0.231 ± 0.016 | 0.569 ± 0.038 |
| RBBR, 1; H2O2, 0.5; ABTS, 0.2 | PO; ABTS derivative | 0.264 ± 0.024 | 0.638 ± 0.044 |
| RBBR, 1; H2O2, 0.5; ABTS, 0.1; ABTS derivative, 0.1 | PO; ABTS derivative | 0.422 ± 0.036 | 0.704 ±0.032 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gramss, G. Reappraising a Controversy: Formation and Role of the Azodication (ABTS2+) in the Laccase-ABTS Catalyzed Breakdown of Lignin. Fermentation 2017, 3, 27. https://doi.org/10.3390/fermentation3020027
Gramss G. Reappraising a Controversy: Formation and Role of the Azodication (ABTS2+) in the Laccase-ABTS Catalyzed Breakdown of Lignin. Fermentation. 2017; 3(2):27. https://doi.org/10.3390/fermentation3020027
Chicago/Turabian StyleGramss, Gerhard. 2017. "Reappraising a Controversy: Formation and Role of the Azodication (ABTS2+) in the Laccase-ABTS Catalyzed Breakdown of Lignin" Fermentation 3, no. 2: 27. https://doi.org/10.3390/fermentation3020027
APA StyleGramss, G. (2017). Reappraising a Controversy: Formation and Role of the Azodication (ABTS2+) in the Laccase-ABTS Catalyzed Breakdown of Lignin. Fermentation, 3(2), 27. https://doi.org/10.3390/fermentation3020027