1. Introduction
The rising demand for renewable energy sources induced the development of new technologies to produce biofuels [
1,
2]. Among them, microbial biotechnologies have been largely developed, allowing the development and production of several different biofuels, also using effluents and wastes as substrates: by this way, the costs of the processes are reduced, improving their economical competitiveness and simultaneously reducing the environmental load for wastes disposal [
3,
4].
Bioethanol can be used as a fuel, either pure or blended with gasoline (gasohol). In the United States, it is used as 10% solution in gasoline (E-10) while in Brazil it is used both blended (24% ethanol, 76% gasoline) and hydrated in flexible-fuel vehicles [
5]. Others mixtures are E-15 (15% ethanol, 85% gasoline) and E-85 (85% ethanol e 15% gasoline). Bioethanol can also replace other additives, as octane boosters, in gasoline fuel, and ethanol–gasoline blend provides the highest brake power [
6]. Other benefits come from using bioethanol as biofuel: it is totally biodegradable and sulphur free, and the products from its incomplete oxidation (acetic acid and acetaldehyde) are less toxic in comparison to other alcohols [
7].
The raw materials that can be used for alcoholic fermentations are sugar crops (sugar cane, sugar beet and sorghum, fruits), starchy crops (corn, wheat and barley), and cellulosic crops (stems, leaves, trunks, branches, husks), the latter needing a pre-treatment to make fermentation possible. They vary in relation to geographic areas: corn is generally used in USA and China, while in tropical areas (India, Brazil, Colombia) sugar cane is more diffused [
8]. Nowadays, the use of ligno-cellulosic biomasses, as forest management residues, food industry wastes, or specific plants, is expanding [
9].
Among fruits, grape fermentation is well known worldwide; other fruit fermentation is typical of peculiar areas. Apple (
Malus domestica) fermentation product is called cider and it is typical of the United Kingdom, France, Spain, Germany, Ireland, The Netherlands, Finland, and Switzerland. The alcoholic fermentation of peach (
Prunus persica) and kiwifruit (
Actinidia chinensis) is extremely rare [
10,
11], but possible due to the sugar content of these fruits. Until now, the fermentation of these fruits has mainly been addressed for nutritional uses; bioethanol destination is less diffused, due to ethical and economical considerations; however, much of fruit residue that is disposed as waste could become low cost substrates for bio ethanol production.
Starch from starchy crops, such as cereals, to become fermentable needs a pre-treatment composed of three steps: gelatinization, to allow the starch to lose its crystallinity and become an amorphous gel; liquefaction, where starch is hydrolyzed to dextrins by an alfa-amylase and viscosity is reduced; and saccharification, where a gluco-amylase is added to convert dextrins to glucose [
12,
13,
14]. Saccharification can be managed to be simultaneous to fermentation: this makes the glucose gradually available to microorganisms and reduces contamination risks, process duration, and costs [
15,
16].
The microorganisms chosen for alcoholic fermentation are usually yeasts, mainly belonging to
Saccharomyces genus. The preferred characteristics for industrial bioethanol production are: high ethanol yield; high ethanol tolerance; high ethanol productivity (>5.0 g/L/h); aptitude to grow in simple, inexpensive, and undiluted media; aptitude to grow in presence of inhibitors, at low pH, or high temperature [
17].
S. cerevisiae is usually considered the typical yeast of wine and cider fermentations; among other species of the genus
Saccharomyces,
S. bayanus—characterized by high ethanol tolerance—is used for the production of wine, sparkling wines, and cider, and it can also be used in industrial applications for bioethanol production [
18].
The temperature is a fundamental parameter of the fermentation process. According to some authors, the ethanol production increases with increasing temperature [
14], under a limit above which the production rate decreases, because high temperatures can become a stress factor for micro-organisms. The temperatures that allow a good microbial growth and a good ethanol yield generally range between 20 and 35 °C. As fermentation is an exergonic process, particular attention is required for fermentation temperature control [
5]. Then, to maximize the ethanol yield, yeast strains resistant to high temperatures should be chosen [
19]; this is the best choice for bioethanol production, allowing high yields and low costs, while it could be unsuitable for fermentations aimed to reach alcoholic beverages, because sensory properties could be compromised [
20,
21].
The fermentation duration must also be chosen to obtain an adequate microbial growth and ethanol yield, taking into account that the shorter the duration is, the lower the costs are. The study of the microbial growth and ethanol production kinetics in relation to the substrate allows the identification of the correct duration of the fermentation process, also in consideration of volumetric productivity (g/L/h), both in batch and in continuous fermentations.
The distillation of ethanol formed during fermentation from ethanol-water solution will lead finally to production of hydrous (azeotropic) ethanol (theoretical maximum achievable 95.5% wt. ethanol and 4.5% water). To remove the remaining water, special processes are required to reach anhydrous ethanol, that include: chemical dehydration process, dehydration by vacuum distillation process, azeotropic distillation process, extractive distillation processes, membrane processes, adsorption processes, and diffusion distillation process [
22]. The evaluation of the energy balance of bioethanol production reveals that most of the energy is required for the distillation, also because of the low concentration of ethanol in the fermented broth [
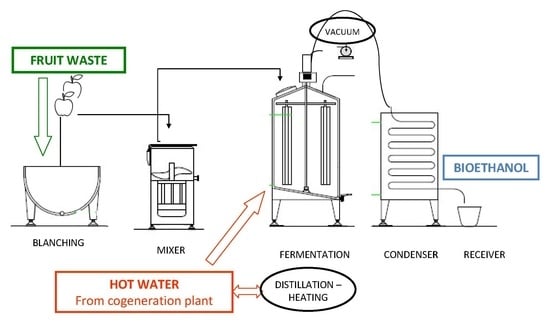
5]. Energy consumption can be reduced during distillation if lower heating is necessary; this can be reached using residual thermal energy from other processes to warm the fermented broth. Moreover, distillations carried out under vacuum enable good ethanol yields even at lower temperatures. The aim of this study was to evaluate the exploitation of the residual thermal energy of a cogeneration plant, producing residual hot water from plant chilling systems, in downstream phases of the fermentation process, by warming the fermented broth with the hot water of the cogeneration plant; vacuum distillation was also tested at temperatures lower than that usually employed in traditional simple batch distillation plants. The possible role of fruit blanching pretreatment, carried out with the hot water from the cogeneration plant—mainly aimed at facilitating the grinding phase and reducing contaminations during alcoholic fermentation—was also tested. Moreover, to further reduce the costs of the process, agricultural wastes were checked as feedstock for fermentations: unmarketable residues of apple, kiwifruit, peaches, and corn threshing residue (CTR).
Saccharomyces bayanus, characterized by high ethanol tolerance and largely used in cider fermentation, has been chosen as starter. The results obtained support the feasibility of a fermentation process, coupled to a cogeneration plant, fed throughout the year with apple wastes, when available, and with CTR when apple wastes are unavailable, where residual thermal energy from a cogeneration plant is used in fruit blanching and in distillation phases.
2. Materials and Methods
2.1. Strain and Culture Media
Fermentation medium was inoculated with a Saccharomyces bayanus commercial strain (Zymoferm Bayanus, Chimica FRANKE, Susa, Torino, Italy). The starter cultures were prepared by inoculating the yeast in 100 mL of YEPD (yeast extract 1%, bacto-peptone 2%, glucose 2%) and incubating it at 25 °C under static conditions in an incubation chamber for 24 h.
The unmarketable residues of apples, kiwifruits, and peaches were cut and ground separately for 2 min (
Tables S1 and S2: raw material characterization). The mash obtained from each fruit was stored at −20 °C. Samples of the same fruits were blanched in boiling water: the apples and the peaches for 15 min, the kiwi for 5 min, then the fruits were cut and ground for 2 min and the mash obtained from each fruit stored at −20 °C. The concentrations of sugars (glucose and fructose) were determined in raw materials to check the homogeneity of starting conditions, and throughout the fermentations, to check the trend of fermentation and sugar consumption (
Table S2), by
d-Fructose/
d-Glucose Assay Kit and Ethanol Assay Kit (Megazyme, Bray, Co., Wicklow, Ireland).
The CTR was milled and water was added to the flour in order to obtain a mash 30%
w/
v powder/water ratio. The gelatinization was conducted for 4 h at 85 °C until the complete water absorption. For the liquefaction the Liquozyme
® SC DS (Novozymes, Dittingen, Switzerland) was added (0.020% weight of enzyme/dried weight of CTR). The saccharification was carried out at the same time as the fermentation by adding Spirizyme
® Ultra (Novozymes, Dittingen, Switzerland) (0.030% weight of enzyme/dried weight of RTC). Starch concentration was analyzed by Kit Total Starch (Megazyme, Bray, Co., Wicklow, Ireland) (
Table S3: CTR characterization).
2.2. Fermentations
Lab-scale batch fermentations were carried out on 600 g mash of each kind of fruit and of liquefied CTR, that were inoculated with the starter yeast culture (1 × 106 cell/g of substrate), in 1 liter flask, under static conditions, at 28 and 35 °C. Samples were taken at 24, 48, 72, 96, and 168 h to quantify ethanol production.
Batch and semi-continuous fermentations were also carried out with liquefied CTR and with an apple:kiwifruit 1:1 mix, the latter in a 2 L flask filled with 1200 g mashed fruits, inoculated with the starter yeast culture (1 × 106 cell/g of substrate) and incubated under static conditions at 35 °C. In semi-continuous fermentations, at 120 h, 400 g fermented substrate was withdrawn and substituted with fresh substrate; the same procedure was repeated every 48 h. Samples were withdrawn once a week after one, two, three, and four weeks to determine ethanol and residual sugars concentrations. The concentrations of ethanol were determined by Ethanol Assay Kit (Megazyme, Bray, Co., Wicklow, Ireland). All the assays were carried out in triplicate.
2.3. Downstream
In order to evaluate the amount of ethanol that can be recovered by evaporation/distillation under vacuum at low temperatures from the fermented fruit biomasses heated with the residual hot water from the plant chilling systems of a cogeneration plant, samples of fermented broth were distilled combining different temperatures to different vacuum levels. Temperatures were chosen lower than the temperature of the chilling water of cogeneration plant (83–85 °C). Different water bath temperatures and vacuum levels were obtained in a rotary evaporation system (Laborota 4000, Heidolph Instruments GmbH & Co, Schwabach, Germany). The combinations of temperature and pressure tested are shown in
Table 1.
2.4. Scale-Up
Scale-up was done for apple and kiwi fruits, fresh or blanched, in a 1000 L thermostated fermenter, loaded with about 600 kg mashed fruits each time. Batch fermentations were carried out for five days under controlled temperature, lower than 35 °C. Exterior air-lock of the bioreactor was loaded with cold water during the fermentation, to avoid excessive warming, and with hot water (83–85 °C), coming from the cooling of the cogeneration plant, during the distillation phase. The same hot water from cogeneration plant was also used to blanch fruits during pretreatment, blanching at 80 °C for 5 min.
3. Results and Discussion
Lab scale fermentations were carried out in order to check the best conditions to reach high ethanol yields; as fruit wastes are available only seasonally, CTR was also evaluated. CTR is an agricultural residue that is easy to store and available throughout the whole year, then usable to supply the fermentation plant when fruit wastes are unavailable.
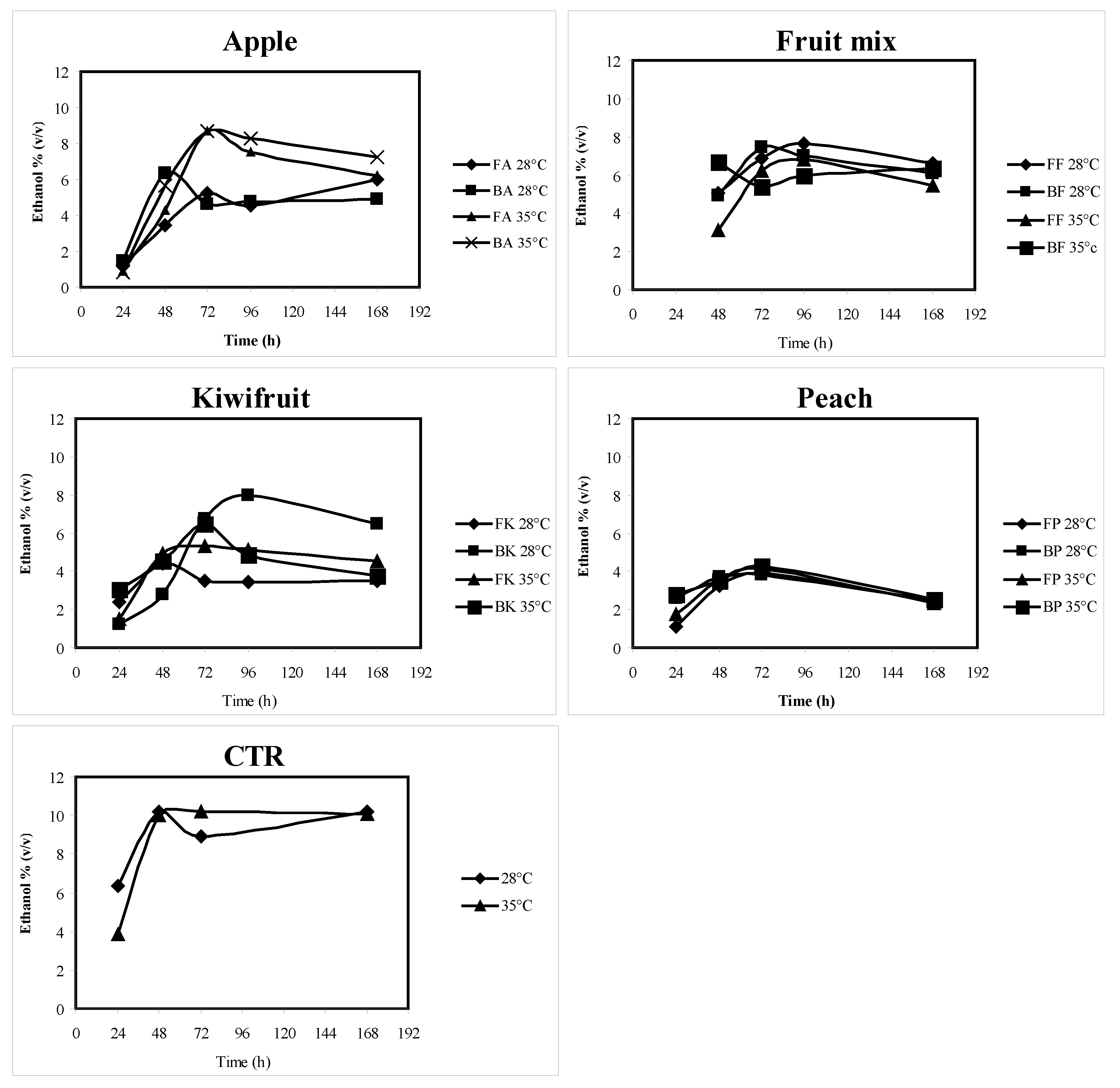
The ethanol concentrations checked throughout batch fermentations are reported in
Figure 1.
Among fruits, the highest ethanol concentrations were reached with blanched fruits, at 35 °C for apple and peach, at 28 °C for kiwifruit and fruit mix. The apple showed, among fruits, the maximum ethanol concentration: 8.71 ± 0.83% (v/v). The kiwifruits produced 7.97 ± 0.39% (v/v) and peaches produced 4.26 ± 0.27% (v/v). The ethanol concentration produced from CTR was 10.22 ± 0.70% (v/v). Apples were shown to give the best ethanol production among the tested fruits, but kiwifruit also gave good results, while the amount of ethanol obtained from peach was low. CTR showed to be very suitable as substrate of fermentation for bioethanol production.
The ethanol productivity, expressed as g/L/h, is reported in
Table 2.
The optimal duration of fermentation was three days for fruits, two days for CTR. A summary of the best selected batch fermentation conditions are reported in
Table 3. The short fermentation duration contributes to the economical sustainability of the process.
Semi-continuous fermentations can be sometimes preferred in industrial fermentations, due to several advantages, such as shorter induction times due to the suppression of the lag phase, better control of contaminations and higher yields; however, in our assays, semi-continuous fermentations gave lower productions of ethanol than batch fermentations (
Table 4). Then, due to short duration and high yield, batch fermentations should be preferred to semi-continuous fermentations; batch fermentations are also suitable, taking into account that the fermentation plant should be fed with seasonally different substrates.
Scale-up fermentations were carried out with the best conditions selected from the lab-scale fermentations, with blanched apple and kiwifruit. As fruits were ground after blanching at 80 °C, it was necessary to cool down the temperature at the start and throughout the whole fermentation to maintain it lower than 35 °C. An average ethanol concentration of 6.58% and an ethanol productivity of 0.30 g/L/h were obtained with apple (three replications), while with kiwifruit no ethanol production was detected, even if sugars (glucose and fructose) were completely exhausted at the end of the fermentation; a possible explanation could be that an aerobic respiratory catabolism from contaminating microorganisms predominated, preventing yeast growth.
The product of a batch apple fermentation (626 kg, 6.44% ethanol concentration at the end of fermentation) was used to test the ethanol yield that can be reached with distillations at low temperatures. The results of the distillation tests carried out under controlled conditions, at atmospheric pressure and under vacuum, are reported in
Table 1. Temperatures of heating water bath ≤80 °C were tested to reproduce the conditions of warming obtained with the hot water coming from the chilling system of a cogeneration plant. The ethanol yielded at 80 °C at atmospheric pressure was only 43.4%, while the yield augmented to 89.59% under vacuum at 400 mbar; a further increase to 93.35% was obtained, raising the vacuum until 200 mbar. A strong pressure decrease (175 mbar) was not sufficient to produce an improvement of ethanol recovery at 60 °C.
In scale-up assay, 47.53% of the produced ethanol was recovered (19.16 L distilled from 40.31 L produced) with distillation at air pressure, warming the whole tank at the end of fermentation with 85 °C hot water. The use of residual hot water from the cogeneration plant allows an energy saving, in each batch fermentation, of 28,000 kcal for warming 500 kg fermented biomass from 35 to 85 °C, and of 6000 kcal to maintain the same biomass at 85 °C during a 12 h distillation; this corresponds to 1775 kcal saved per liter of ethanol produced. If distillation is applied under vacuum, distilled ethanol recovered would rise to 37.49 L; energy consumption for vacuum production would be 2438 kcal per fermentation, then the global energy saving would decrease from 34,000 to 31,562 kcal, but considering the higher distilled ethanol recovery, the energy saved per liter of ethanol would be 842 kcal.