Abstract
This study examines the enhancement of dark sequential fermentation and photofermentation of organic solid waste using magnetite and substrate pre-treatment for hydrogen production within the context of transitioning to cleaner energy sources, particularly low-carbon hydrogen. Experimental dark fermentation and photofermentation apparatuses were used, utilizing microorganisms to decompose biomass at a mesophilic temperature (35 °C) of Organic Fraction of Municipal Solid Waste (OFMSW), inoculated with UASB sludge and enhanced with magnetite. A dosage of 120 mg/L of magnetite was the most effective, yielding an average value of 4144 mL H2/gVS. Additionally, the analysis revealed that the levelized cost of hydrogen (LCOH) decreases as more organic waste is utilized, making biohydrogen production a sustainable option, reaching USD 5/kg of OFMSW. Ultimately, generating hydrogen from organic waste can help reduce greenhouse gas emissions and promote a cleaner energy matrix.
1. Introduction
Currently, many countries still have energy matrices and production systems based predominantly on fossil fuels, that is, an energy matrix still largely dependent on non-renewable fuels with alarming, adverse environmental effects. Such effects are especially evident during climate change due to greenhouse gas (GHG) emissions, such as carbon dioxide (CO2). In Brazil, the management of organic waste, including the Organic Fraction of Municipal Solid Waste (OFMSW), poses an increasing challenge due to population growth and the resulting waste production. Given this scenario, energy transition to cleaner and more sustainable energy sources becomes essential. Low-carbon hydrogen stands out as a viable alternative, offering high calorific value and potential applications in several sectors, including transportation and heavy industry. The combustion of H2 fuels produces water and does not contribute to the GHG effect. The calorific value (61,100 Btu/Ib) of H2 is also almost three-times greater than that of methane (23,879 Btu/Ib), and it has the highest energy content per unit weight: 44 MJ/kg for gasoline, 50 MJ/kg for CH4, and 26.8 MJ/kg for ethanol [1]. In Brazil, the transportation sector is one of the main contributors to CO2 emissions, and low-carbon hydrogen can be an effective solution to reduce such emissions, in line with the reduction targets established at the 21st United Nations Climate Change Conference (COP21) in Paris. Negotiators aimed to define financing mechanisms for actions focused on decarbonizing the global economy and establishing deadlines for reducing GHG emissions. An agreement was signed by 195 countries, including Brazil, to combat global warming caused by climate change. The signatory countries committed to contributions, known as iNDCs (Intended Nationally Determined Contributions), which are set by each nation. The agreement aims to limit the increase in the global average temperature to below 2 °C above pre-industrial levels, with the aspiration to reach 1.5 °C by 2100. According to the projections established at COP 21, global emissions are expected to reach 55 GtCO2e by 2030 and to be reduced to 40 GtCO2e to align with the target of limiting warming to 1.5 °C above pre-industrial levels [2]. Brazil’s iNDC, presented at COP 21, includes a commitment to reduce GHG emissions by 37% from 2005 levels by 2025 and by 43% by 2030 [3]. After the National Congress approved the ratification of the Paris Agreement on 12 September 2016, Brazil’s climate goals transitioned from being “intended” to “official” commitments. On 21 September of the same year, the instrument was delivered to the United Nations. This change in terminology was marked by the removal of the “i” (intended) from the acronym, now referred to as NDC (Nationally Determined Contribution). The National Hydrogen Program (PNH2) was established in June 2022 by the National Energy Policy Council in Brazil to promote the hydrogen industry as a key energy vector and market [4,5]. The National Low-Carbon Hydrogen Policy was sanctioned by Law 14948, of 2 August 2024 [6]. The policy establishes guidelines for the production, transportation, and use of green hydrogen. Additionally, it establishes voluntary certification and offers federal tax incentives to the industry, promoting the production of low-carbon hydrogen in Brazil. The production of hydrogen from organic waste presents itself as a sustainable and economically promising strategy. These residues, which represent approximately 45.3% of urban waste in Brazil [7], can be converted into biohydrogen through biotechnological processes, such as dark fermentation. In this process, microorganisms decompose biomass, generating hydrogen, which is notable for its ability to produce hydrogen from organic waste and therefore control and stabilize biological waste, mitigating the potential risk of contamination [8]. A key advantage of dark fermentation is its fast hydrogen production rate—hydrogen evolution in dark fermentation (DF) can be orders of magnitude faster than in photobiological processes [1]. Another way to obtain hydrogen through bioprocesses is by applying photofermentation (PF), a process where photosynthetic bacteria convert organic substrates into hydrogen under illumination, which triggers hydrogenase enzymes reducing protons to H2; a significant benefit of photofermentative hydrogen production is its high theoretical yield and effective COD removal [1,9,10,11]. Another method that stands out is the integration of DF and PF, as together they are capable of maximizing organic matter degradation and, consequently, H2 recovery. Studies have reported that these two-stage systems achieve a much higher fraction of the substrate’s theoretical H2 potential, in some cases nearly doubling the yield obtained with single-stage fermentation [1,11]. However, to achieve the economic viability of this method on a large scale, it requires careful analysis to determine its costs, such as obtaining real laboratory data on H2 production per mass of organic material added. For this purpose, the present study will determine the production of hydrogen through the DF and PF method in organic substrates enhanced with iron particles in order to evaluate its potential. For this purpose, statistical and economic analyses will be carried out, such as internal rate of return (IRR),
Net present value (NPV), levelized cost of hydrogen (LCOH), and payback period. It is expected that with such data it will be possible to strengthen the narrative about the importance and viability of the application of bioprocesses that promote the production of possible biofuels and energy vectors, thus mitigating the environmental crises related to this issue.
2. Biomass for Biohydrogen Production and Enhancement with Additives
Biomass sources, including energy, agricultural, forestry, industrial, and municipal wastes, are utilized in microbially assisted hydrogen (H2) production. The microbial capability to decompose biomass has been extensively studied to optimize hydrogen yield [1]. Hydrogen production from organic waste offers the potential to reduce the amount of waste (like wastewater) and do so readily [8]. Dark fermentation presents advantages, including the utilization of diverse waste streams and a simple reactor technology [8] while the hydrogen production rate (H2 volume per reactor volume per unit time) can be an order of magnitude higher than those achieved other methods [1]. However, according to known fermentative reactions, the maximum theoretical hydrogen yield is limited to 4 mol H2/mol glucose when the only electron sinks are hydrogen and acetate [1]. It also has drawbacks, such as significant byproduct generation, reactor-to-reactor variation, and low chemical oxygen demand (COD) removal. Additionally, according to [1], the primary challenge in dark fermentative production is its low hydrogen yield. For instance, when glucose is the substrate, converting 100% of its equivalents to hydrogen could theoretically yield 12 mol H2/mol glucose. Nevertheless, based on the Gibbs free energy variation, butyrate fermentation is more dominant than the acetate reaction. It produces only 3.3 ATP molecules, and the maximum H2 production is 2.5 mol H2/mol glucose, as shown in Equations (1) and (2), on a stoichiometric basis [8,9]. The expected maximum hydrogen yield is limited to values lower than 4 and 2.5 mol H2/mol glucose for acetate and butyrate fermentation, respectively, since the inoculum itself consumes hydrogen. There is mention of values only in the range of 1–2.5 mol H2 mol/glucose produced in practice [12].
Under certain conditions, metabolic pathways result in the production of ethanol and acetate, thereby reducing the stoichiometric yield of hydrogen to two moles of H2 per mole of glucose (equivalent to 272 mL of H2 per gram of hexose at 25 °C), as depicted in Equation (3) [9].
A two-stage system (dark and photofermentation) can extract additional energy and improve COD removal. In photofermentation, readily available waste streams can serve as a stock, enabling complete substrate conversion; however, this process is hindered by a low volumetric production rate and limited conversion efficiencies [8]. Theoretically, in photofermentation and under appropriate physicochemical conditions, four moles of hydrogen can be generated from one mole of acetate [1], as shown in Equation (4). Purple non-sulfur bacteria produce molecular hydrogen (H2) through a process catalyzed by nitrogenase under nitrogen-deficient conditions, utilizing light energy and reduced compounds, such as organic acids [10].
Additionally, hydrogenases involved in dark fermentation are primarily categorized into two groups based on their content [13]: [Fe–Fe]-hydrogenase and [Ni–Fe]-hydrogenase.
After using food waste as feed, heat shock-treated anaerobic sludge as the inoculum in a leaching bed reactor in a two-stage process, at 37 °C, with a pH between 5.5 and 7, the maximum H2 yield was 310 (mL H2/g VSadded), the maximum H2 production rate was 151.25 mL H2/L/h, and the H2 in biogas was between 10% and 55% [13].
A diverse range of bacteria can produce [Ni–Fe]-hydrogenase, while some are capable of synthesizing [FeFe]-hydrogenase. According to the authors [13], adequate levels of iron and nickel ions are essential for the expression and functionality of these enzymes within dark fermentation. Numerous studies have investigated the impact of nickel and iron ions on the dark fermentation process, demonstrating enhanced efficiency at specific concentrations of Fe and Ni ions. The effects of Fe° nanoparticles (NPs) and Fe2+ ions were significant (p < 0.005), enhancing the hydrogen yield by 37% and 15%, respectively, in the study by Taherdanak et al. [14]. According to Taherdanak et al. [14], cultures containing iron oxide NP exhibited a 38% increase in production with a maximum hydrogen production rate 58% higher than cultures without NP or containing just silica particles. When used in a 2.5 L sequential batch reactor, iron oxide NP demonstrated no significant impact on yields (maintained at 2.2 molhydrogen/molglucose) but resulted in a +113% improvement in H2 production rate, and a peak rate of 86 mL/L·h [15]. The incorporation of 200 mg/L Fe2+ and 200 mg/L magnetite nanoparticles into the inoculum (heat-treated sludge) resulted in a 62.1% and 69.6% enhancement in hydrogen yield, respectively [15], using sugarcane bagasse as feed. For magnetite addition, the maximum hydrogen yield was 1.211 mol/mol [16]. The study by Pérez-Barragán et al. [17] investigated the effect of magnetite supplementation at concentrations of 50, 100, and 200 ppm on hydrogen production in a continuous dark fermenter using rice straw acid hydrolysate. The optimal production rate, 2.6 ± 0.3 NL H2/L-d, was obtained by Pérez-Barragán et al. [17] with the addition of 100 ppm nanoparticles, reflecting a 53% increase from the rate without magnetite supplementation. Nevertheless, the hydrogen production rate decreased to 1.6 ± 0.2 NL H2/L-d when the NP concentration increased to 200 ppm.
BioH2 generated through dark fermentation is a gas mixture primarily consisting of H2, CO2, and moisture, with H2 concentrations typically ranging between 40% and 60% [12]. The integration of dark fermentation and photofermentation is proposed as a solution to address the issue of limited hydrogen yield in dark fermentation, since the volatile fatty acids generated during the process cannot be fully oxidized [18].
3. Materials and Methods
3.1. OFMSW Substrate and Inoculum Preparation
3.1.1. Dark Fermentation
As advocated by Jariyaboon et al. [19], food waste can be utilized for the feasible generation of mixed volatile fatty acids (VFAs) and bio-hydrogen through dark fermentation. Theoretical and laboratory research methods were employed to conduct this study, yielding expected results in the production of biogas through dark fermentation in biodigesters using potato wastewater in the absence of light.
This study was conducted by collecting samples of food waste from the Federal University of Itajubá (UNIFEI) Restaurant at the Itajubá Campus, which would otherwise be discarded in a landfill, along with sludge from the COPASA wastewater treatment plant (WWTP) in Itajubá, MG, Brazil. Organic waste from the university restaurant at UNIFEI’s Itajubá Campus has already been studied by Barros et al. [20] for use in composting, by Cruz et al. [21] for anaerobic digestion, and by Barros et al. [22] in a lysimeter. However, the possibility of producing BioH2 from such waste has not yet been studied. Additionally, magnetite was added to the biodigester mixture. According to Sun et al. [23], the addition of magnetite/reduced graphene oxide nanocomposites (Fe3O4-rGO NCs) and magnetite nanoparticles (Fe3O4 NPs) enhances biohydrogen (BioH2) production during dark fermentation. The authors found that a supplement concentration between 10 and 100 mg/L is optimal for increasing BioH2 production, while concentrations above 100 mg/L may lead to inhibition.
The wastewater treatment plant (WWTP) sludge served as the inoculum to initiate fermentation, while magnetite acted as a catalyst to boost BioH2 production. As noted by Sun et al. [23], iron-based and carbon-based nanoparticles possess desirable physicochemical properties, including the quantum dimensional effect, a large specific surface area, non-toxicity, and biocompatibility. These properties make them effective additives for enhancing dark fermentation systems. Iron-based nanoparticles positively influence the production of fermentative hydrogen through the slow release of bioavailable Fe2+ and Fe3+ ions, which are crucial trace elements that stimulate enzymatic activity for BioH2 production; however, excessive amounts can lead to inhibition.
On 15 June 2023, approximately 3.5 kg of food waste was collected from the UNIFEI University Restaurant. The sample included rice, beans, chicken, polenta (made from corn flour), tomatoes, eggs, chard, cucumbers, and beets, following that day’s menu. Since the food waste was collected on a regular day from the restaurant, all items were mixed without any separation by type. As a result, it was not possible to measure the individual quantities of each food item present in the sample. The sludge used as inoculum was collected from the average register of the COPASA WWTP, located in Itajubá, MG, on 16 June 2023, at approximately 8:00 a.m.
The substrate was prepared by adding approximately 800 mL of water to the food sample. The mixture was then blended to homogenize it and break it down into smaller particles, which facilitates the fermentation process, following the methodology outlined by Cruz et al. [21]. Mechanical reduction is a type of physical pretreatment that alters the particle size, thereby increasing the surface area available for a reaction.
The mixture used in this experiment consisted of 350 mL of substrate, 150 mL of inoculum, and 0, 100, or 120 mg/L of magnetite in each biodigester. The magnetite dosages were determined according to the methodology of Sun et al. [23], using an optimal dosage of 100 mg/L and an upper dosage of 120 mg/L to evaluate the behavior of biogas production.
3.1.2. Photofermentation
The digestates from dark fermentation served as substrates for the subsequent photofermentation process after 90 days.
3.2. Construction of Biodigesters (Reactors)
3.2.1. Dark Fermentation
Following the methodology of Cañote et al. [24], nine biodigesters were constructed using polyethylene terephthalate (PET) bottles with a total volume of 2.1 L. These bottles were coated with black paint and sealed using thread sealant, insulating tape, silicone, and equipped with air chamber valves in the lids for gas control and measurement. The effective volume of the reactors was allocated as follows: 0.35 L for the substrate, 0.15 L for the inoculum, and 1.6 L for storing the generated biogas (Figure 1a).
The nine biodigesters were divided into three groups of three (triplicate), as follows: (a) a blank control; (b) a concentration of 100 mg/L of magnetite; and (c) a concentration of 120 mg/L of magnetite. Throughout the experiment, the biodigesters were kept approximately one-third submerged in water heated to around 35 °C by a thermostat (Figure 1b). This methodology was chosen because it creates favorable conditions for the development of fermentative microorganisms.
3.2.2. Photo Fermentation
The digestate mass from dark fermentation was sent to the nine biodigesters for photofermentation (Figure 1c), which were assembled based on the design by Castro and Silva et al. [25]. Each photofermentation biodigester was filled with the corresponding digestate for each set with triplicates, classified as: (1) without magnetite (blank); (2) 100 mg/L of magnetite; and (3) 120 mg/L of magnetite. In the photofermentation process, the temperature was maintained at 35 °C using a thermostat.
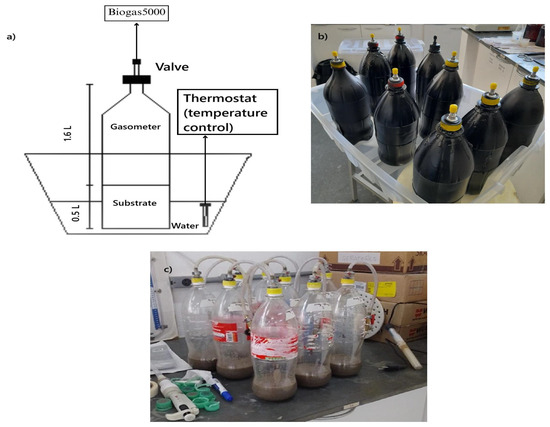
Figure 1.
Experimental apparatus for sample digestion at the pilot scale: (a) schematic representation for dark fermentation; (b) nine dark fermentation reactors; and (c) nine photofermentation reactors. A light illuminated the photo-reactor, which was in an environmental lamp.
3.3. Physicochemical Analyses of the Substrate, Digestate, and Inoculum
Following the methodology outlined by Sun et al. [23], laboratory analyses were conducted to characterize both the substrate and the wastewater treatment plant (WWTP) sludge, as well as the digestate. The analyses included measurements of mass, total solids (TSs), volatile solids (VSs), fixed solids (FSs), pH, and chemical oxygen demand (COD). The physicochemical analysis of the digestate included solid series (TS, FS, and VS), pH, and COD after 90 days of dark fermentation of the substrate. These evaluations were conducted by the Standard Methods for the Examination of Water and Wastewater, as established by the American Public Health Association (APHA) [26].
3.4. Biogas Quality and Quantity
Biogas readings were taken using the Geotech© Biogas 5000© equipment, sold by QED Environmental Systems Ltd. located in Coventry, UK, which utilizes control valves and suction hoses. This device analyzes the percentage of each gas present in the biodigester, measures the internal pressure of the reactors, monitors the temperature of the water in which the reactors are immersed, and performs various other functions. With the device, it is possible to measure the different gases present (oxygen, methane, hydrogen sulfide, carbon dioxide, and hydrogen), as well as the pressure in the system. Measurements were taken at 5, 25, 60, and 90 days after the start of the fermentation process. The experiment reached its maximum generation peak at 30 days of fermentation, with the best results at 60 days for biohydrogen, which was the most promising for this research. This equipment reads H2 up to 1000 ppm, which corresponds to a range of 0.18–0.22 mL of H2/gVS, depending on the total pressure of the system. When the analysis exceeded this maximum value, samples were collected and analyzed using an elementary gas analyzer (Asea Brown Boveri ABB®, model number AO2040, Frankfurt, Hesse, Germany). It can analyze CO, CO2, CH4, and H2 levels in a range from 0 to 60% by volume. This equipment continuously monitors various gases and consists of two measurement modules: URAS 26 and CALDOS 27. The first module, URAS 26, is an analyzer that operates on the principle of infrared absorption. Therefore, compound gases such as CO, CO2, and CH4 absorb infrared radiation (Beer-Lambert law), and the equipment can measure this absorption. While the CALDOS 27 analyzer measures the thermal conductivity of gases such as H2 or its compounds, its operating principle is to measure the current variation between the sample gas and a standard gas using a Wheatstone bridge. available at the Fuel Characterization Laboratory of the Center of Excellence in Thermoelectric and Distributed Generation (NEST) at UNIFEI.
The Boyle and Gay-Lussac law, as advocated by Ribeiro et al. [27] and Castro and Silva et al. [25], were applied to the measurements and corrections of the generated biogas volumes in both dark fermentation and photofermentation. The standard pressure and temperature (STP) values were considered for the corrections from the local conditions of Itajubá-MG (Equation (5)).
where:
- VCNTP = corrected volume (m3) for STP;
- pCNTP = corrected biogas pressure for 1 atm—10,332.72 mm H2O;
- TCNTP = corrected biogas temperature for 20 °C—293.15 K;
- V1 = biogas volume in the gasometer;
- p1 = pressure of the biogas at the time of measurement in the reactors, which consists of the average atmospheric pressure at Itajubá-MG on the day of measurement, in addition to the pressure added by the gasometer, measured by the Geotech© Biogas 5000© equipment; and
- T1 = biogas temperature at the time of measurement, temperature in mesophilic conditions, 308 K.
For the calculation from volume (liters) to mass (in grams) of H2 per gram of VS of substrate, a density of hydrogen was considered at 0.08235 kg/m3.
The assessment of statistical significance was carried out using statistical analyses implemented in Python® (version 3.11), utilizing the libraries pandas, numpy, scipy.stats, statsmodels, and seaborn.
Initially, the sample standard deviation was calculated to estimate the internal variability within each experimental group. Subsequently, an Analysis of Variance (ANOVA) was performed, considering the experimental units—defined by the variation in magnetite concentration—as the independent factor, and the volume of hydrogen produced (mL H2/g SV) as the dependent variable.
To identify specific differences between the groups, the Tukey’s Honestly Significant Difference (HSD) test was applied using the pairwise_tukeyhsd function. Boxplots were generated using the seaborn library to visualize the dispersion and compare the data across treatments.
3.5. Economic Analysis—LOCH
Parameters such as net present value (NPV) and internal rate of return (IRR) are commonly used to assess the attractiveness of an investment. The levelized cost of hydrogen (LCOH) has become a key indicator in the economic evaluation of low-carbon hydrogen projects. It is a comprehensive assessment of the cost-effectiveness of H2 production over its life cycle. The analysis includes a range of costs, from the initial capital investment (CAPEX) required to operate the infrastructure and technology to the operating expenses (OPEX) associated with maintenance, energy consumption, and labor costs. Levelized cost of hydrogen is unique in its ability to provide a holistic view, considering all aspects of H2 production and utilization. Such analyses play a crucial role in the decision-making process, enabling stakeholders to compare the costs of low-carbon hydrogen with those of traditional production methods and other energy carriers [28,29]. Crispim et al. [30] also utilized LOCH to analyze the economic viability of low-carbon H2 projects from the team methane reforming of biomethane derived from landfill biogas purified in the state of Minas Gerais, Brazil. Financial analysis was carried out using this methodology [30], as shown in Equation (6).
where:
- LCOH = the levelized cost of H2 (USD/kg);
- = the sum of associated costs to produce H2 (sum of CAPEX and OPEX about the purification of biogas into H2, according to Nemestóthy et al. [31]);
- n = a 20-year analysis, where i is the interest rate equal to 8% per year [32];
- = H2 flow generated during the period of analysis.
The currency was converted to USD 1.00, equivalent to RUSD 5.6, according to the BCB [33]. The calculation of the LCOH was based on the annual amount of organic waste generated by the University Restaurant at UNIFEI, Campus Itajubá-MG, Brazil. For this, the average waste production in August was calculated, which was then distributed throughout the remainder of the 2022 academic year, accounting for 181 days. The calculations considered biodigestion for different volumes of organic waste, ranging from 4772.82 kg to 40,000 kg in increments of 5000 kg.
4. Results
4.1. Biogas Quantity and Quality
4.1.1. Dark Fermentation
Table 1 presents the results obtained during the four measurements performed over time for dark fermentation.

Table 1.
Results for bottles with the addition of 100 mg/L, 120 mg/L of magnetite, and a blank for dark fermentation.
Biogas analyses using an elementary gas analyzer, ABB®, were conducted on the 90th day, and the results are presented in Table 2.

Table 2.
Proportion (in percentage) and volume (in liters) of H2 in biogas after analyses in an elementary gas analyzer, ABB®, on the 90th day of dark fermentation.
4.1.2. In Photofermentation
Table 3 presents the results obtained from the four measurements performed over time for photofermentation.

Table 3.
Results obtained for bottles with the addition of 100 mg/L, 120 mg/L of magnetite, and a blank for photofermentation.
4.2. Physical–Chemical Analysis Results
4.2.1. The Dark Fermentation Process
The physical–chemical analysis results for the substrate, inoculum, and the substrate mixture (OFMSW from UNIFEI Restaurant) from the dark fermentation process are presented in Table 4. Figure 2 presents the results of the physical–chemical analyses of the digestate from dark fermentation. Part A of Figure 2 presents the levels of COD and VS removal in each of the experimental units. Part B presents a general overview of the analysis of total solids, also quantified in volatile and fixed solids of the respective experimental units.

Table 4.
Physical–chemical analysis results for the substrate and inoculum of the dark fermentation process.
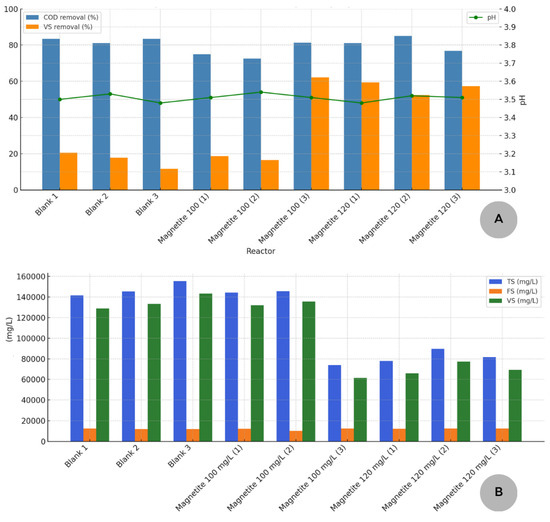
Figure 2.
(A) COD and SV removal rates after dark fermentation. (B) Concentrations of TS, VS, and FS in the experimental units after dark fermentation.
4.2.2. The PhotoFermentation Process
Figure 3 presents the results of the physicochemical analyses of the digestate from photofermentation. Part A of Figure 3 represents the COD removal levels after photofermentation, as well as the pH variation in the experimental units, while part B represents the results of the concentrations of total, volatile, and fixed solids analyses.
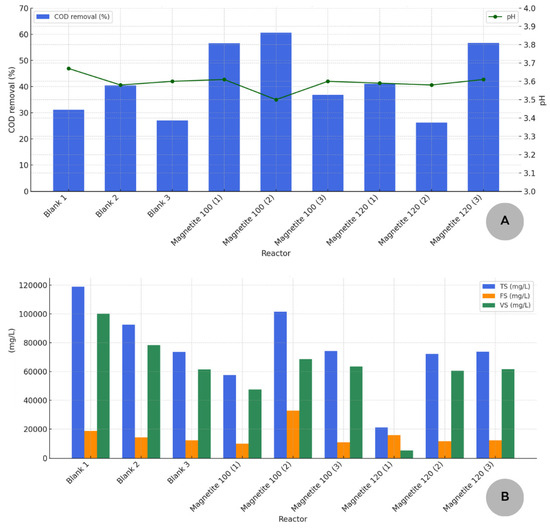
Figure 3.
(A) COD removal rates after photofermentation. (B) Results of TS, FS, and VS analysis after photofermentation.
4.3. Hydrogen Production Results in the Dark Fermentation Process
The hydrogen production results concerning VS added are presented for the dark fermentation process, as shown in Table 5.

Table 5.
Hydrogen production in the dark fermentation process with VS added.
The results of the ANOVA statistical analysis reveal a calculated F value of 17.593 and a p-value of 0.003. Since the p-value is lower than the 0.05 significance level, this indicates that there is a statistically significant difference among the groups.
The Tukey’s Honestly Significant Difference (HSD) test identified which treatments resulted in significantly higher H2 production. The outcome is illustrated in Figure 4, where each horizontal line represents the 95% confidence interval of the mean difference between a pair of groups. The central dot indicates the observed mean difference; red lines denote statistically significant differences, whereas the blue line represents a pair without significant difference. In summary, the treatments with magnetite (100 and 120 mg/L) showed statistically significant differences when compared to the control group; however, no significant difference was found between the two magnetite concentrations.
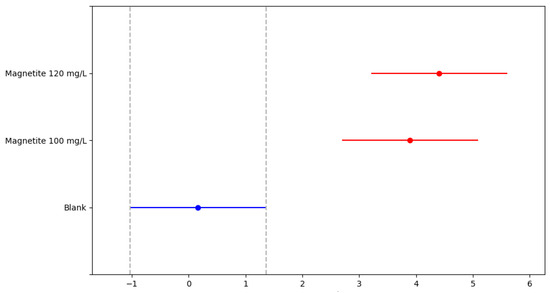
Figure 4.
Results of the statistical analysis for the Tukey’s test.
4.4. Economic Analysis for Biohydrogen Production
Table 6 presents the total investment and Operation and Maintenance (O&M) costs for BioH2 production, including dark fermentation reactor, purification, and compression. Figure 5 shows LCOH variation as a function of OFMSW to be digested in the dark fermentation reactor.

Table 6.
Total investment and operation and maintenance (O&M) costs for BioH2 production.
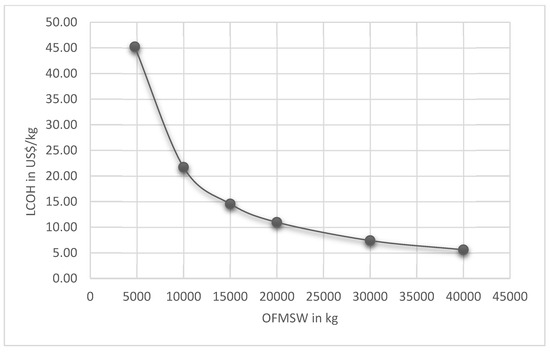
Figure 5.
LCOH variation as a function of OFMSW to be digested in the dark fermentation reactor.
To improve the reliability of the results, Figure 6 and Figure 7 show sensibility curves for the LCOH per mass of the two factors. This represents the cost of investment along with the discounted rate.
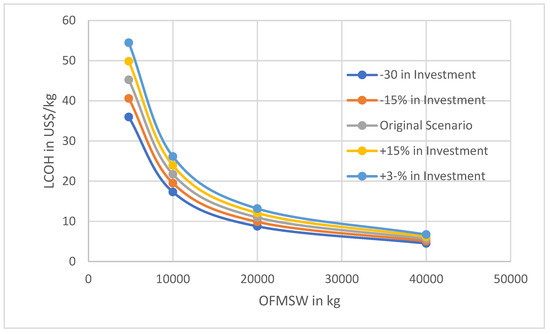
Figure 6.
LCOH variation as a function of OFMSW to be digested in the dark fermentation reactor in function of the investment.
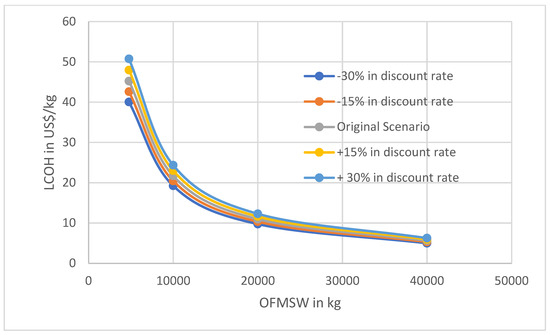
Figure 7.
LCOH variation as a function of OFMSW to be digested in the dark fermentation reactor in function of the discount rate.
5. Discussion
5.1. Biogas Quantity and Quality
5.1.1. Dark Fermentation
Upon analyzing Table 1, even without the addition of magnetite, hydrogen levels exceeded the reading limit of 1000 ppm, greater than 0.22 mL H2/g VS, (greater than 0.1%) in all three reactors after approximately 30 days of fermentation. Methane gas (CH4) was detected in low proportions, ranging from 0% to 1.1%. This is a positive outcome for this research, as it was expected; dark fermentation typically leads to the formation of its precursors, butyrate and acetate. However, carbon dioxide (CO2) stands out in considerable proportions, with values ranging from 0.9% to 81.1%, as expected in a dark fermentation process. This fact is due to the natural dark fermentation process, where there is evident formation of H2 + CO2 gases, corresponding to what was expected, as it was described by authors as Ghimire et al. [9], Akhlaghi and Najafpour-Darzi [11], García-Depraect [12], and Jariyaboon et al. [19]. According to Ren et al. [34], for mixed acid fermentation, glucose is converted into acetate, lactate, propionate, ethanol, hydrogen, and carbon dioxide. Oxygen gas (O2) appears in small proportions at the end of the generation cycle, becoming more prominent in the final reading of the experiment, representing approximately 18%. This fact may indicate a small amount at the beginning of the process. The nitrogen balance, represented by the nomenclature “Bal”, accounted for a significant portion of the total gases generated, ranging from 0% to 80%, and varying throughout the experiment according to anoxic to strictly anaerobic environments. Hydrogen sulfide (H2S) represented low proportions, varying between 0.007% and >0.5%. According to Sun et al. [23], the optimal dosage of magnetite, which enhanced the H2 generation process, was 100 mg/L. Nevertheless, Beckers et al. [15] achieved the optimal dosage of 200 mg/L of magnetite. Therefore, this dosage was adopted to evaluate biogas generation over time with and without the addition of magnetite. Analyzing Table 1, it is also possible to compare the results for magnetite dosages of 100 and 120 mg/L and verify that hydrogen generation occurred in greater proportions from the first reading, reaffirming the potential of magnetite to catalyze the process. In addition to enhancing hydrogen production, magnetite also caused changes in the other gases, although these changes were of little relevance. The dosage of 120 mg/L showed the best results.
Biogas measurements were taken on days 5, 25, 60, and 90 following the start of the fermentation process. The experiment reached its peak in biogas generation at 30 days of fermentation. However, the most promising results for biohydrogen production were observed in 60 days. Hydrogen levels quickly reached the maximum capacity of the measurement equipment, with readings exceeding 0.22 mL H2/g VS after 30 days. The hydrogen generation cycle is prolonged, with levels still above 0.22 mL H2/g VS observed even after more than 90 days of fermentation.
By analyzing Table 2, it is evident that the results obtained with a dosage of 120 mg/L of magnetite were the optimal dosage, indicating that the methodology of adding magnetite is effective for maximizing BioH2 production during dark fermentation.
5.1.2. In PhotoFermentation
When undergoing a second photo digestion process (Table 3), the potential for hydrogen generation is not significant, as most of the fermentable compounds have already been converted during the previous dark fermentation process. Most of the values were lower than 0.22 mL/gVS. Only two measurements greater than 0.22 mL/gVS were observed in one of the blank reactors, rather than what was expected. Perhaps the magnetite had been inhibitory in the photofermentation process, as it hindered light from crossing the substrate. Due to the limited production of BioH2 in photofermentation, this study focuses solely on hydrogen production through dark fermentation. Small amounts of oxygen (O2) were also detected. The nitrogen balance, labeled as “Bal,” constitutes a significant portion of the total gases generated. Additionally, there is a small production of methane (CH4), with a concentration of 120 mg/L of magnetite.
5.2. Physical–Chemical Analysis Results
5.2.1. The Dark Fermentation Process
According to Table 4, it is possible to verify that VS accounts for 86% and FS accounts for 14% of TS. The pH values indicate that both the WWTP and the substrate are slightly acidic. These values are crucial for subsequent calculations and system efficiency in organic matter removal, as shown in Figure 2. According to Table 4, it is evident that COD removal accounts for 81.07–85.08% and VS removal accounts for 52.32–59.40% for a magnetite dosage of 120 mg/L. The pH values indicate that both the WWTP and the substrate were slightly acidic after the dark fermentation process, indicating, as expected, an acidic environment (3.48–3.52 for a magnetite dosage of 120 mg/L). As advocated by Jayalakshmi et al. [35], the higher concentration of VFA was the motive for the drop in pH values in the reactors.
5.2.2. The PhotoFermentation Process
Figure 3 presents values for COD removal in the range of 72.48–85.08%; however, there are still high values of COD ranging from 12,520 mg/L (magnetite dosage of 120 mg/L) to 15,035 mg/L (blank) and of VS from 5305 mg/L (magnetite dosage of 120 mg/L) to 100,140 mg/L (blank).
Figure 3 shows a significant decrease in all measurements in parameters, with input analyses conducted through dark fermentation and output analyses performed after photo digestion. According to Jayalakshmi et al. [35], photosynthetic bacteria, such as purple non-sulfur bacteria, carry out this photofermentation process in the presence of light, using VFAs as substrates, including acetate (HAc) and butyrate (HBu). The presence of only ambient light limited the potential of photofermentation. Additionally, the high concentration of VFAs generated during dark fermentation is evident from the low pH observed in the metabolites at the end of the process (Figure 2). Therefore, the digestate from photofermentation may still contain numerous soluble metabolites and VFAs, as reflected in the consistently low pH values of the photofermentation digestate (Figure 3), ranging from 3.50 to 3.67. Future studies may utilize liquid chromatography to assess VFAs in metabolites and potentially pre-treat these metabolites before photofermentation. This process may also require a more intense light source.
Several factors may have contributed to the low hydrogen production during photofermentation. An excess of carbon compared to nitrogen (C/N) can hinder cell growth and reduce hydrogen production. Additionally, high concentrations of magnetite can be toxic to photofermentation processes, as it may block light and promote the development of competing microorganisms that consume substrates without producing hydrogen. If the UASB sludge used as inoculum from the Itajubá wastewater treatment plant lacks a significant population of purple phototrophic bacteria, hydrogen production is likely to be low. Furthermore, a pH outside the ideal range (typically between 6.5 and 7.5) can inhibit essential enzymes involved in photofermentation [19,35,36,37,38].
5.3. Hydrogen Production Results
By observing Table 5, it can be seen that the optimal dosage of 120 mg/L magnetite achieves an average volume of 4.144 mL H2/gVS, slightly below the values found in the literature, since, according Lay et al. [39], as also presented in Ren et al. [34], using food waste as a substrate, grass composts as an inoculum, and Fe2+ (50–250 mg/L) as an additive, at pH 7, and 37 °C, an optimal production of 61.7 mL H2/g VS (+ 14.9%) was achieved for the optimal dosage of 125 mg/L. These authors [39] conducted an experiment using grass composts at high temperatures and durations (80 °C and 3 h, respectively), which could yield high numbers of hydrogen-producing microorganisms. In addition, Yin et al. [40] reported that BioH2 production from macroalgae was significantly increased by 6.5 times compared to the control test, reaching 20.25 mL H2/g VS with the addition of 200 mg/L NZVI (Zerovalent Iron). In another study, Camacho et al. [41] achieved hydrogen production from organic market waste by adding 0–2000 mg/L Fe0 NPs, and an improved hydrogen yield of 101.7 mL H2/g VS was obtained with a 2000 mg/L Fe0 NPs dosage. An optimal pH of 6.5 and a TS content of 7.5% resulted in a hydrogen yield of 103.4 LH2/gVS obtained by Regueira-Marcos et al. [42]. Using kitchen waste as a substrate and heat-treated inoculum at 100 °C, at a feeding rate of 7 kg kitchen waste/day, Jayalakshmi et al. [35] obtained a value of 0.072 molH2/mol substrate; according to Jayalakshmi et al. [35] the reactor pH stabilized at 5.6 after 21 days after its operation, in which 40% VS degradation was attained. It produced 72 mL H2/gVS.
The low hydrogen production yield may be related to several factors, such as limitations of the dark fermentation method, hydrogen consumption by the inoculum, inadequate process conditions, among others [1,8,9,14]. However, given the results obtained, probable causes stand out: the occurrence of alternative metabolic pathways, a low rate of organic matter degradation, and an acidic pH during the process.
Depending on the experimental conditions, some metabolic pathways may be facilitated, leading to the formation of byproducts or the co-production of hydrogen and methane. The data in Table 1 demonstrate methane formation, in some cases even greater than hydrogen production. Figure 2A shows that VS removal did not exceed 65% in any of the experimental units, indicating low organic matter consumption. According to Regueira-Marcos et al. (2023) [42], studies with degradation greater than 70% obtained good results of up to 103.4 mL H2/gVS.
The low pH of the digestate may also have impacted the yield, as it was between 4.48 and 3.52, shown in Figure 2A, a scenario that appears to be an inhibitor for the nitrogenase and hydrogenase enzymes, essential for the production of H2. The ideal, according to the literature, is for the pH to be in the range of 5.5 to 7.0 for dark fermentation [16,42].
Combining dark and photofermentation increases the hydrogen yield from cassava starch from a range of 9.3–10.7 mmol H2/g to 17.5–18.0 mmol H2/g, marking an improvement of 63.1% to 93.7%. This is significantly higher than the 4.0 to 9.47 mmol H2/g reported in the literature [36]. Sequential fermentation in a photo shadow environment can increase the theoretical hydrogen yield from 4 to 12 mol H2 per mol of glucose, and the experimental hydrogen yield from 1.72 to 5.48 mol H2 per mol of glucose [35]. According to Su et al. [36], several causes of inhibition in photofermentation exist, making it essential to determine the optimal concentration of VFAs for enhancing hydrogen yield. At acetate concentrations below 15 mM, it mainly supports bacterial growth with minimal hydrogen production. However, high acetate concentrations can cause substrate inhibition. There is the possibility of inhibitory effect of on nitrogenase. Hydrogen yields decreased as soluble metabolite concentrations rise. Acetate and butyrate are almost entirely utilized during photofermentation, with utilization ratios in the range of 74.6–89.3% for acetate and 95.6–98.5% for butyrate [36].
In this sense, we emphasize the potential alternative route identified by Castagnoli et al. [43], who investigated an alternative method for producing polyhydroxyalkanoates (PHAs) by using VFAs as intermediates. Their research focuses on utilizing mixed microbial cultures in sequencing batch reactors (SBRs) that are fed with VFA-rich solutions derived from fermented organic waste, such as cheese whey, food waste, and sewage sludge. Their approach takes advantage of low-cost waste streams and facilitates the conversion of VFAs into PHAs, which are biodegradable bioplastics, within the framework of a circular bioeconomy. The decoupled feeding strategy, where bacterial accumulation and replication phases are separated, has proven effective in maximizing the conversion efficiency of VFAs into PHAs. This method offers a sustainable and economically viable alternative to fossil-based plastics [43].
5.4. LCOH
The UASB reactor has a capacity of up to 30 m3, and the cylinder for storing biogas has a volume of 1000 l. In addition, the annual cost varies from year to year (Table 6), which is caused by the lifespan of specific process components, such as the crusher and the separation membrane, among others. It is noted that as the amount of organic waste increases, the production cost decreases (Figure 2). Still, there is a limitation on the amount of biomass that can be inserted in the anaerobic reactor. The reactor has a maximum capacity of 30,000 L; however, not all the space in the reactor can be occupied by biomass, as the gases generated also occupy part of this space in the gasometer.
As illustrated in Figure 6 and Figure 7, sensitivity analyses were conducted with respect to initial investment and discount rate. The results indicate that variations in levelized cost of hydrogen (LCOH) are more pronounced when altering the initial investment than when adjusting the discount rate. Another noteworthy observation is that for lower waste mass inputs, the influence of both parameters on LCOH becomes more significant. For instance, with a waste mass of 4773 kg, the hydrogen price fluctuates by approximately 20 USD/kg when the initial investment varies by ±30%, and by about 10 USD/kg for a ±30% change in the discount rate. In contrast, for a waste mass of 40,000 kg, the variation in hydrogen price is around 2 USD/kg. This suggests that utilizing larger quantities of waste can mitigate the impact of economic uncertainties on project outcomes. Sensitivity analyses were not performed for minor component costs, such as the cost of magnetite, due to their negligible influence on total project costs in the scenario under study.
6. Conclusions
The results of this research, presented in this paper, highlight the significant potential of dark sequential fermentation and photofermentation of OFMSW and all organic wastes for hydrogen production. This sequential process is particularly effective when combined with the addition of magnetite and substrate pretreatment. Statistical analyses, particularly ANOVA, revealed that the experimental units containing magnetite presented statistically significant differences compared to the control units, demonstrating the efficiency of iron particles in the hydrogen production process, reaching an average production of 4144 mL H2/g VS. However, Tukey’s test revealed no significant difference between the magnetite concentrations, suggesting that both concentrations were efficient compared to the control units. Additionally, the economic analysis shows that the levelized cost of hydrogen (LCOH) decreases as the amount of organic waste increases, making biohydrogen production a sustainable and economically viable strategy reaching USD 5.6/kgOFMSW for 40,000 kg.
A significant challenge identified during the process is the accumulation of volatile fatty acids (VFAs), which can inhibit hydrogen production. VFAs, such as acetate and butyrate, lead to a decrease in pH, creating an acidic environment that negatively impacts the efficiency of the photofermentation process. This situation underscores the need for strategies to control VFA levels and maintain optimal conditions for hydrogen production.
Accordingly, using organic waste to generate biohydrogen not only helps reduce liquid GHG gas emissions, but also promotes a cleaner and more sustainable energy matrix in the context of the energy transition. This study emphasizes the importance of continuing to invest in innovative and sustainable technologies for energy production, aiming to overcome the problems that result in low biohydrogen production and seeking a greener future that is less dependent on fossil fuels.
Author Contributions
Conceptualization, R.M.B.; methodology, R.M.B., R.d.S.C., G.L.T.F. and E.E.S.L.; software, D.M.Y.M., I.F.S.d.S. and A.J.M.d.O.P.; validation, R.M.B., D.M.Y.M., A.J.M.d.O.P. and J.V.R.d.F.; formal analysis, R.M.B., D.M.Y.M., A.J.M.d.O.P. and J.V.R.d.F.; investigation, G.C.d.S., J.S.S., I.F.S., A.J.M.d.O.P. and J.V.R.d.F.; resources, R.M.B., D.M.Y.M. and E.E.S.L.; data curation, R.M.B., R.d.S.C., G.L.T.F. and E.E.S.L.; writing, R.M.B.; writing—review and editing, R.M.B., R.d.S.C., G.L.T.F., I.F.S.d.S., A.J.M.d.O.P. and E.E.S.L.; visualization, R.M.B., R.d.S.C., G.L.T.F. and E.E.S.L.; supervision, R.M.B. and R.d.S.C.; project administration, R.M.B. and R.d.S.C.; funding acquisition, R.M.B., D.M.Y.M. and E.E.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq; in Portuguese) for the Research Productivity Grant to Prof. Regina Mambeli Barros (PQ1D, Process Number 303036/2021-4), for the Research Productivity Grant to Prof. Electo Eduardo Silva Lora (PQ1A), and for granting a Scientific Initiation scholarship to Gabriela Cadete de Souza. This research was funded by the Minas Gerais State Agency for Research and Development (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG, in Portuguese) for granting financial support by Project: RED-00090-21, “Theoretical–experimental evaluation of the production and use of green hydrogen in Minas Gerais” and for granting a Scientific Initiation scholarship to Isabela Faria Silva. This research was funded by the Federal University of Itajubá, which granted a Scientific Initiation scholarship to Jessica Silva Souza through its institutional scholarship program. We are thankful to Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, in Portuguese) for granting the Master’s scholarship (Finance Code I) to Aylla Joani Mendonça de Oliveira Pontes.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors would like to thank Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) for the Project UNIFEI/GIZ/FAPEPE: “Green Hydrogen Cluster CH2V” (“Centro de Hidrogênio Verde CH2V”, in Portuguese).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of Variance |
| APHA | American Public Health Association |
| BCB | Brazilian Central Bank |
| CAPEX | Capital Expenditure |
| CH4 | Methane |
| CO2 | Carbon Dioxide |
| COD | Chemical Oxygen Demand |
| Fe2+ | Ferrous Ion |
| Fe3+ | Ferric Ion |
| FSs | Fixed Solids |
| GHG | Greenhouse Gas |
| H2S | Hydrogen Sulfide |
| iNDC | Intended Nationally Determined Contribution |
| LCOH | Levelized Cost of Hydrogen |
| NDC | Nationally Determined Contribution |
| NPs | Nanoparticles |
| NPV | Net Present Value |
| OFMSW | Organic Fraction of Municipal Solid Waste |
| OPEX | Operating Expenses |
| PNH2 | National Hydrogen Program |
| STP | Standard Temperature and Pressure |
| TSs | Total Solids |
| UASB | Upflow Anaerobic Sludge Blanket |
| VFAs | Volatile Fatty Acids |
| VSs | Volatile Solids |
References
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- United Nations. Framework Convention on Climate Change: Adoption of the Paris Agreement—FCCC/CP/2015/L.9/Rev.1. UNFCCC. 12 December 2015. Available online: http://unfccc.int/files/bodies/awg/application/pdf/draft_paris_agreement_5dec15.pdf (accessed on 31 December 2024).
- Brazil. Intended Nationally Determined Contribution Towards the Achievement of the United Nations Framework Convention on Climate Change. Available online: https://antigo.mma.gov.br/images/arquivos/clima/convencao/indc/BRASIL_iNDC_portugues.pdf (accessed on 8 June 2023).
- Brazil. Ministry of Mines and Energy—MME. National Hydrogen Program—PNH2. Available online: https://www.gov.br/mme/pt-br/programa-nacional-do-hidrogenio-1 (accessed on 31 December 2024).
- Brazil. Ministry of Mines and Energy—MME. Proposed Guidelines for the National Hydrogen Program—PNH2. 2021. Available online: https://www.gov.br/mme/pt-br/assuntos/noticias/mme-apresenta-ao-cnpe-proposta-de-diretrizes-para-o-programa-nacional-do-hidrogenio-pnh2/HidrognioRelatriodiretrizes.pdf (accessed on 31 December 2024).
- Brazil. Law N°. 14,948 of 2 August 2024. Institutes the Legal Framework for Low-Carbon Hydrogen; Provides for the National Policy for Low-Carbon Hydrogen; Institutes Incentives for the Low-Carbon Hydrogen Industry; Institutes the Special Incentive Regime for the Production of Low-Carbon Hydrogen (Rehidro); Creates the Low-Carbon Hydrogen Development Program (PHBC); and Amends Laws No. 9427 of 26 December 1996, and 9478 of 6 August 1997. Federal Official Gazette. 02/08/2024—Extra Edition. Available online: https://www.planalto.gov.br/ccivil_03/_ato2023-2026/2024/lei/l14948.htm (accessed on 2 September 2024).
- Abrelpe. Panorama of Solid Waste in Brazil—2020. 2020. Available online: https://abrelpe.org.br/panorama-2020/ (accessed on 21 August 2023).
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- García-Depraect, O.; Vargas-Estrada, L.; Muñoz, R.; Castro-Muñoz, R. Membrane-Assisted Dark Fermentation for Integrated Biohydrogen Production and Purification: A Comprehensive Review. Fermentation 2025, 11, 19. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Performance of an innovative two-stage process converting food waste to hydrogen and methane. J. Air Waste Manag. Assoc. 2004, 54, 242–249. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The effects of Fe° and Ni° nanoparticles versus Fe2+ and Ni2+ ions on dark hydrogen fermentation. Int. J. Hydrogen Energy 2016, 41, 167–173. [Google Scholar] [CrossRef]
- Beckers, L.; Hiligsmann, S.; Lambert, S.D.; Heinrichs, B.; Thonart, P. Improving effect of metal and oxide nanoparticles encapsulated in porous silica on fermentative biohydrogen production by Clostridium butyricum. Bioresour. Technol. 2013, 133, 109–117. [Google Scholar] [CrossRef]
- Reddy, K.; Nasr, M.; Kumari, S.; Kumar, S.; Gupta, S.K.; Enitan, A.M.; Bux, F. Biohydrogen production from sugarcane bagasse hydrolysate: Effects of pH, S/X, Fe2+, and magnetite nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 8790–8804. [Google Scholar] [CrossRef]
- Pérez-Barragán, J.; Martínez-Fraile, C.; Muñoz, R.; Vargas-Estrada, L.; Maya-Yescas, R.; León-Becerril, E.; Castro-Muñoz, R.; García-Depraect, O. Iron (Magnetite) Nanoparticle-Assisted Dark Fermentation Process for Continuous Hydrogen Production from Rice Straw Hydrolysate. Appl. Sci. 2024, 14, 9660. [Google Scholar] [CrossRef]
- Sarkar, O.; Modestra, J.A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Waste-Derived Renewable Hydrogen and Methane: Towards a Potential Energy Transition Solution. Fermentation 2023, 9, 368. [Google Scholar] [CrossRef]
- Jariyaboon, R.; Hayeeyunu, S.; Usmanbaha, N.; Ismail, S.B.; O-Thong, S.; Mamimin, C.; Kongjan, P. Thermophilic Dark Fermentation for Simultaneous Mixed Volatile Fatty Acids and Biohydrogen Production from Food Waste. Fermentation 2023, 9, 636. [Google Scholar] [CrossRef]
- Barros, R.M.; Tiago Filho, G.L.; Moura, J.S.; Pieroni, M.F.; Vieira, F.C.; Lage, L.R.; Mohor, G.S.; Bastos, A.S. Design and Implementation Study of a Permanent Selective Collection Program (PSCP) on a University campus in Brazil. Resour. Conserv. Recycl. 2013, 80, 97–106. [Google Scholar] [CrossRef]
- Da Cruz, H.M.; Barros, R.M.; Dos Santos, I.F.S.; Tiago Filho, G.L. Estudo do potencial de geração de energia elétrica a partir do biogás de digestão anaeróbia de resíduos alimentares. Res. Soc. Dev. 2019, 8, 3785811. [Google Scholar] [CrossRef]
- Barros, R.M.; Tiago Filho, G.L.; Santos, A.H.M.; Ferreira, C.H.; Pieroni, M.F.; Moura, J.S.; Abe, H.S.D.S.; Brito, L.M.; Santos, I.F.S.D.; Ribeiro, E.M.; et al. A potential of the biogas generating and energy recovering from municipal solid waste. Renew. Energy Focus 2018, 25, 4–16. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Y.; Zhang, B.; Sun, H.; Wang, N.; Wang, L.; Zhang, J.; Xue, R. Comparison of magnetite/reduced graphene oxide nanocomposites and magnetite nanoparticles on enhancing hydrogen production in dark fermentation. Int. J. Hydrogen Energy. 2022, 47, 22359–22370. [Google Scholar] [CrossRef]
- Cañote, S.J.B.; Barros, R.M.; Lora, E.E.S.; del Olmo, O.A.; dos Santos, I.F.S.; Piñas, J.A.V.; Ribeiro, E.M.; de Freitas, J.V.R.; de Castro e Silva, H.L. Energy and Economic Evaluation of the Production of Biogas from Anaerobic and Aerobic Sludge in Brazil. Waste Biomass Valor 2021, 12, 947–969. [Google Scholar] [CrossRef]
- De Castro e Silva, H.L.; Silva, A.M.L.; Barros, R.M.; Dos Santos, I.F.S.; De Freitas, J.V.R. Addition of iron ore tailings to increase the efficiency of anaerobic digestion of pig manure: A technical and economic analysis. Biomass Bioenergy 2021, 148, 106013. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; APHA: Washington, DC, USA, 2022. [Google Scholar]
- Ribeiro, E.M.; Barros, R.M.; Tiago Filho, G.L.; Santos, I.F.S.; Sampaio, L.C.; dos Santos, T.V.; Da Silva, F.d.G.B.; Silva, A.P.M.; De Freitas, J.V.R. Feasibility of biogas and energy generation from poultry manure in Brazil. Waste Manag. Res. 2018, 36, 221–235. [Google Scholar] [CrossRef]
- Cormos, C.-C. Techno-economic and environmental assessment of green hydrogen production via biogas reforming with membrane-based CO2 capture. Int. J. Hydrogen Energy 2025, 101, 702–711. [Google Scholar] [CrossRef]
- Tahir, M.M.; Abbas, A.; Dickson, R. Green hydrogen and chemical production from solar energy in Pakistan: A geospatial, techno-economic, and environmental assessment. Int. J. Hydrogen Energy 2025, 116, 613–626. [Google Scholar] [CrossRef]
- Crispim, A.M.D.C.; Barros, R.M.; Tiago Filho, G.L.; Dos Santos, I.F.S. An economic study of hydrogen and ammonia generation from the reforming of biogas from co-digestion of municipal solid waste and wastewater sludge in a Brazilian state. Int. J. Hydrogen Energy 2024, 67, 312–326. [Google Scholar] [CrossRef]
- Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Enhancement of dark fermentative H2 production by gas separation membranes: A review. Bioresour. Technol. 2020, 302, 122828. [Google Scholar] [CrossRef] [PubMed]
- Brazil. Ministry of Mines and Energy, Energy Research CompanyTen-Year Energy Expansion Plan 2034/Ministry of Mines and Energy. Energy Research Company. Brasília: MME/EPE. 2024. Available online: https://www.epe.gov.br/sites-pt/publicacoes-dados-abertos/publicacoes/PublicacoesArquivos/publicacao-804/topico-758/PDE2034_Aprovado.pdf (accessed on 2 September 2024).
- Brazilian Central Bank BCB. Currency Converter. Available online: https://www.bcb.gov.br/conversao (accessed on 28 July 2025).
- Ren, Y.; Si, B.; Liu, Z.; Jiang, W.; Zhang, Y. Promoting dark fermentation for biohydrogen production: Potential roles of iron-based additives. Int. J. Hydrogen Energy 2022, 47, 1499–1515. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Joseph, K.; Sukumaran, V. Bio hydrogen generation from kitchen waste in an inclined plug flow reactor. Int. J. Hydrogen Energy 2009, 34, 8854–8858. [Google Scholar] [CrossRef]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Improving hydrogen production from cassava starch by combination of dark and photo fermentation. Int. J. Hydrogen Energy 2009, 34, 1780–1786. [Google Scholar] [CrossRef]
- Androga, D.D.; Ozgur, E.; Gunduz, U.; Yucel, M.; Eroglu, I. Factors affecting the longterm stability of biomass and hydrogen productivity in outdoor photofermentation. Int. J. Hydrogen Energy 2011, 36, 11369–11378. [Google Scholar] [CrossRef]
- Putatunda, C.; Behl, M.; Solanki, P.; Sharma, S.; Bhatia, S.K.; Walia, A.; Bhatia, R.K. Current challenges and future technology in photofermentation-driven biohydrogen production by utilizing algae and bacteria. Int. J. Hydrogen Energy 2023, 48, 21088–21109. [Google Scholar] [CrossRef]
- Lay, J.; Fan, K.; Hwang, J.; Chang, J.; Hsu, P. Factors Affecting hydrogen production from food wastes by Clostridium-Rich Composts. J. Environ. Eng. 2005, 131, 595–602. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Enhanced biohydrogen production from macroalgae by zero-valent iron nanoparticles: Insights into microbial and metabolites distribution. Bioresour. Technol. 2019, 282, 110–117. [Google Scholar] [CrossRef]
- Camacho, C.E.G.; Romano, F.I.; Ruggeri, B. Macro approach analysis of dark biohydrogen production in the presence of zero valent powered Fe degrees. Energy 2018, 159, 525–533. [Google Scholar] [CrossRef]
- Regueira-Marcos, L.; García-Depraect, O.; Muñoz, R. Elucidating the role of pH and total solids content in the co-production of biohydrogen and carboxylic acids from food waste via lactate-driven dark fermentation. Fuel 2023, 338, 127238. [Google Scholar] [CrossRef]
- Castagnoli, A.; Falcioni, S.; Touloupakis, E.; Pasciucco, F.; Pasciucco, E.; Michelotti, A.; Iannelli, R.; Pecorini, I. Influence of Aeration Rate on Uncoupled Fed Mixed Microbial Cultures for Polyhydroxybutyrate Production. Sustainability 2024, 16, 2961. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).