Towards the Potential of Using Downstream-Separated Solvents as the Pulping Liquor of Upstream Lignocellulose Fractionation for Enhanced Acetone–Butanol–Ethanol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Organosolv Pulping
2.3. Enzymatic Hydrolysis
2.4. ABE Fermentation
2.5. Analytical Methods
3. Results and Discussions
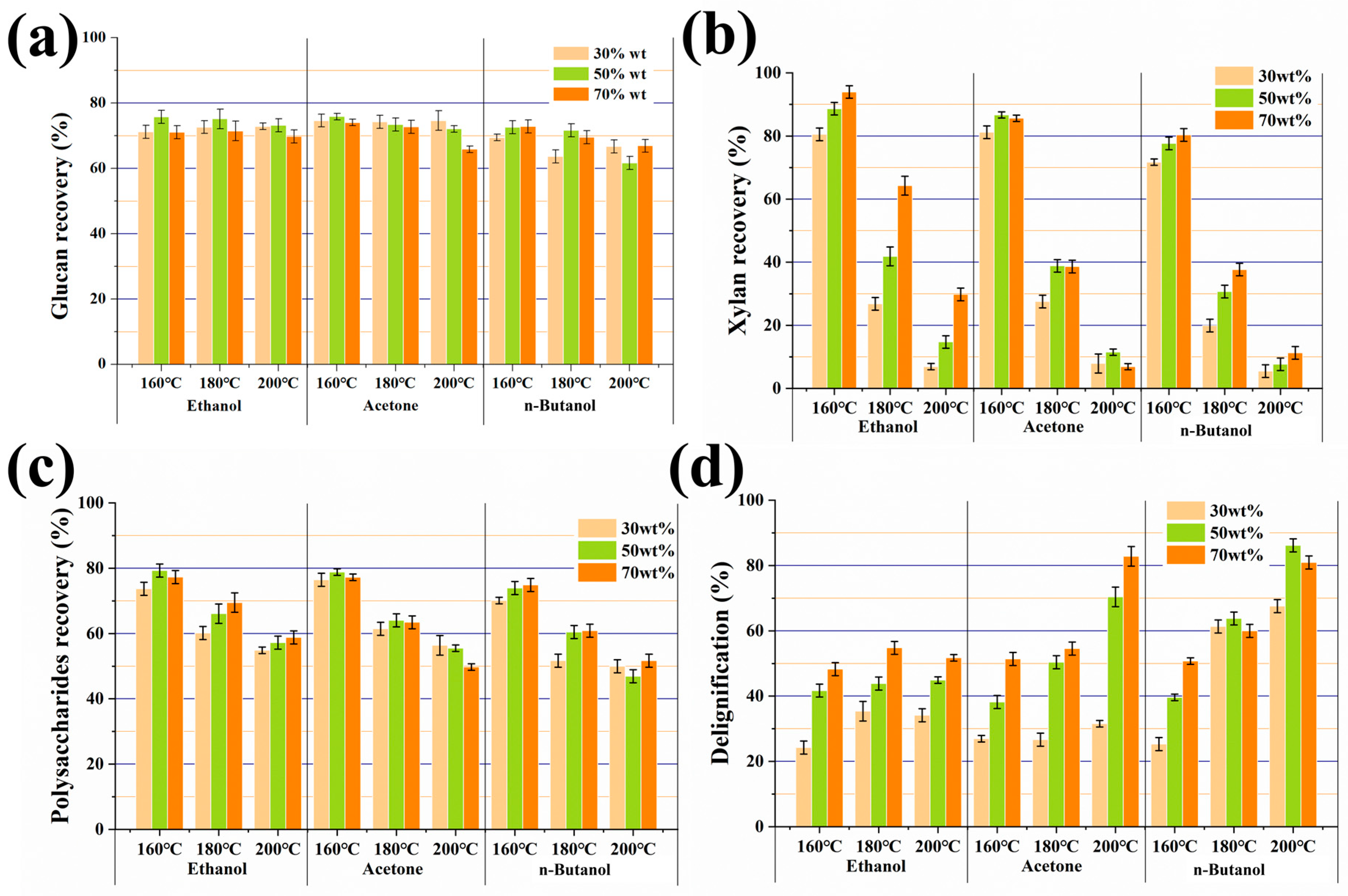
3.1. Organosolv Pulping Using Binary A-B-E and Water Solution
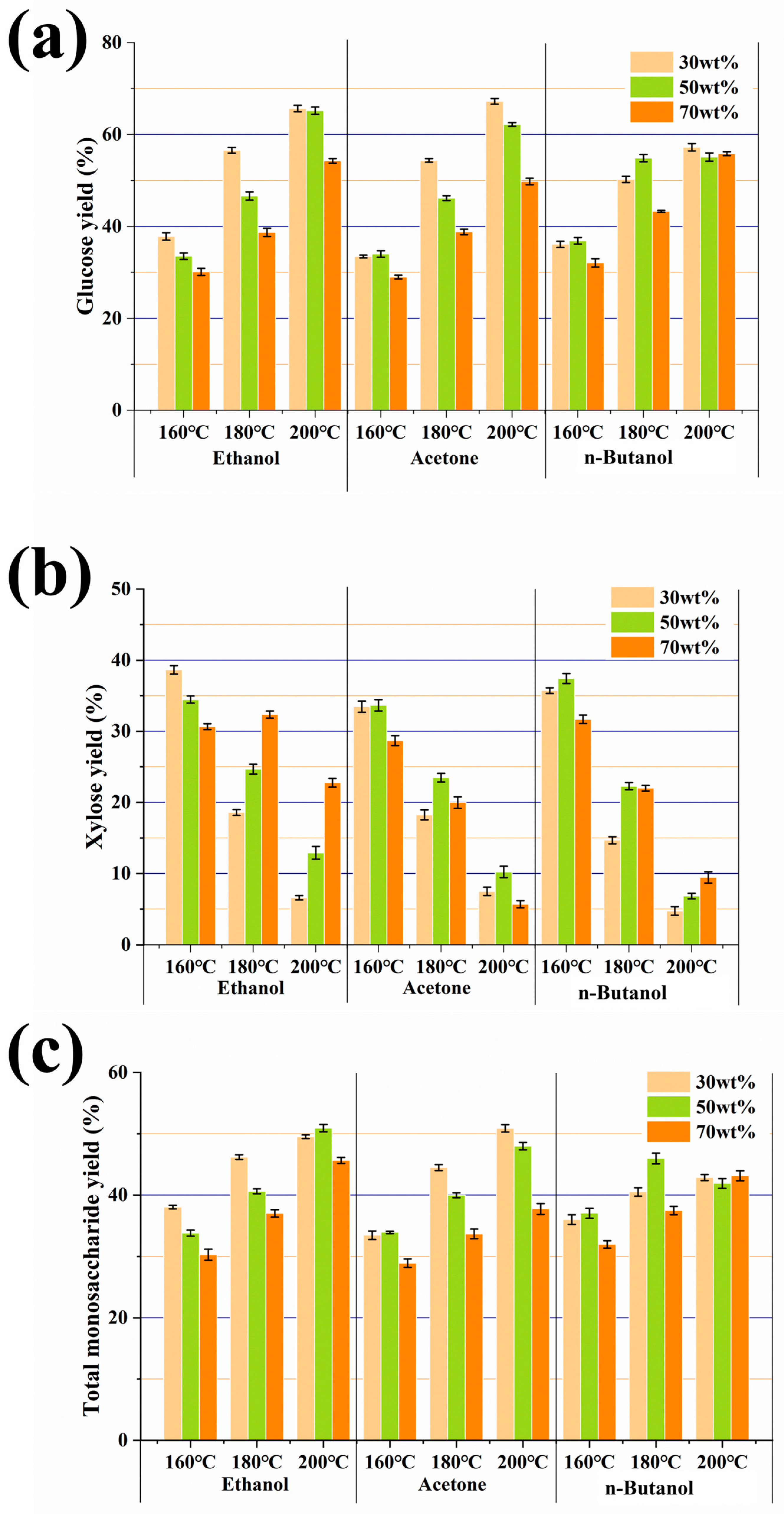
3.2. Enzymatic Hydrolysis of Pretreated Pulps
3.3. Characterization of the Fractionated Lignin
3.4. ABE Fermentation Using the Enzymatic Hydrolysate of Pulps
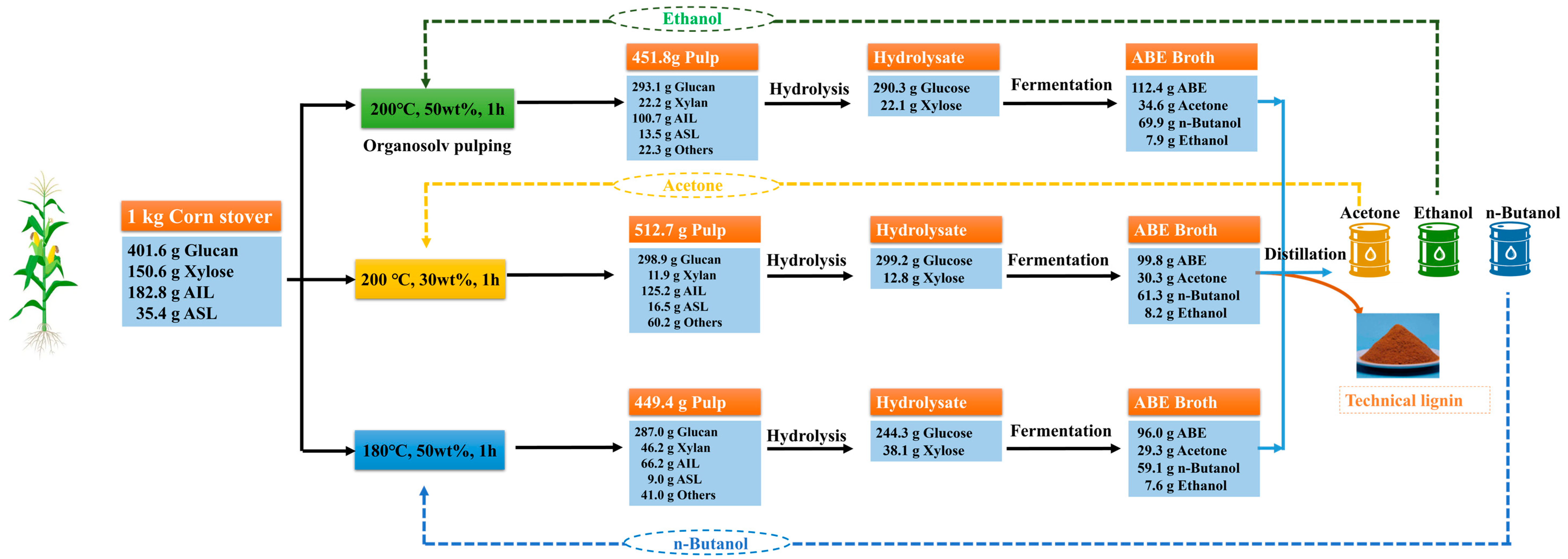
3.5. Mass Balance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Zhong, H.; Chen, Z.; Wu, M.; Cheng, K. Enhanced biobutanol production through online product separation technology. Renew. Sustain. Energy Rev. 2025, 215, 115637. [Google Scholar] [CrossRef]
- Lin, Z.; Cong, W.; Zhang, J. Biobutanol production from acetone–butanol–ethanol fermentation: Developments and prospects. Fermentation 2023, 9, 847. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Li, X.; Shi, J.; Liu, L. Exploring the potential of multiple lignocellulosic biomass as a feedstock for biobutanol production. Fuel 2024, 357, 129697. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, Y.; Wu, R.; Sui, Y.; Chen, C.; Xin, F.; Zhou, J.; Dong, W.; Jiang, M. Current status and perspectives on biobutanol production using lignocellulosic feedstocks. Bioresour. Technol. Rep. 2019, 7, 100245. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefinery 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Panovic, I.; Deuss, P.J.; Barta, K.; Westwood, N.J. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: Towards complete biomass valorisation. Green Chem. 2017, 19, 202–214. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef]
- Agwu, K.A.; Belmont, S.R.; Enguita, J.M.; Sheehan, J.D. Polar aprotic solvent properties influence pulp characteristics and delignification kinetics of CO2/Organic base organosolv pretreatments of lignocellulosic biomass. Chem. Eng. Sci. 2024, 288, 119808. [Google Scholar] [CrossRef]
- Duarte, L.C.; Sampaio, B.; Carvalheiro, F. Organosolv Pretreatment of Lignocellulosic Biomass. In Handbook of Biorefinery Research and Technology: Biomass Logistics to Saccharification; Springer: Berlin/Heidelberg, Germany, 2024; pp. 487–514. [Google Scholar]
- Bozell, J.J.; Black, S.K.; Myers, M.; Cahill, D.; Miller, W.P.; Park, S. Solvent fractionation of renewable woody feedstocks: Organosolv generation of biorefinery process streams for the production of biobased chemicals. Biomass Bioenergy 2011, 35, 4197–4208. [Google Scholar] [CrossRef]
- Schulze, P.; Leschinsky, M.; Seidel-Morgenstern, A.; Lorenz, H. Continuous separation of lignin from organosolv pulping liquors: Combined lignin particle formation and solvent recovery. Ind. Eng. Chem. Res. 2019, 58, 3797–3810. [Google Scholar] [CrossRef]
- Jafari, Y.; Amiri, H.; Karimi, K. Acetone pretreatment for improvement of acetone, butanol, and ethanol production from sweet sorghum bagasse. Appl. Energy 2016, 168, 216–225. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 2014, 152, 450–456. [Google Scholar] [CrossRef]
- Nan, Y.; Yang, M.; Xin, D.; Li, K.; Kuittinen, S.; Pappinen, A.; Zhang, J. Acetone-butanol-ethanol solvents improved enzymatic hydrolysis of pretreated energy grass. Fuel 2019, 245, 406–412. [Google Scholar] [CrossRef]
- Teramura, H.; Sasaki, K.; Oshima, T.; Matsuda, F.; Okamoto, M.; Shirai, T.; Kawaguchi, H.; Ogino, C.; Hirano, K.; Sazuka, T. Organosolv pretreatment of sorghum bagasse using a low concentration of hydrophobic solvents such as 1-butanol or 1-pentanol. Biotechnol. Biofuels 2016, 9, 27. [Google Scholar] [CrossRef]
- Li, R.; Zheng, Y.; Zhao, X.; Yong, Q.; Meng, X.; Ragauskas, A.; Huang, C. Recent advances in biomass pretreatment using biphasic solvent systems. Green Chem. 2023, 25, 2505–2523. [Google Scholar] [CrossRef]
- Jang, J.H.; Callejón Álvarez, J.; Neuendorf, Q.S.; Román-Leshkov, Y.; Beckham, G.T. Reducing Solvent Consumption in Reductive Catalytic Fractionation through Lignin Oil Recycling. ACS Sustain. Chem. Eng. 2024, 12, 12919–12926. [Google Scholar] [CrossRef]
- Yang, M.; Guo, X.; Liu, G.; Nan, Y.; Zhang, J.; Noyazzesh, H.; Kuittinen, S.; Vepsäläinen, J.; Pappinen, A. Effect of solvent mixture pretreatment on sugar release from short-rotation coppice Salix schwerinii for biobutanol production. Bioresour. Technol. 2022, 344, 126262. [Google Scholar] [CrossRef]
- Cai, D.; Li, P.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Wang, Z.; Tan, T. Effect of dilute alkaline pretreatment on the conversion of different parts of corn stalk to fermentable sugars and its application in acetone–butanol–ethanol fermentation. Bioresour. Technol. 2016, 211, 117–124. [Google Scholar] [CrossRef]
- Cai, D.; Wen, J.; Wu, Y.; Su, C.; Bi, H.; Wang, Y.; Jiang, Y.; Qin, P.; Tan, T.; Zhang, C. Surfactant-assisted dilute ethylenediamine fractionation of corn stover for technical lignin valorization and biobutanol production. Bioresour. Technol. 2024, 394, 130231. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Gao, Y.; Zhang, G.; Zhang, X.; Li, Y.; Zhang, H.; Wen, H.; Ren, W.; Zhang, C.; Cai, D. Circulating of In Situ Recovered Stream from Fermentation Broth as the Liquor for Lignocellulosic Biobutanol Production. Fermentation 2025, 11, 453. [Google Scholar] [CrossRef]
- Cai, D.; Chen, H.; Chen, C.; Hu, S.; Wang, Y.; Chang, Z.; Miao, Q.; Qin, P.; Wang, Z.; Wang, J. Gas stripping–pervaporation hybrid process for energy-saving product recovery from acetone–butanol–ethanol (ABE) fermentation broth. Chem. Eng. J. 2016, 287, 1–10. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Su, C.; Cai, D.; Zhang, H.; Wu, Y.; Jiang, Y.; Liu, Y.; Zhang, C.; Li, C.; Qin, P.; Tan, T. Pilot-scale acetone-butanol-ethanol fermentation from corn stover. Green Carbon 2024, 2, 81–93. [Google Scholar] [CrossRef]
- Cai, D.; Hu, S.; Miao, Q.; Chen, C.; Chen, H.; Zhang, C.; Li, P.; Qin, P.; Tan, T. Two-stage pervaporation process for effective in situ removal acetone-butanol-ethanol from fermentation broth. Bioresour. Technol. 2017, 224, 380–388. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Q.; Wang, W.; Zhuang, X.; Deng, Y.; Yuan, Z. Comparison study of organosolv pretreatment on hybrid pennisetum for enzymatic saccharification and lignin isolation. Fuel 2019, 249, 334–340. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Liu, C.; Sun, R. Comparative study of alkali-and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydr. Res. 2006, 341, 253–261. [Google Scholar] [CrossRef]
- Song, Y.; Ji, H.; Shi, X.; Yang, X.; Zhang, X. Successive organic solvent fractionation and structural characterization of lignin extracted from hybrid poplar by deep eutectic solvent for improving the homogeneity and isolating narrow fractions. Renew. Energy 2020, 157, 1025–1034. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, a.T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Katahira, R.; Mittal, A.; McKinney, K.; Ciesielski, P.N.; Donohoe, B.S.; Black, S.K.; Johnson, D.K.; Biddy, M.J.; Beckham, G.T. Evaluation of clean fractionation pretreatment for the production of renewable fuels and chemicals from corn stover. Acs Sustain. Chem. Eng. 2014, 2, 1364–1376. [Google Scholar] [CrossRef]
- Huijgen, W.J.; Reith, J.H.; den Uil, H. Pretreatment and fractionation of wheat straw by an acetone-based organosolv process. Ind. Eng. Chem. Res. 2010, 49, 10132–10140. [Google Scholar] [CrossRef]
- Sun, D.; Lv, Z.-W.; Rao, J.; Tian, R.; Sun, S.-N.; Peng, F. Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef]
- Gabhane, J.; William, S.P.; Vaidya, A.N.; Das, S.; Wate, S.R. Solar assisted alkali pretreatment of garden biomass: Effects on lignocellulose degradation, enzymatic hydrolysis, crystallinity and ultra-structural changes in lignocellulose. Waste Manag. 2015, 40, 92–99. [Google Scholar] [CrossRef]
- Jeoh, T.; Ishizawa, C.I.; Davis, M.F.; Himmel, M.E.; Adney, W.S.; Johnson, D.K. Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 2007, 98, 112–122. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst. Eng. 2015, 38, 1959–1972. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Zhang, Y.-H.P. Cellulose solvent-and organic solvent-based lignocellulose fractionation enabled efficient sugar release from a variety of lignocellulosic feedstocks. Bioresour. Technol. 2012, 117, 228–233. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Wang, W.; Yu, Q.; Wang, Q.; Miao, C.; Guo, Y.; Zhuang, X.; Yuan, Z. Screening solvents based on Hansen solubility parameter theory to depolymerize lignocellulosic biomass efficiently under low temperature. ACS Sustain. Chem. Eng. 2019, 7, 8678–8686. [Google Scholar] [CrossRef]
- Ni, H.; Ren, S.; Fang, G.; Ma, Y. Determination of alkali lignin solubility parameters by inverse gas chromatography and Hansen solubility parameters. BioResources 2016, 11, 4353–4368. [Google Scholar] [CrossRef]
- Novo, L.P.; Curvelo, A.A. Hansen solubility parameters: A tool for solvent selection for organosolv delignification. Ind. Eng. Chem. Res. 2019, 58, 14520–14527. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sun, H.; Sui, W.; Si, C. Lignin fractionation: Effective strategy to reduce molecule weight dependent heterogeneity for upgraded lignin valorization. Ind. Crops Prod. 2021, 165, 113442. [Google Scholar] [CrossRef]
- Parot, M.; Rodrigue, D.; Stevanovic, T. High purity softwood lignin obtained by an eco-friendly organosolv process. Bioresour. Technol. Rep. 2022, 17, 100880. [Google Scholar] [CrossRef]
- Wen, J.-L.; Xue, B.-L.; Xu, F.; Sun, R.-C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- You, T.-T.; Mao, J.-Z.; Yuan, T.-Q.; Wen, J.-L.; Xu, F. Structural elucidation of the lignins from stems and foliage of Arundo donax Linn. J. Agric. Food Chem. 2013, 61, 5361–5370. [Google Scholar] [CrossRef]
- Sun, S.-N.; Li, M.-F.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Effect of ionic liquid/organic solvent pretreatment on the enzymatic hydrolysis of corncob for bioethanol production. Part 1: Structural characterization of the lignins. Ind. Crops Prod. 2013, 43, 570–577. [Google Scholar] [CrossRef]
- Wang, N.; Xu, B.; Wang, X.; Lang, J.; Zhang, H. Chemical and structural elucidation of lignin and cellulose isolated using DES from bagasse based on alkaline and hydrothermal pretreatment. Polymers 2022, 14, 2756. [Google Scholar] [CrossRef]
- Yang, S.; Chen, K.; Zhu, Z.; Guan, Q.; Zhou, H.; He, L. A green pretreatment approach of corn stalk wastes for obtaining micro/nano-cellulose fibers, monosaccharides and lignin fractions. Renew. Energy 2022, 194, 746–759. [Google Scholar] [CrossRef]
- Mingliu, L.; Zhang, H.; Zheng, H.; Yuanyuan, L.; Huang, H.; Rong, X. Characterization of lignins isolated from alkali treated prehydrolysate of corn stover. Chin. J. Chem. Eng. 2013, 21, 427–433. [Google Scholar] [CrossRef]
- Miao, Z.; Li, Z.; Teng, X.; Wang, H.; Zhou, Y.; Qiu, Y.; Li, C.; Liu, C.; Tan, Y. Density Functional Theory Calculations and Infrared Spectral Analysis of Lignin. Molecules 2024, 29, 5683. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, J.; Wang, X.; Guo, Y.; Han, Y.; Zhou, J. Lignin structure and solvent effects on the selective removal of condensed units and enrichment of S-type lignin. Polymers 2018, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, Z.; Wang, X.; Sheng, Y.; Liu, Y. Demethylation of ethanol organosolv lignin by Na2SO3 for enhancing antioxidant performance. Sustain. Chem. Pharm. 2023, 36, 101312. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Zhang, H.; Wu, Y.; Su, C.; Guo, B.; Liu, Y.; Sun, M.; Li, C.; Cai, D. Hydrogen-Transfer Reductive Catalytic Depolymerization of the Solid Residue from a Demonstration Lignocellulosic Ethanol Plant. ACS Sustain. Chem. Eng. 2025, 13, 6936–6945. [Google Scholar] [CrossRef]
- Reiter, J.; Strittmatter, H.; Wiemann, L.O.; Schieder, D.; Sieber, V. Enzymatic cleavage of lignin β-O-4 aryl ether bonds via net internal hydrogen transfer. Green Chem. 2013, 15, 1373–1381. [Google Scholar] [CrossRef]
- Wen, J.-L.; Xue, B.-L.; Xu, F.; Sun, R.-C. Unveiling the structural heterogeneity of bamboo lignin by in situ HSQC NMR technique. BioEnergy Res. 2012, 5, 886–903. [Google Scholar] [CrossRef]
- Xu, G.; Shi, Z.; Zhao, Y.; Deng, J.; Dong, M.; Liu, C.; Murugadoss, V.; Mai, X.; Guo, Z. Structural characterization of lignin and its carbohydrate complexes isolated from bamboo (Dendrocalamus sinicus). Int. J. Biol. Macromol. 2019, 126, 376–384. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y. Recent advances in the application of alcohols in extracting lignin with preserved β-O-4 content from lignocellulosic biomass. Bioresour. Technol. 2023, 384, 129238. [Google Scholar] [CrossRef]
- Wu, M.; Pang, J.; Zhang, X.; Sun, R. Enhancement of lignin biopolymer isolation from hybrid poplar by organosolv pretreatments. Int. J. Polym. Sci. 2014, 2014, 194726. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, J.; Su, C.; Jiang, C.; Zhang, C.; Wang, Y.; Jiang, Y.; Ren, W.; Qin, P.; Cai, D. Inhibitions of microbial fermentation by residual reductive lignin oil: Concerns on the bioconversion of reductive catalytic fractionated carbohydrate pulp. Chem. Eng. J. 2023, 452, 139267. [Google Scholar] [CrossRef]
- Chen, H.; Cai, D.; Chen, C.; Wang, J.; Qin, P.; Tan, T. Novel distillation process for effective and stable separation of high-concentration acetone–butanol–ethanol mixture from fermentation–pervaporation integration process. Biotechnol. Biofuels 2018, 11, 286. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Integration of autohydrolysis and organosolv delignification for efficient acetone, butanol, and ethanol production and lignin recovery. Ind. Eng. Chem. Res. 2016, 55, 4836–4845. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Liu, J.; Shen, T.; Cao, Z.; Shan, J.; Zhu, C.; Ying, H. Sustainable biobutanol production using alkali-catalyzed organosolv pretreated cornstalks. Ind. Crops Prod. 2017, 95, 383–392. [Google Scholar] [CrossRef]
- Li, J.; Shi, S.; Tu, M.; Via, B.; Sun, F.F.; Adhikari, S. Detoxification of organosolv-pretreated pine prehydrolysates with anion resin and cysteine for butanol fermentation. Appl. Biochem. Biotechnol. 2018, 186, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ren, W.; Guo, F.; Wang, H.; Yu, Y. Structural elucidation of lignin, hemicelluloses and LCC from both bamboo fibers and parenchyma cells. Int. J. Biol. Macromol. 2024, 274, 133341. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, M.; Jiao, L.; Dai, H. Molecular weight distribution and dissolution behavior of lignin in alkaline solutions. Polymers 2021, 13, 4166. [Google Scholar] [CrossRef]
- Wen, J.; Yuan, T.; Sun, S.; Xu, F.; Sun, R. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]




| Sample | Mw (g/mol) | Mn (g/mol) | PDI (Mw/Mn) |
|---|---|---|---|
| Ethanol-200 °C-50% | 1270 | 1150 | 1.10 |
| Acetone-200 °C-30% | 1080 | 1030 | 1.05 |
| Butanol-180 °C-50% | 1280 | 1160 | 1.10 |
| Solvent Condition | Raw Materials | Pretreatment Conditions | Enzymatic Hydrolysis Efficiency (%) | Strain | ABE Concentration (g/L) | ABE Yield from Monosaccharide (g/g) | Solvent Yield per kg of Straw (g/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 75% (v/v)Ethanol + 1% (w/w) H2SO4 | Elm wood | 180 °C, 60 min | — | Clostridium acetobutylicumnrrl B-591 | 11.6 | — | 121.1 | [40] |
| 75% (v/v) Ethanol + 1% (w/w) H2SO4 | Rice straw | 180 °C, 30 min | — | Clostridium acetobutylicumnrrl B-591 | 10.5 | — | 123.9 | [16] |
| 75% (v/v) Ethanol + 1% (w/w) H2SO4 | Elm wood | Autohydrolysis (180 ℃, 60 min) + Organosolv (180 ℃, 60 min) | — | Clostridium acetobutylicumnrrl B-591 | 12.7 | — | 133 | [64] |
| 60% Ethanol + 4% NaOH | Cornstalks | 110 °C, 90 min | Cellulose 85%, hemicellulose 82% | Clostridium beijerinckii NCIMB 4110 | 12.8 | 0.43 | — | [65] |
| 50% Acetone + 0.1% H2SO4 | Sweet sorghum bagasse | 180 °C, 60 min | 94.2% | Clostridium acetobutylicumnrrl B-591 | 11.4 | 0314 | 125 | [15] |
| 65% (v/v) Ethanol + 1% (w/w) H2SO4 | Loblolly pine | 170 °C, 60 min | 92.0% | Clostridium acetobutylicum ATCC 824 | 11.11 | 0.21 | — | [66] |
| 168.24 g/l ABE solvent a +1% NaOH | Corn stover | 160 °C, 1 h | 92.6% | Clostridium acetobutylicum ABE1401 | 13.5 | 0.331 | 106.6 | [24] |
| 50%wt Ethanol | Corn stover | 200 ℃, 1 h | 89.01% | Clostridium acetobutylicum ABE1401 | 15.03 | 0.36 | 112.4 | This study |
| 30%wt Acetone | Corn stover | 200 ℃, 1 h | 90.26% | Clostridium acetobutylicum ABE1401 | 11.68 | 0.32 | 99.8 | This study |
| 50%wt Butanol | Corn stover | 180 ℃, 1 h | 76.05% | Clostridium acetobutylicum ABE1401 | 12.82 | 0.34 | 96.0 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Gao, Y.; Zhang, G.; Wen, H.; Chen, R.; Wang, J.; Li, Y.; Sun, M.; Cao, J.; Cai, D. Towards the Potential of Using Downstream-Separated Solvents as the Pulping Liquor of Upstream Lignocellulose Fractionation for Enhanced Acetone–Butanol–Ethanol Production. Fermentation 2025, 11, 514. https://doi.org/10.3390/fermentation11090514
Su C, Gao Y, Zhang G, Wen H, Chen R, Wang J, Li Y, Sun M, Cao J, Cai D. Towards the Potential of Using Downstream-Separated Solvents as the Pulping Liquor of Upstream Lignocellulose Fractionation for Enhanced Acetone–Butanol–Ethanol Production. Fermentation. 2025; 11(9):514. https://doi.org/10.3390/fermentation11090514
Chicago/Turabian StyleSu, Changsheng, Yunxing Gao, Gege Zhang, Hao Wen, Rui Chen, Jiajing Wang, Yujie Li, Mingyuan Sun, Jikang Cao, and Di Cai. 2025. "Towards the Potential of Using Downstream-Separated Solvents as the Pulping Liquor of Upstream Lignocellulose Fractionation for Enhanced Acetone–Butanol–Ethanol Production" Fermentation 11, no. 9: 514. https://doi.org/10.3390/fermentation11090514
APA StyleSu, C., Gao, Y., Zhang, G., Wen, H., Chen, R., Wang, J., Li, Y., Sun, M., Cao, J., & Cai, D. (2025). Towards the Potential of Using Downstream-Separated Solvents as the Pulping Liquor of Upstream Lignocellulose Fractionation for Enhanced Acetone–Butanol–Ethanol Production. Fermentation, 11(9), 514. https://doi.org/10.3390/fermentation11090514





